Abstract
Purpose:
There is considerable evidence regarding the efficacy and effectiveness of BRCA genetic testing programs, but whether they represent good use of financial resources is not clear. Therefore, we aimed to identify the main health-care programs for BRCA testing and to evaluate their cost-effectiveness.
Methods:
We performed a systematic review of full economic evaluations of health-care programs involving BRCA testing.
Results:
Nine economic evaluations were included, and four main categories of BRCA testing programs were identified: (i) population-based genetic screening of individuals without cancer, either comprehensive or targeted based on ancestry; (ii) family history (FH)-based genetic screening, i.e., testing individuals without cancer but with FH suggestive of BRCA mutation; (iii) familial mutation (FM)-based genetic screening, i.e., testing individuals without cancer but with known familial BRCA mutation; and (iv) cancer-based genetic screening, i.e., testing individuals with BRCA-related cancers.
Conclusions:
Currently BRCA1/2 population-based screening represents good value for the money among Ashkenazi Jews only. FH-based screening is potentially very cost-effective, although further studies that include costs of identifying high-risk women are needed. There is no evidence of cost-effectiveness for BRCA screening of all newly diagnosed cases of breast/ovarian cancers followed by cascade testing of relatives, but programs that include tools for identifying affected women at higher risk for inherited forms are promising. Cost-effectiveness is highly sensitive to the cost of BRCA1/2 testing.
Genet Med 18 12, 1171–1180.
Keywords: BRCA, breast cancer, cost-effectiveness, health-care programs, systematic review
According to the Centers for Disease Control and Prevention, breast cancer susceptibility gene (BRCA) mutation testing is one of the few genetic applications with evidence of validity and utility that supports its implementation into practice.1 However, such recommendations do not take into account the economic perspective. Furthermore, the available assessment tools for evaluating genetic tests, such as the Evaluation of Genomic Applications in Practice and Prevention model, do not provide adequate information on the economic aspects.2 Moreover, there has been little research on the effectiveness or return on investment of implementation and dissemination research.3,4,5 Economic evaluation may be useful in this regard because it analyzes the delivery of a specific genomic test under real-life conditions within a genomic health-care program that has the underlying objective of maximizing the health of the population with the available resources.6
Inheritance of a dominant mutation in one of two breast/ovarian cancer susceptibility genes (BRCA1 and BRCA2) is responsible for 2–7% of breast cancers and 10–15% of ovarian cancers.7,8,9 Implementation of prevention programs would reduce the burden of such inherited diseases, and in some countries public health genomics policies have already been formulated and genetic interventions included in public health strategies for preventive medicine. For example, in Italy, the 2014–2018 National Prevention Plan contemplates the development of regional BRCA genetic testing programs because such programs can increase awareness of hereditary cancer risk in the population and reduce incidence and mortality of BRCA-related cancers.10 However, it is not clear which BRCA genetic programs are the most efficient and therefore which should be implemented. Can population-based screening for BRCA mutations be considered good value for the money, and under what conditions? Does limiting BRCA testing to at-risk individuals yield the most health gains at an acceptable cost? Or could it be more cost-effective to begin testing individuals with breast or ovarian cancers to find probands and to follow up with cascade screening among relatives?
This study has two purposes. The first is to identify the BRCA genetic testing programs whose cost-effectiveness has been analyzed in published economic evaluations. The second is to provide an overview of which BRCA testing programs are potentially ready for implementation on the basis of their cost-effectiveness, structure, and main assumptions, together with a discussion of the difficulties of transferring context-specific tools such as economic evaluations to other settings.11
Materials and Methods
This review was conducted according to the Center for Reviews and Dissemination guidance on undertaking systematic reviews of economic evaluations12 and the Cochrane Handbook for systematic reviews of interventions.13
Inclusion criteria
Broad inclusion criteria were used to encompass all economic evaluations of BRCA testing programs in the literature. A BRCA testing program was defined as any type of health intervention that includes BRCA testing in a target population with the purpose of reducing the risk of breast and ovarian cancer (initial and second primary), for example, by prophylactic surgery or intense surveillance of mutation-positive patients. According to this definition, a BRCA testing program should consist of the following components: a target population to test, genetic counseling, genetic testing to identify mutation carriers, and specific health-care pathways for individuals with and without genetic mutations. In particular, studies considered suitable for inclusion should evaluate the costs and benefits of a BRCA testing program, as well as the consequent health-care pathways specified.
We included studies that used standard full economic evaluation designs such as cost-effectiveness analysis (CEA), cost-utility analysis (CUA), cost-benefit analysis, or cost-minimization analysis.6 All health-related and cancer-related outcomes were included. It was anticipated that the majority of studies would measure the number of cases of cancer detected, life-years gained (LYG), or quality-adjusted life-years (QALYs). Studies were included regardless of the perspective of the evaluation (health-care system or a broad societal perspective).
Search strategy
Relevant studies (from inception to December 2014) were searched using the following databases: Medline, Embase, Scopus, the Health Economic Evaluations Database (HEED), the CEA Registry, the Economics Literature Index (EconLit), the Health Technology Assessment (HTA) Database, and the National Health Service Economic Evaluation Database (NHS EED). The literature search was rerun in February 2015 to identify any relevant studies published since the original search date. Two investigators conducted the literature search independently, to enhance sensitivity. The search terms were developed in relation to the intervention, outcomes, and designs of the studies: “BRCA1 OR BRCA2” AND “genetic* OR gene OR genom*” AND “economic* OR cost-effectiveness OR cost-utility OR cost-benefit OR cost-minimization OR cost*”. The strings were adjusted for each database while maintaining a common overall architecture. The search strategy for Medline and Embase included both MeSH terms and free texts of the primary search terms. The reference lists of retrieved articles were also searched to identify potentially relevant studies.
Selection of studies
Two reviewers selected relevant studies, removed duplicates, and screened titles and abstracts of the returned citations. Studies that clearly did not meet the inclusion criteria were excluded. Full texts of potentially relevant articles were retrieved and independently examined by two pairs of reviewers to determine the eligibility of papers. Disagreements were resolved through discussion and reasons for exclusion recorded.
Data extraction and quality assessment
Data were collected from the included studies by two reviewers independently and checked by a third reviewer. Data extraction focused on key methodological features (type of economic evaluation, analytical approach, outcome measures, study perspective, collection of cost and effectiveness data, time horizon, discounting, sensitivity analyses), key characteristics of the intervention (setting, target population, gene and clinical condition, scope of testing), and health-care pathways. Additional information, such as authors, journal, funding declaration, and year of publication, was also extracted.
Quality was assessed using two tools: the BMJ checklist14 and the Quality of Health Economic Studies (QHES) list.15 The former gives a qualitative assessment of quality of economic evaluations, and the second is quantitative, enabling us to integrate both approaches. The BMJ tool is more detailed than the QHES instrument (35 vs. 16 items); each item on the BMJ checklist reflects a specific aspect of methodological process, whereas the QHES list includes double-barreled items (multiple methodological aspects under the same item). The BMJ tool specifically identifies every potential methodological error and produces a descriptive critical assessment, whereas the QHES tool gives an overall score for each included study.
Three reviewers performed the quality assessment of studies using both checklists independently. Cohen's kappa was calculated to quantify the level of agreement among reviewers, and disagreements were resolved by peer discussion.16
Because quality assessment primarily reflects the extent to which the studies adhere to standard methods, an effort was made to evaluate the plausibility of the basic assumptions of each study to better appraise the quality of its findings.
Data synthesis
Owing to substantial heterogeneity among studies, a meta-analysis was not possible. A narrative synthesis of the identified studies was performed to summarize the key features of the included studies and to compare study questions, interventions, methods, and results. In both CEA and CUA, the main result is usually expressed as an incremental cost-effectiveness ratio (ICER), defined as the ratio that relates difference in costs to difference in outcomes between two alternative interventions. Policymakers may decide if an intervention is an efficient use of resources using a threshold of maximally acceptable cost per unit of outcome. Because there is no universally accepted ICER threshold, we adopted the most common cost-effectiveness thresholds used in the United States ($50,000–100,000/QALY or LYG)17,18 and the United Kingdom (£20,000–30,000/QALY or LYG).19 The ICERs provided by the CEA registry, maintained by Tufts Medical Center, were also extracted.20 In the Tufts database, all the original ICERs are converted into US dollars and adjusted for inflation.
Results
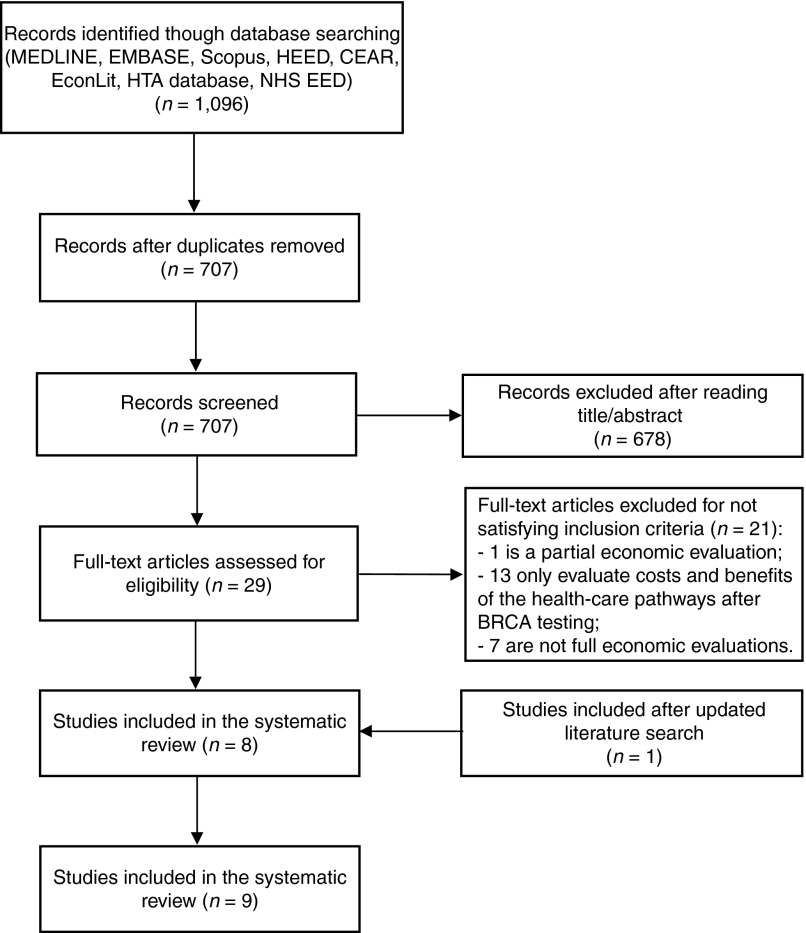
The electronic search identified 707 studies. Title and abstract screening reduced this number to 29 potential studies, which were retrieved for full text review. After full text review, a further 21 studies were excluded, leaving 8 studies.20,21,22,23,24,25,26,27,28 An additional relevant study was identified in the updated literature search.29 Therefore, a total of nine economic evaluations were included in the systematic review, all published in peer-reviewed journals. A flow diagram of the selection of studies is shown in Figure 1, and the list of studies excluded at the full text review stage, including reasons for exclusion, is available on request.
Figure 1.
Flow diagram for selection of full economic evaluations of BRCA1/2 testing programs. (Adapted from ref. 30)
Characteristics of studies
The key characteristics of the included studies are reported in Table 1. Three studies used CEA,21,22,24 four used CUA,23,25,27,29 and two used both CEA and CUA.26,28 All the most recent studies, published from 2009 until 2015, included a CUA.25,26,27,28,29 Six economic evaluations were performed in the United States,21,23,25,26,27,28 and three were performed in Europe (United Kingdom, Spain, and Norway).22,24,29 All but one study evaluated BRCA testing programs for both BRCA1 and BRCA2 genes; the exception included only a BRCA1 test.22 Six economic evaluations considered both breast and ovarian cancers, two referred only to breast cancer,22,24 and one study related only to ovarian cancer.25
Table 1. General characteristics of full economic evaluations of BRCA1/2 testing programs.
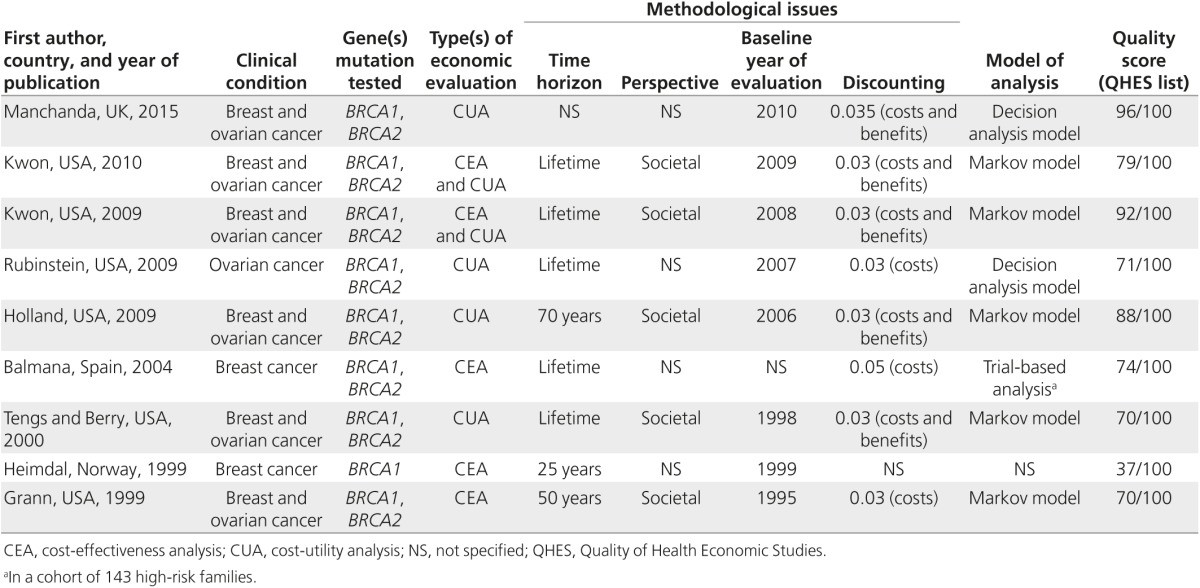
Nearly all studies were based on hypothetical BRCA genetic screening programs and used assumptions and probabilities extracted by other studies to model the impact of genetic testing.21,24,25,26,27,28 Two derived benefits from internal databases.22,23 Only one study performed a trial-based analysis and extrapolated the outcomes of that experimental study to obtain long-term cost-utility estimates.22
The time horizon was lifetime in five studies,23,24,26,27,28 between 25 and 70 years in three studies,21,22,27 and in one case it was not specified.23 However, the time horizon in all studies was long enough to reflect differences in costs and outcomes. The most frequent viewpoint declared by the authors was the societal perspective,21,23,25,26,28 but in four studies the perspective was not stated clearly.22,24,27,29
Quality assessment and basic assumptions
The main criticism of the study design is that the viewpoint of analysis was stated in only five of the nine economic evaluations21,23,25,26,28; the perspective declared by the authors was consistent with the costs included in the analysis in only two evaluations.25,26 By contrast, all other aspects of the study design were fulfilled in almost all economic evaluations (Table 2). Concerning data collection, there were several issues. Although the clinical effectiveness of the interventions was stated in the majority of studies, none gave details of how the effectiveness data were combined from several sources (Table 2). Health benefits were valued in six studies in which the main effect was on the quality of life, whereas in the remainder the health measures were LYG or cancer detected.21,22,24 Among studies using CUA, details of the methods used (e.g., time trade-off, standard gamble, contingent valuation) were given only in two studies,25,29 and the subjects from whom valuations were obtained (e.g., patients, members of the general public, health-care professionals) were specified only in one study.25 All studies made an appropriate measurement of costs, but none considered price adjustments for inflation or currency conversion (Table 2). Regarding the analysis and interpretation of results, the main criticism is how sensitivity analyses were used because only three economic evaluations tested results using two-way (or more complex) analyses.25,26,29 Another critical point is the lack of incremental analysis (Table 2). In three economic evaluations, the programs described were only compared with the situation where no testing was performed21,22,23; in another case, there was no incremental analysis at all.25
Table 2. Quality assessment of full economic evaluations of BRCA1/2 testing programs according to the BMJ checklist.
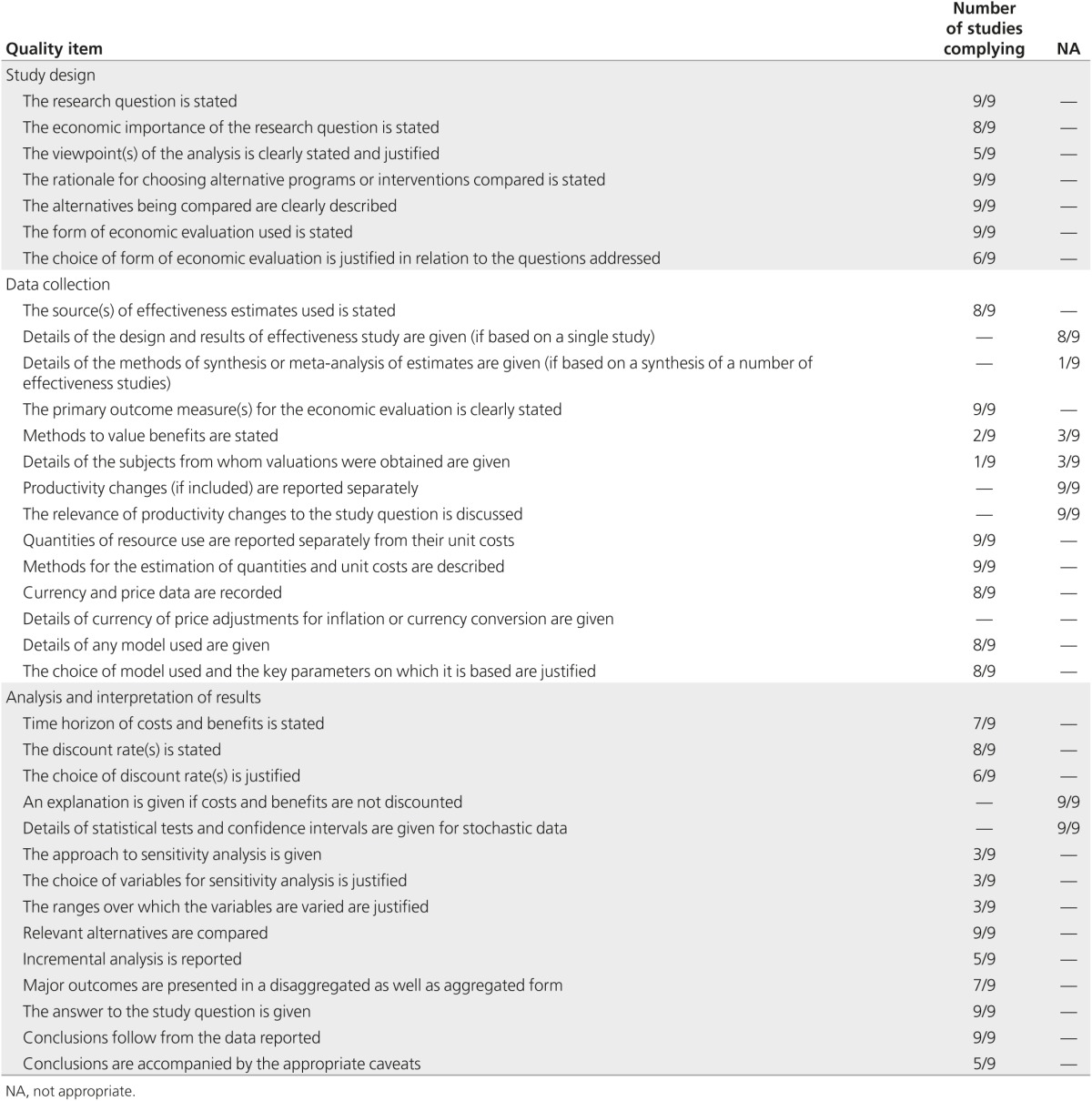
In general, studies were well conducted and the average quality was good. The median QHES score was 74, with a range of 37–96 (Table 1). There has been a gradual increase in the quality of studies over time, and those economic evaluations of higher quality were published in the past 5 years of the study period (from 2009 to 2014) (Table 1). The mean scores (±SD) awarded by the three reviewers for the assessment set were 73.8 (±19.2), 76.0 (±18.6), and 74.4 (±17.1) (P value for ANOVA = 0.966). The agreement for dichotomous scoring was high (κ = 0.8). The three reviewers scored 85% of the studies in the test set within 10 points, showing very good agreement. The criteria that required the most objective response (disclosing funding source and conduct of sensitivity analyses) received the highest level of agreement. The criteria with the lowest agreement levels were the discussion of magnitude and direction of potential biases and the inclusion of short-term, long-term, and negative outcomes.
Basic assumptions and main model inputs have been extracted to assess their impact on the results (see Supplementary Table S1 online). The main critical issues are the entity of risk reduction after preventive interventions, the compliance of patients with prophylactic surgery, the uptake of BRCA testing among at-risk relatives of index cases, and the costs of genetic testing. Risk reductions for breast and ovarian cancer after preventive interventions were higher in the most recent studies,25,26,27,28,29 because they were based on updated guidelines. This means that the cost-effectiveness ratios resulting from less recent studies, in which risk reductions were lower, may be overestimated.21,24 Patient compliance with prophylactic surgery was assumed to be 100% in some studies,21,22,24,28 and only one study tested patient compliance in the sensitivity analysis, showing a moderate impact on the results.28 The level of uptake of BRCA testing among relatives of index cases was specified only in two studies and varied between 5026 and 100%,22 but it was not tested in the sensitivity analysis. However, the 50% rate of compliance with genetic testing among relatives provided some evidence of cost-effectiveness.26 Concerning BRCA1/2 genetic testing costs, substantial differences exist between full sequence analysis of BRCA genes23,24,25,26,28 and tests of specific BRCA mutations.21,22,27,29 These specific tests are far less expensive, but because they are context-specific the results of their studies are difficult to generalize to other settings.
Evidence of cost-effectiveness
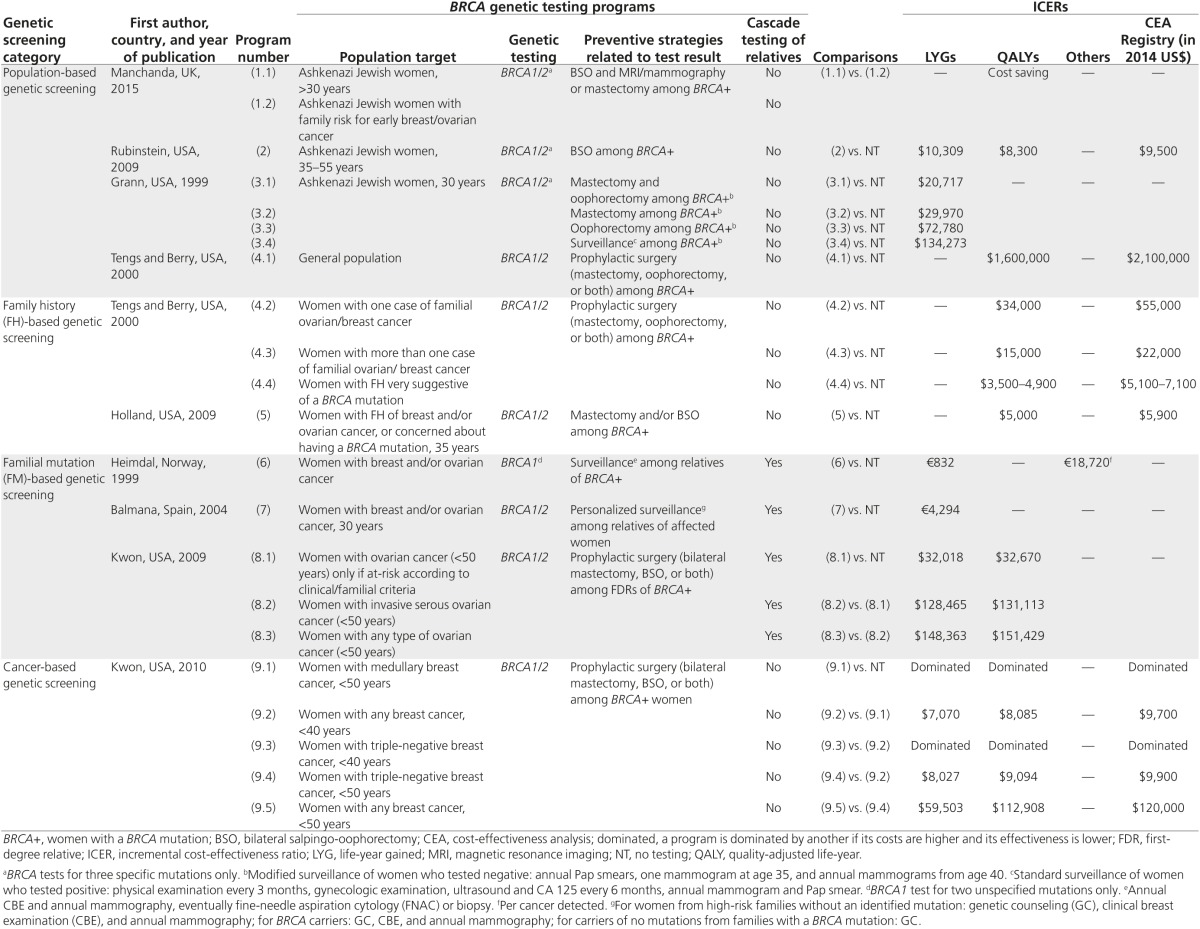
To aid interpretation, four main categories of the BRCA testing program were identified on the basis of target populations: (i) population-based genetic screening of individuals without cancer, either comprehensive or targeted based on ancestry or ethnicity; (ii) family history (FH)-based genetic screening, i.e., testing unaffected individuals with FH suggestive of BRCA mutation but in whom a familial mutation (FM) has not been identified; (iii) FM-based genetic screening, i.e., testing unaffected individuals with a known familial mutation; and (iv) cancer-based genetic screening, i.e., testing individuals with BRCA-related cancers to prevent the occurrence of further primary cancers (Table 3).
Table 3. Evidence of cost-effectiveness of different BRCA 1/2 testing programs.
Population-based genetic screening. The broadest approach involves population-based genetic screening of individuals without cancer, either comprehensive or subpopulations. Three studies focused on a specific ethnic group, the Ashkenazi Jewish (AJ) communities in Israel, United States, and United Kingdom,21,27,29 and one program targeted the general population.23 Studies targeted at the AJ community included, as a routine genetic test, the “BRCA founder-mutation test,”21,27,29 which tests only three specific mutations in BRCA (BRCA1*185delAG, BRCA1*5382insC, and BRCA2*6174delT); these mutations account for ~95% of detectable BRCA mutations in dominantly inherited early-onset breast and ovarian cancer in families of AJ ancestry.31,32,33
In the first study, two strategies were compared.29 In the first of these, BRCA genetic testing was offered to all women belonging to the AJ community. For this ethnicity, the probability of having a mutation was assumed to be 2.45% by the authors. The second strategy was consistent with current recommendations: genetic testing was offered only to women in the AJ community with a definite risk, which was determined from information on familial and personal history (FH-based screening). In this subpopulation, the prevalence of BRCA mutation was higher than in the previous target group (9.38%). In both strategies, those AJ women with positive results in the BRCA genetic test (carriers) were encouraged to undertake specific preventive health-care pathways, which may include prophylactic surgery to reduce ovarian and breast cancer risk or, as an alternative to preventive mastectomy, early detection of breast cancer by surveillance with magnetic resonance imaging and mammography each year, followed by curative mastectomy. For both strategies, the authors assumed that slightly more than 50% of carriers would decide to undergo preventive surgery or surveillance. The population-based approach was cost-saving compared with FH-based screening in AJ women, but no comparison was made with a no-testing strategy.29
The second study focused on ovarian cancer risk only in AJ women, and a comparison between population-based genetic screening and no testing was conducted.27 The preventive intervention in BRCA carriers was bilateral salpingo-oophorectomy (BSO), assuming a carrier compliance with surgery of 50%. Also, in this case, favorable conclusions were reached regarding the cost-effectiveness of the BRCA testing ($10,309/LYG and $8,300/QALY).27
In the third study, four hypothetical scenarios were compared with no testing.21 In all scenarios, the founder mutations were tested in AJ women, assuming a BRCA mutation prevalence of 2.5%. Four types of preventive interventions were analyzed. In the first, all carriers underwent combined surgery (mastectomy and oophorectomy). In the second and third scenarios, carriers were encouraged to undergo prophylactic bilateral mastectomy or bilateral oophorectomy, respectively. In the last, only surveillance was offered to them. In all scenarios carrier compliance was assumed to be 100%, and this has not been assessed by sensitivity analysis. Taking into account the current thresholds, the analysis suggested that screening AJ women for BRCA mutations would be cost-effective only if those testing positive underwent combined surgery ($20,717/LYG), or mastectomy ($29,717/LYG).21 The authors considered bilateral oophorectomy to reduce the risk of ovarian cancer to 45%. Currently, the recommended prophylactic surgery for inherited BRCA cancers is BSO, with a risk reduction of 96%; this was considered in the previous two studies discussed here.27,29
Tengs and Berry23 considered BRCA testing screening of the general population (0.02–0.06% probability of carrying a BRCA mutation) with an option for identified BRCA carriers to decide whether to undergo prophylactic surgery. The results clearly suggest that population-based screening is too expensive to be justified by the health gains achieved.
FH-based genetic screening. In an FH-based screening approach, the information is obtained by interviewing individuals about their personal and familial history; women who may have an increased risk of hereditary breast and ovarian cancer are offered genetic testing.34 Two economic evaluations analyzed this approach.23,25 The target population was unaffected women with FH suggestive of a BRCA mutation and with an unknown mutation in their families (Table 3).
The first study included target populations of women who differed in the strength of their FH of breast or ovarian cancer, with probabilities of identifying a BRCA mutation being 5, 10, or 25–50%.23 Prophylactic surgery was offered to BRCA carriers in all programs. Incremental analysis was not performed, and all strategies were compared with a no-testing situation. The results showed that testing unaffected women with greater than 10% probability of a BRCA mutation was very cost-effective ($3,500–15,000/QALY).23
In the second study, the target population comprised 35-year-old women, including women at high risk based on their FH and those who were concerned they might carry a BRCA mutation. In both cases the probability of identifying a BRCA gene mutation was 10% and no FM had been characterized.25 Prophylactic surgery was offered to BRCA mutation-positive women. This strategy was cost-effective ($5,000/QALY) when compared with no intervention. Using data from a sensitivity analysis, the authors established that the “test strategy” is cost-effective even when the pretest probability of mutation was less than 10%, as long as a negative mutation result leads to a utility gain for at-risk women.25
FM-based genetic screening. The third potential screening approach is FM-based genetic screening, in which genetic tests are performed on the close relatives of previously identified index cases. Three economic evaluations analyzed this approach (Table 3).22,24,26
In the first study, only one of the two BRCA programs analyzed met our inclusion criteria because the other program did not include genetic analysis to identify women with cancer-predisposing mutations.22 In the included program, a BRCA1 genetic test was offered by physicians to all women with breast and/or ovarian cancer, without pretest counseling. If a BRCA1 mutation was found (index case), then the genetic test was performed on relatives (cascade screening). The preventive intervention for positive relatives was surveillance. This program, compared with no intervention, was cost-effective both in terms of life-years saved (€832/LYG) and number of inherited breast cancer diagnoses (€18,720 per cancer detected).22
The second study evaluated a BRCA genetic program for inherited breast cancer carried out in a Spanish hospital.24 A dedicated hospital unit performed genetic counseling to identify women at high risk for breast cancer. After counseling, the index cases of families who met the criteria for hereditary breast cancer were tested; if a BRCA mutation was found, then all close relatives were encouraged to undergo genetic analysis. BRCA carriers and females from high-risk families (without an identified mutation in their families) received intensive surveillance until age 80 or early onset of breast cancer. Women who tested negative followed the standard population breast cancer screening program. This program was shown to be cost-effective (€4,294/LYG) compared with no screening. The cost-effectiveness analysis was focused only on breast cancer early detection with surveillance; neither the impact on costs nor the reduction of breast and ovarian cancer associated with preventive surgery was considered.24
The third study targeted women with ovarian cancer.26 Four strategies were considered: no testing; testing at-risk women for inherited ovarian cancer identified using the Society of Gynecologic Oncologists criteria (pretest probability 10%); testing only women with invasive serous cancer; and testing all women with ovarian cancer. If the women tested positive, then their first-degree relatives were tested and risk-reducing interventions were performed. The only program shown to be cost-effective according to current thresholds was that involving testing of women with cancer ($32,018/LYG and $32,670/QALY) after performing a risk assessment based on the Society of Gynecologic Oncologists criteria.26
Cancer-based genetic screening. In the last screening approach, genetic tests are offered to individuals with a cancer diagnosis for the purpose of diagnosing inherited cancer predisposition. Only one economic evaluation analyzed this approach (Table 3).28 This study estimated costs and benefits of different BRCA testing programs in women younger than 50 years old with breast cancer, regardless of their FH and ethnicity.28 Several testing strategies were considered according to type of breast cancer (medullary, triple-negative) and age of onset (women younger than 40 or 50 years old). In the baseline analysis, the authors assumed that all carriers would choose to undergo surgery, whereas compliance levels varied in the sensitivity analysis, ranging up to 20% for mastectomy and 55% for BSO. Cascade testing among relatives was not included in the model. The results showed that testing women younger than 40 years old with any type of breast cancer and testing women younger than 50 years old with triple-negative breast cancer were both cost-effective if the preventive treatment for carriers was prophylactic surgery (ipsilateral and/or contralateral mastectomy and BSO) ($7,070 and $8,027/LYG and $8,085 and $9,094/QALY, respectively).28
Discussion
The main challenge in conducting a systematic review of economic evaluations derives from their high degree of heterogeneity, meaning that results cannot be pooled across studies by meta-analysis or other quantitative synthesis methods.12,13 Consequently, the majority of such reviews have presented the results obtained in a narrative format.11 In the case of genetic testing, previously published systematic reviews have focused principally on assessing the methodological quality of economic evaluations, or simply on identifying which genetic testing programs were supported by economic evidence; therefore, they were able to include a wide range of genetic tests.35,36,37,38,39 The outcome of these systematic reviews was that most genetic testing programs result in better health care, but usually at a higher cost, and save money only in a small minority of cases.18,40 These findings are similar to those from other health-care areas, where it was estimated that only a low percentage of preventive measures and treatments for existing conditions are cost-saving.41,42 Relatively few systematic reviews have focused on a specific genetic test and only one analyzed BRCA1/2 testing, with the main purpose of identifying available economic evidence to support the use of a specific laboratory method for detecting BRCA mutations in UK genetic services.43 By contrast, our systematic review used a qualitative approach to identify genetic health-care programs for BRCA-related diseases that are ready for implementation in clinical practice based on the cost-effectiveness evidence. Four categories of genetic screening programs were identified: population-based, FH-based, FM-based, and cancer-based genetic screening.
Estimates of cost-effectiveness of population-based BRCA1/2 testing indicate that this strategy is currently too expensive. It has been calculated that even if every woman with a detected mutation underwent bilateral mastectomy and salpingo-oophorectomy, universal screening would still cost in excess of $1 million per QALY gained.44 By contrast, population-based screening targeted at Ashkenazi Jewish women seems to be reasonable and cost-effective if carriers undergo prophylactic surgery.21,27,29 The difference is explained not only by the prevalence of mutations (AJ, 2.5%; general population, 0.002–0.006%) but also by the price of the test, because testing a woman from the general population costs at least five times more than testing a AJ woman. Despite its cost-effectiveness, the current guidelines do not explicitly recommend population-based screening of AJ women, and AJ heritage is considered only one of several risk factors that may increase the total risk of having a deleterious BRCA mutation.45,46
In FH-based screening, the unit of analysis is not the individual but the entire family. Accordingly, three steps should be followed: identify high-risk individuals through an assessment of personal and FH, test the affected family member to identify a significant FM, and in the case of a positive test result, perform direct gene testing on apparently unaffected family members. The economic evaluations of FH-based screening showed two important deficiencies: none discussed how to select high-risk women in the general population, thereby failing to detail the related costs, and none modeled cascade screening among relatives of carriers following the detection of index cases. For these reasons, the cost-effectiveness ratios calculated in these studies should be considered of limited utility; more information is needed for a complete evaluation. Despite this, the possibility that this screening approach is cost-effective appears very high if the probability of mutation in the at-risk population is 10% or higher.
The category of FM-based genetic screening detects index cases by directly testing women with breast and/or ovarian cancers. In the event that a mutation is identified in index subjects, genetic analysis is performed on unaffected relatives. All three economic evaluations that analyzed this approach showed some evidence of cost-effectiveness. Problems such as the lack of a defined pretest probability of affected women, the decision to study only two BRCA1 mutations, the unknown percentage of at-risk relatives of index cases who elect to undergo testing, and the unrealistically high expected compliance with preventive strategies among carriers limit the amount of useful information gathered by testing all women with breast and/or ovarian cancers that can be drawn from the first study.22 Assumptions seem to be more robust in the second and third studies, in which genetic tests to identify index cases were offered to women with breast and/or ovarian cancers at age 30 and to women with ovarian cancer younger than 50 years.24,26 If a mutation was found, then unaffected relatives were tested and prophylactic interventions were offered to them. In both studies, the cost-effective strategies were those in which genetic tests were offered to affected women at risk for inherited breast/ovarian cancer according to personal and familial criteria.24,26 Currently, there is no evidence for testing all individuals with BRCA-related cancers to identify index cases regardless of their clinical and familial histories.
The only study included in the last category, i.e., cancer-based genetic screening, proposed genetic screening of several populations of women with breast cancer, stratified by subtypes and age of onset, with the purpose of preventing the occurrence of further primary cancers, without considering cascade testing of relatives.28 This approach was cost-effective only under particular conditions.28
Our survey of the literature found only nine full economic evaluations of BRCA genetic testing. Most of these were of acceptable quality, although some methodological limitations should be mentioned. For example, the included costs were frequently inadequate. Thus, although the societal perspective is the most appropriate for the analysis of health-care programs and five economic evaluations adopted this perspective, none of the authors included social costs. The following other limitations are worth highlighting: (i) few details were reported on data abstraction for the base-case estimates, (ii) none of the evaluations considered price adjustments for inflation or currency conversion, and (iii) only three economic evaluations performed appropriate sensitivity analysis (two-way or more complex). Finally, the evaluations were conducted in a limited range of countries (United States, United Kingdom, Norway, and Spain), and whether the results are generally applicable was not addressed in the included studies. A previous review of studies on the cost-effectiveness of BRCA genetic testing identified similar limitations, pointing out the considerable variety in methodologies and hence problems of transparency, comparability, and generalizability.43
In conclusion, the results of this systematic review indicate that, although BRCA1/2 population-based screening is currently an inefficient use of health-care resources, population-based screening of the AJ community appears to be a good value for the money. Furthermore, it is highly likely that FH-based screening will prove cost-effective, although further economic evaluations that include the costs of identifying high-risk women are needed to fully justify this conclusion. This point is crucial because counseling strategies to detect at-risk individuals could involve primary-care physicians, and currently physicians seem to be not yet adequately prepared about hereditary breast cancer and BRCA1/2 testing.47,48 Finally, in contrast to genetic testing for hereditary colorectal cancer (i.e., Lynch syndrome),49,50,51 there is no evidence for the cost-effectiveness of screening for BRCA1/2 of newly diagnosed cases of breast and ovarian cancers, followed by cascade testing of relatives. However, cancer-based genetic screening programs for BRCA1/2 that includes tools for identifying women at higher risk for inherited forms are very promising in terms of cost-effectiveness. On the contrary, more high-quality studies are needed to prove the cost-effectiveness of BRCA genetic testing as an instrument of secondary prevention in affected women with predisposing gene mutation.
In any case, the price of BRCA1/2 testing is of paramount importance in determining the cost-effectiveness of BRCA1/2 testing programs.44 If the cost of testing falls significantly, then all BRCA1/2 testing strategies analyzed in this review—perhaps including population-based screening—are likely to become highly cost-effective interventions.
Disclosure
Part of this work is connected to the project “L'impatto economico dei test genetici sul Servizio Sanitario Nazionale (SSN): valutazione dei percorsi diagnostico-assistenziali, stime di costi-efficacia e costi-utilità e analisi delle politiche sanitarie a livello europeo” (The economic impact of genetic testing on the National Health Service: evaluation of diagnostic care pathways, estimates of cost-effectiveness and cost-utility, and investigation of health policies in Europe), funded by the Italian Ministry of Health; and to the project “Personalized pREvention of Chronic DIseases consortium (PRECeDI)” funded by the European Research and Innovation programme Horizon 2020, under grant agreement no. 645740. All authors have taken part in both projects.
Supplementary Material
References
- Centers for Disease Control and Prevention. Public Health Genomics. Genomic Tests and Family History by Levels of Evidence. www.cdc.gov/genomics/gtesting/tier.htm. Accessed 5 August 2015.
- Khoury MJ, Coates RJ, Evans JP. Evidence-based classification of recommendations on use of genomic tests in clinical practice: dealing with insufficient evidence. Genet Med 2010;12:680–683. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med 2007;9:665–674. [DOI] [PubMed] [Google Scholar]
- Schully SD, Benedicto CB, Gillanders EM, Wang SS, Khoury MJ. Translational research in cancer genetics: the road less traveled. Public Health Genom 2011;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury MJ, Coates RJ, Fennell ML, et al. Multilevel research and the challenges of implementing genomic medicine. J Natl Cancer Inst Monogr 2012;2012:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes, 3rd edn. Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer 2000;83:1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 2005;104:2807–2816. [DOI] [PubMed] [Google Scholar]
- Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst 2006;98:1694–1706. [DOI] [PubMed] [Google Scholar]
- Ministry of Health. 2014–2018 National Prevention Plan, Italy. www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2285. Accessed 5 August 2015.
- Anderson R. Systematic reviews of economic evaluations: utility or futility? Health Econ 2010;19:350–364. [DOI] [PubMed] [Google Scholar]
- Centre for Reviews and Dissemination (CRD). Systematic reviews of economic evaluations. In: Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care. CRD: York, UK, 2009. [Google Scholar]
- Shemilt I, Mugford M, Byford S, et al. Incorporating economics evidence. In: Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. The Cochrane Collaboration, 2011.http://handbook.cochrane.org/ [Google Scholar]
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou CF, Hay JW, Wallace JF, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care 2003;41:32–44. [DOI] [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37–46. [Google Scholar]
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Ann Sakowski J, Trosman J, Douglas MP, Liang SY, Neumann P. The economic value of personalized medicine tests: what we know and what we need to know. Genet Med 2014;16:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton K, Martin S, Soares M, et al. Methods for the estimation of the NICE cost effectiveness threshold. Health Technol Assess 2015;19(14):1–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for the Evaluation of Value and Risk in Health at the Institute for Clinical Research and Health Policy Studies. Cost-Effectiveness Analysis Registry (https://research.tufts-nemc.org/cear4/). Tufts Medical Center: Boston, MA, 2015. Accessed 5 August, 2015. [Google Scholar]
- Grann VR, Whang W, Jacobson JS, Heitjan DF, Antman KH, Neugut AI. Benefits and costs of screening Ashkenazi Jewish women for BRCA1 and BRCA2. J Clin Oncol 1999;17:494–500. [DOI] [PubMed] [Google Scholar]
- Heimdal K, Maehle L, Møller P. Costs and benefits of diagnosing familial breast cancer. Dis Markers 1999;15:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengs TO, Berry DA. The cost effectiveness of testing for the BRCA1 and BRCA2 breast-ovarian cancer susceptibility genes. Dis Manag Clin Outcomes 2000;1:15–24. [Google Scholar]
- Balmaña J, Sanz J, Bonfill X, et al. Genetic counseling program in familial breast cancer: analysis of its effectiveness, cost and cost-effectiveness ratio. Int J Cancer 2004;112:647–652. [DOI] [PubMed] [Google Scholar]
- Holland ML, Huston A, Noyes K. Cost-effectiveness of testing for breast cancer susceptibility genes. Value Health 2009;12:207–216. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Daniels MS, Sun CC, Lu KH. Preventing future cancers by testing women with ovarian cancer for BRCA mutations. J Clin Oncol 2010;28:675–682. [DOI] [PubMed] [Google Scholar]
- Rubinstein WS, Jiang H, Dellefave L, Rademaker AW. Cost-effectiveness of population-based BRCA1/2 testing and ovarian cancer prevention for Ashkenazi Jews: a call for dialogue. Genet Med 2009;11:629–639. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Gutierrez-Barrera AM, Young D, et al. Expanding the criteria for BRCA mutation testing in breast cancer survivors. J Clin Oncol 2010;28:4214–4220. [DOI] [PubMed] [Google Scholar]
- Manchanda R, Legood R, Burnell M, et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi jewish women compared with family history-based testing. J Natl Cancer Inst 2015;107:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997;336:1401–1408. [DOI] [PubMed] [Google Scholar]
- Oddoux C, Struewing JP, Clayton CM, et al. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet 1996;14:188–190. [DOI] [PubMed] [Google Scholar]
- Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med 1996;2:1179–1183. [DOI] [PubMed] [Google Scholar]
- European Society of Human Genetics (ESHG). Population genetic screening programmes: technical, social and ethical issues. Recommendations of the European Society of Human Genetics. Eur J Hum Genet 2003;11:S5–S7. [DOI] [PubMed] [Google Scholar]
- Giacomini M, Miller F, O'Brien BJ. Economic considerations for health insurance coverage of emerging genetic tests. Community Genet 2003;6:61–73. [DOI] [PubMed] [Google Scholar]
- Carlson JJ, Henrikson NB, Veenstra DL, Ramsey SD. Economic analyses of human genetics services: a systematic review. Genet Med 2005;7:519–523. [DOI] [PubMed] [Google Scholar]
- Rogowski W. Genetic screening by DNA technology: a systematic review of health economic evidence. Int J Technol Assess Health Care 2006;22:327–337. [DOI] [PubMed] [Google Scholar]
- Djalalov S, Musa Z, Mendelson M, Siminovitch K, Hoch J. A review of economic evaluations of genetic testing services and interventions (2004–2009). Genet Med 2011;13:89–94. [DOI] [PubMed] [Google Scholar]
- Assasi N, Schwartz L, Tarride JE, Goeree R, Xie F. Economic evaluations conducted for assessment of genetic testing technologies: a systematic review. Genet Test Mol Biomarkers 2012;16:1322–1335. [DOI] [PubMed] [Google Scholar]
- D'Andrea E, Marzuillo C, Pelone F, De Vito C, Villari P. Genetic testing and economic evaluations: a systematic review of the literature. Epidemiol Prev 2015;39(suppl 1):S45–S50. [PubMed] [Google Scholar]
- Cohen JT, Neumann PJ, Weinstein MC. Does preventive care save money? Health economics and the presidential candidates. N Engl J Med 2008;358:661–663. [DOI] [PubMed] [Google Scholar]
- Chokshi DA, Farley TA. The cost-effectiveness of environmental approaches to disease prevention. N Engl J Med 2012;367:295–297. [DOI] [PubMed] [Google Scholar]
- Sullivan W, Evans DG, Newman WG, Ramsden SC, Scheffer H, Payne K. Developing national guidance on genetic testing for breast cancer predisposition: the role of economic evidence? Genet Test Mol Biomarkers 2012;16:580–591. [DOI] [PubMed] [Google Scholar]
- Long EF, Ganz PA. Cost-effectiveness of universal BRCA1/2 screening: evidence-based decision making. JAMA Oncol 2015;3:1–2. [DOI] [PubMed] [Google Scholar]
- Febbo PG, Ladanyi M, Aldape KD, et al. NCCN Task Force report: evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw 2011;9(suppl 5):S1–S32; quiz S33. [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force (USPSTF). Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer. Systematic Review to Update the US Preventive Services Task Force Recommendation. Evidence Syntheses, No. 101. Agency for Healthcare Research and Quality (US): Rockville, MD, 2013. [PubMed] [Google Scholar]
- Bellcross CA, Kolor K, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among US primary care physicians. Am J Prev Med 2011;40:61–66. [DOI] [PubMed] [Google Scholar]
- Marzuillo C, De Vito C, Boccia S, et al. Knowledge, attitudes and behavior of physicians regarding predictive genetic tests for breast and colorectal cancer. Prev Med 2013;57:477–482. [DOI] [PubMed] [Google Scholar]
- Grosse SD. Economic analyses of genetic tests in personalized medicine: clinical utility first, then cost utility. Genet Med 2014;16:225–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med 2010;12:93–104. [DOI] [PubMed] [Google Scholar]
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med 2011;155:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.