ABSTRACT
Proteinuria in hypertension is an early marker of renal disease and a predictor for the progression of end stage renal disease, and cardiovascular diseases. This study was designed to determine the prevalence of proteinuria and its association with cardiovascular risk factors among adult hypertensive patients in Afghanistan. Five hundred fifty-five patients with a high blood pressure recorded in an outpatient clinic in Andkhoy, Afghanistan from December 2014 to May 2015, were included in this study. Data obtained from each patient, included demographic characteristics, body mass index, blood pressure patterns, cardiovascular history, cardiovascular risk factors, comorbidity, and current drug-therapy. Dipstick screening for proteinuria was performed with reagent test strips. The mean age of the patients was 57.9 ± 13.3 years, and a female predominance was observed (n = 333, 60%). The prevalence of proteinuria was 67.2%. The predictors of proteinuria were found to be age ≥65 years (odds ratio [OR] 1.02, 95% confidence interval [CI] 1.00–1.04), smoking (OR 1.88, 95% CI 1.17–3.02), heart failure (OR 2.23, 95% CI 1.13–4.41), and diabetes mellitus (OR 3.41, 95% CI 1.49–7.81). In conclusion, this study shows that proteinuria is highly prevalent among hypertensive outpatients in an outpatient clinic in Andkhoy, Afghanistan, especially in those with high cardiovascular risk.
Key Words: proteinuria, hypertension, Afghanistan
INTRODUCTION
The World Health Organization (WHO) has estimated that about 17 million people die annually from cardiovascular disease worldwide, which accounts for approximately 33% of all deaths. In addition, 9.4 million deaths are attributed to complications of hypertension.1) According to WHO statistics in 2008, hypertension was diagnosed in almost two-fifths of people aged 25 years and older worldwide.2) If current trends continue, the number of patients with hypertension will increse by ≥ 500 million and reach a total of 1.56 billion in 2025.3)
Hypertension is becoming a major public health problem in both developed and developing countries.4) There are about 639 million hypertensive people, which equals almost 75% of the population in developing countries with limited resources. These hypertensive persons also have a very low awareness of their condition, as well as poor blood pressure (BP) control.3) Non-optimal BP control accounts for 66.6% of all strokes and 50% of coronary artery disease cases.5) Furthermore, hypertension is known to be one of the main risk factor of deaths in South Asia.6) According to a study conducted between December 2011 and March 2012 in Kabul, Afghanistan the prevalence of hypertension was 46.2% for the age group of 40years and older.7)
Several epidemiological studies have reported an association between proteinuria and a poor prognosis in hypertensive patients.8-11) The burden of proteinuria and its association with hypertension have not been studied in the adult hypertensive population of Afghanistan. Therefore, this study was aimed at determining the prevalence of proteinuria, and the association of different variables with proteinuria, among adult hypertensive patients attending an outpatient clinic in Afghanistan.
MATERIALS AND METHODS
Subjects
Five hundred fifty-five patients with a high BP recorded at an outpatient clinic in Andkhoy, Afghanistan from December 2014 to May 2015. The case subjects included patients aged 18 years and older, who were receiving treatment for newly diagnosed arterial hypertension, defined as seated systolic/diastolic hypertension 140/90 mm Hg at rest on the day of the study visit.12) Self-reported use of antihypertensive drugs at the time of interview was noted. Patients who had engaged in strenuous physical activity in the preceding 24 h, as well as female participants who were pregnant or menstruating, were ineligible because of the likely presence of false-positive results. This was an observational study. The subjects were evaluated during their clinic visit, and the study protocol included assessment of data obtained from each patient, including demographic characteristics, body mass index (BMI; weight in kilograms divided by height in meters squared), BP pattern, cardiovascular history, presence of cardiovascular risk factors, comorbidity and current drug-therapy. The study was approved by the scientific review committee of Balkh Regional Hospital.
For this study, diabetes was defined as a fasting blood sugar level of ≥ 126 mg/dl,13) at the visit or self-reported use of hypoglycaemia medications. Hypercholesterolemia was defined as a total cholesterol level of ≥ 200 mg dl.14) The results were obtained by using the reagent tests strips of the Accu-Trend Plus kit for cholesterol/glucose (Roche Diagnostic USA).
The definitions of obesity varies across studies, even among from the same country. For this study, overweight was defined as a BMI of 25 to < 30 kg/ m2 and obesity as BMI ≥ 30.15) Dipstick screening for proteinuria and glycosuria were performed with the reagent strips (Roche Diagnostic USA). The urinary protein levels of dipstick urinary analyses were recorded as follows; negative (–), trace (+/–) or proteinuria (1+, 2+, 3+ or 4 +).16) Cigarette smoking was assessed through a face to face interview. Patients were grouped into three categories: (i) patients who had smoked 100 cigarettes in his or her lifetime and currently smokes cigarettes (current smoker); (ii) patients who had smoked at least 100 cigarettes in his or her lifetime but had stopped smoking at the time of the interview (past smokers); (iii) patients who have never smoked, or who had smoked < 100 cigarettes in his or her lifetime (non-smoker).
Other factors that were considered were regular aerobic physical activity a minimum of 30 min for 5 days/week or vigorous physical activity for a minimum of 20 min for 3 days/week.17) The results from this measurement were entered onto each patient’s case report form.
Statistical analysis
The means of variables are presented with standard deviation (SD). Categorical variables are expressed as count and percentages. Continuous data were compared by using a t-test. Categorical data were compared by means of a χ-test. Univariate and multivariate logistic regression analyses were performed to observe the association between proteinuria and the risk factors as well as target organ lesions. Odds ratios (ORs) with 95% confidence intervals (CIs) were estimated by using a logistic regression model. All the prognostic valuables with p < 0.05 in a univariate logistic regression analysis were entered into a multivariate logistic regression analysis in order to determine independent predictors.
A p value of < 0.05 was considered statistically significant. All analyses were performed with the SPSS 20.0 software package (SPSS, Chicago, IL, USA).
RESULTS
The sociodemographic characteristics of all patients are summarized in Table 1. The mean age of the patients was 57.9 ± 13.3 years (range 18–92 years). Most of the patients (59.1%) were aged between 40 and 65 years, 222 were men (40.0%) and 333 were women (60.0%). Concerning cardiovascular risk factors, 42.6% had hypercholesterolemia and 11.2% had diabetes. The prevalence of current smokers among female patients (18.6%) was significantly (p < 0.001) lower than that among male patients (37.8%). The prevalence of proteinuria was 67.2% and considerably higher in male than in female patients (p < 0.001). Among all patients, 14.9% had a family history of ischemic heart disease, 14.8% had family history of diabetes, and 53.4% had a family history of hypertension. Of these patients, 26.3% were current smokers, 33.7% had a BMI of ≥25, 78.2% had uncontrolled BP, and 59.2% had regular physical exercise.
Table 1.
Fequency distribution of sociodemographic characteristics of study participants (n=555)
| Characteristics | Total N (%) | Male (n=222) N (%) | Female (n=333) N (%) | p | |||
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <40 | 31 (5.6) | 21 (9.5) | 10 (3.0) | <0.001 | |||
| 40–65 | 328 (59.0) | 159 (71.6) | 169 (50.7) | <0.001 | |||
| >65 | 196 (35.3) | 42 (18.9) | 154 (46.2) | <0.001 | |||
| Level of education | |||||||
| Illiterate | 311 (56.0) | 0 (0.0) | 311 (93.4) | <0.001 | |||
| Primary/private education | 51 (9.2) | 29 (13.1) | 22 (6.6) | <0.001 | |||
| Secondary | 99 (17.8) | 99 (44.6) | 0 (0.0) | <0.001 | |||
| High school or more | 94 (16.9) | 94 (42.3) | 0 (0.0) | <0.001 | |||
| Marital status | |||||||
| Single | 20 (3.6) | 18 (8.1) | 0 (0.0) | <0.001 | |||
| Married | 492 (88.6) | 161 (72.5) | 333 (99.4) | <0.001 | |||
| Others | 43 (7.7) | 43 (19.3) | 0 (0.0) | <0.001 | |||
| Occupation | |||||||
| Employed | 125 (22.5) | 98 (44.1) | 27 (8.1) | <0.001 | |||
| Unemployed | 124 (22.3) | 124 (55.8) | 0 (0.0) | <0.001 | |||
| House wife | 306 (55.1) | 0 (0.0) | 306 (91.9) | <0.001 | |||
| Smoking status | |||||||
| Current smoker | 146 (26.3) | 84 (37.8) | 62 (18.6) | <0.001 | |||
| Past smoker | 91 (16.4) | 25 (11.3) | 66 (19.8) | <0.001 | |||
| Non smoker | 318 (57.3) | 113 (50.9) | 205 (61.6) | <0.001 | |||
| BMI ≥ 25 | 187 (33.7) | 97 (43.7) | 90 (27.0) | 0.16 | |||
| Blood pressure control | 121 (21.8) | 54 (24.3) | 67 (20.1) | 0.367 | |||
| Lack of regular physical exercise | 216 (38.7) | 98 (44.1) | 118 (35.4) | <0.001 | |||
| Diabetes mellitus | 62 (11.2) | 31 (14.0) | 31 (9.3) | 0.088 | |||
| Family history of hypertension | 296 (53.3) | 103 (46.4) | 193 (57.9) | 0.015 | |||
| Family history of ischemic heart disease | 83 (14.9) | 40 (18.0) | 43 (12.9) | 0.017 | |||
| Family history of diabetes mellitus | 82 (14.8) | 40 (18.0) | 42 (12.6) | 0.012 | |||
| Hypercholesterolemia | 236 (42.5) | 92 (41.4) | 144 (43.2) | 0.753 |
The mean age of patients in the proteinuria and non-proteinuria groups was 62.0 ± 13.6 years and 57.7 ± 13.4 years, respectively. The male-to-female ratio in the proteinuria group was 1.15 and that in the nonproteinuria group was 2.81. Smokers had higher rates of proteinuria than non-smokers 30.7% vs. 17.5% (p = 0.03). Patients with proteinuria had substantially increased levels of both systolic BP and diastolic BP (155.0 ± 24.8 vs.146.2 ± 24.0 mm Hg, and 94.6 ± 13.8 vs. 88.6 ± 10.8 mm Hg; p < 0.001, respectively). The fasting blood glucose levels were 115.1 ± 34.9 versus 104.5 ± 23.7; p = 0.001). The prevalence of proteinuria was significantly higher among diabetic patients than non-diabetic patients (14.7% vs. 3.8%; p < 0.001). Patients with myocardial infarction had significantly higher proteinuria levels than those without myocardial infarction (25.7% vs.13.2%; p = 0.001). Proteinuria was more common in patients with heart failure (15.2% vs. 6.6%; p = 0.04).
Physical activity was inversely associated with proteinuria (45.0% vs. 25.9%; p < 0.001). There was also a considerable difference with regard to use of angiotensin-receptor blockers (ARBs), angiotensin-converting enzyme (ACE) inhibitors, calcium channel blockers (CCBs), and beta blockers (BBs) between patients with and those without proteinuria (Table 2).
Table 2.
Comparison of factors associated with proteinuria among hypertensive patients
| No proteinuria Mean ± SD/N (%) | Proteinuria Mean ± SD/N (%) | p value | |||
|---|---|---|---|---|---|
| Age, mean, years | 57.8 ± 13.4 | 62.0 ± 13.6 | <0.001 | ||
| Males% | 48 (26.5) | 174(46.5) | <0.001 | ||
| Smokers% | 31 (17.5) | 115 (30.7) | 0.030 | ||
| Systolic blood pressure | 146.2 ± 23.9 | 150.0 ± 24.8 | <0.001 | ||
| Diastolic blood pressure | 88.6 ± 10.8 | 94.6 ± 13.8 | <0.001 | ||
| Body mass index | 24.1 ± 4.4 | 23.5 ± 4.1 | 0.17 | ||
| Fasting blood glucose | 104.5 ± 23.7 | 115.1 ± 34.9 | 0.001 | ||
| History of myocardial infarction | 24 (13.2) | 964 (25.7) | 0.001 | ||
| Diabetes | 7 (3.9) | 55 (14.7) | <0.001 | ||
| Hypercholesterolemia | 85 (47.0) | 151 (40.4) | 0.14 | ||
| Lack of regular physical exercise | 48 (26.5) | 168 (44.9) | <0.001 | ||
| Stroke | 4 (2.2) | 20 (5.3) | 0.12 | ||
| Congestive heart failure | 12 (6.6) | 57 (15.2) | 0.040 | ||
| Family history of hypertension | 76 (42.0) | 220 (58.8) | <0.001 | ||
| Family history of ischemic heart disease | 37 (20.4) | 46 (12.3) | 0.009 | ||
| Family history of diabetes | 29 (16.0) | 53 (14.2) | 0.32 | ||
| Anti-hypertensive drugs | |||||
| Angiotensin II receptor Blocker | 54 (29.7) | 33 (8.5) | <0.001 | ||
| Angiotensin-converting-enzyme inhibitor | 91 (50.0) | 88 (23.6) | <0.001 | ||
| Diuretics | 56 (30.8) | 135 (36.2) | 0.21 | ||
| Calcium channel blocker | 33 (18.1) | 196 (52.4) | <0.001 | ||
| Beta blocker | 26 (14.2) | 188 (50.4) | <0.001 | ||
| Centrally acting agent | 4 (2.2) | 4 (1.1) | 0.45 |
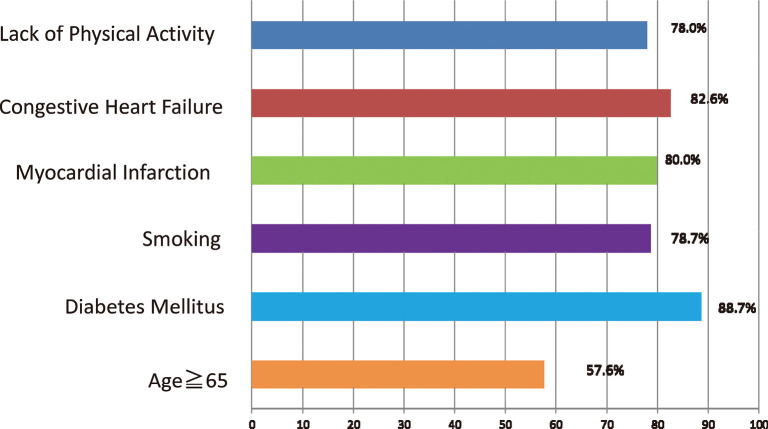
Multivariate logistic regression analysis was used to determine the association between proteinuria and smoking, heart failure, myocardial infarction, diabetes mellitus, stroke, hypercholesterolemia, and regular physical activity. Proteinuria was considered a dependent variable. The odds of proteinuria among smokers were 1.88 times greater than those in non-smokers (95%CI 1.10–2.95). Patients with heart failure had a more than two-fold increase in the odds of proteinuria than those without heart failure (95%CI 1.13–4.45). Patients aged ≥ 65 years showed a 1.02 times increase of in the odds of proteinuria than those < 65 years (95%CI 1.00–1.04). The odds of proteinuria among diabetic patients were 3.41 times higher than those in patients without diabetes (95%CI 1.49–7.80). Myocardial infarction, regular physical activity and diabetes mellitus were other variables that had a significant association with proteinuria in the univariate logistic regression model, however, in the multivariate model, this association was not significant (Table 3). The frequency and associated factors of proteinuria in hypertensive patients is shown in Fig. 1.
Table 3.
Univariate and multivariate logistic regression analysis for prediction of proteinuria
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Logistic Regression | Logistic Regression | |||||
| OR | (C95% CI) | P value | OR | (C95% CI) | P value | |
| Age ≥65 | 1.02 | (1.01–1.04) | 0.001 | 1.02 | (1.01–1.04) | 0.002 |
| Smoking | 2.18 | (1.40–3.41) | 0.001 | 1.88 | (1.17–3.02) | 0.009 |
| Congestive heart failure | 2.11 | (1.34–3.34) | 0.005 | 2.23 | (1.13–4.42) | 0.021 |
| Myocardial infarction | 2.28 | (1.40–3.71) | 0.001 | 1.45 | (0.75–2.79) | 0.271 |
| Lack of regular physical exercise | 2.35 | (1.17–3.04) | ‹0.001 | 1.66 | (0.98–2.82) | 0.061 |
| Diabetes mellitus | 3.68 | (1.71–7.91) | 0.001 | 3.41 | (1.49–7.81) | 0.004 |
| Stroke | 1.90 | (0.70–5.17) | 0.21 | |||
| Hypercholesterolemia | 1.36 | (0.95–1.95) | 0.09 | |||
CI=Confidence Interval
Fig. 1.
DISCUSSION
There has been a lack of data about the prevalence of proteinuria in Afghanistan, which is important information on the evaluation of hypertensive patients. The presence of proteinuria helps in identifying patients who are at high risk and need to receive aggressive treatment for risk factors. The prevalence of proteinuria among patients with hypertension in the northern part of Afghanistan was 67.2%. A similar prevalence, 67.8% was reported in Morocco, as part of an i-SEARCH (Survey for Evaluating Microalbuminuria Routinely by Cardiologists in patients with Hypertension) study, in which the data were collected from 40 cardiology centers with a population of 476 patients.18) In another i-SEARCH study performed in Turkey in an international multi-center, the prevalence of microalbuminuria was found to be 64.7%. The study was designed to evaluate the frequency of microalbuminuria, which was observed in a large number of outpatient clinics, in a total of 21,050 patients that were receiving treatment for hypertension in 26 countries.19) However, in our study, the prevalence of proteinuria was higher than that seen in another study from a tertiary hospital in Uganda (39.5%).20) These differences are most likely associated with the different criteria used in patient selection, such as age, severity of hypertension, race, and coexisting cardiovascular risk factors (e.g., diabetes mellitus, renal disease, dyslipidemia, and obesity), as well as the technique used for proteinuria determination. Moreover, in a study conducted in 26 countries, the highest rates of microalbuminuria were observed in Asia.21)
The prevalence of smoking among female patients was lower than that among male patients. A similar finding has been reported in another study.22) This low prevalence of smoking among female patients may due to social factors that discourage smoking in women.
In our study, we found a significant association between proteinuria and the male which is comparable with the results of others studies.21, 23) In contrast, other studies did not find a relationship between sex and microalbuminuria.19, 21) This discrepancy is most probably due to a combination of risk factors (i.e., diabetes, smoking, and obesity) and the fact that ischemic heart disease is more frequent in men than in women.
In the present investigation, we found the association between high systolic BP and diastolic BP and proteinuria. A similar result was obtained by Ya-Pan et al. who found that an increase in systolic BP and diastolic BP was related to a prevalence of proteinuria24), however, Al-Saffar et al. did not observe this association.25) The likely reason might be due to a substantial reduction in renal perfusion, which leads to proteinuria.
According to our study there was a substantial statistically difference between proteinuria and patient age, which is in agreement with other studies.26, 27) This could be explained by relevant studies that found advancing age to be a risk factor for a higher prevalence of proteinuria.28) Therefore, the early detection of proteinuria and screening of hypertensive patients might prevent complication such as renal and cardiovascular diseases.23, 29)
The prevalence of proteinuria is higher in smoking hypertensive patients than in those who do not smoke. Similar findings have been reported in other studies.30, 31) This could be explained by the presence of endothelial dysfunction, which involves an imbalance between the contracting and relaxing substances produced by the endothelium. For example, the plasma concentration of endothelin-14 is higher in smokers than in nonsmokers.
The present study has shown an independent relationship between proteinuria and the presence of concomitant diseases such as diabetes, congestive heart failure, and myocardial infarction. This observation is in agreement with other studies that found a relationship between albuminuria and cardiovascular risk.21) This relationship could be explained by other relevant studies in which microalbuminuria was found to indicate a generalized dysfunction of the vascular endothelium with permeability changes, which leads to a leakage of albumin through the glomerular membrane. Furthermore, the finding that the prevalence of proteinuria was higher among patients with diabetes is most likely attributable to the fact that these patients had diabetes nephropathy.
The result of our study also showed that physical activity decreased the risk of proteinuria in hypertensive patients. This association has been previously demonstrated by reports that showing physical activity was inversely associated with microalbuminuria.18, 30) Physical activity has protective effects on the vascular endothelium among patients with cardiac diseases.31)
We found a significant association between the use of CCBs and proteinuria risk in the present study. This can be explained by the dilating effect of CCBs on the efferent glomerular arterioles. Systemic hypertension results in glomerular hypertension and could increase urinary albumin excretion.32) The present study also shows that the use of BBs is associated with proteinuria. However, Kozan et al. could not find a significant association between the use of BBs and microalbuminuria risk.19) Our finding show the expected superiority of ACE inhibitors and ARBs in lowering proteinuria. The reason for this association was illustrated by other observations in which different mechanisms were identified to explain the beneficial effect of ACE inhibitors and ARBs on proteinuria. First, ACE inhibitors and ARBs decrease glomerular capillary and intra glomerular pressure. Second, this group of drugs can reduce the permeability of the basement membrane of the glomerulus. Both hemodynamic and non-hemodynamic effects of ACE inhibitors and ARBs on renal activity lead to a reduction in proteinuria among patients with hypertension.32)
This study has some limitations. The samples consisted of patients who were referred to an outpatient clinic for treatment. In addition, the data source was a single center and the study design was an observational analysis. Patients were not randomly selected in our study; therefore, there might be a selection bias. Furthermore, the examination of proteinuria by using our data did not demonstrate the proportion of patients who had positive or negative results based on retesting. Moreover, concerning the study sample, the patients were selected on a convenience basis. Finally, the study only included the Uzbek-Turkmen ethnic group and the findings may not be generalizable to the general population.
We conclude that our results confirm the presence of a high incidence of proteinuria among hypertensive outpatients in a clinic in Andkhoy, Afghanistan, especially in those with a high cardiovascular risk. Therefore, more frequent screening for proteinuria might be imperative for the evaluation
ACKNOWLEDGEMENT
This project was supported in part, by the non-profit organization Epidemiological and Clinical Research Information Network (ECRIN). We would like to thank Dr.Mirwais Rabi, and Dr.Ahmad Arsalam Karimi for their cooperation for this study.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest in this manuscript.
REFERENCES
- 1).World Health Organization; Causes of Death. WHO, Geneva. 2008. at: http://www.who.int/healthinfo/global_burden_disease/cod_2008_sources_methods.pdf.
- 2).A global brief on hypertension: silent killer, global public health crisis. WHO, 2013. at: http://apps.who.int/iris/handle/10665/79059#sthash.ApjACvG7.dpuf.
- 3).Ibrahim MM, Damasceno A. Hypertension in developing countries. Lancet, 2012; 380: 611–619. [DOI] [PubMed]
- 4).Poudel B, Yadav BK, Nepal AK, Jha B, Raut KB. Prevalence and association of microalbuminuria in essential hypertensive patients. N Am J Med Sci, 2012; 4: 331–335. [DOI] [PMC free article] [PubMed]
- 5).Perkovic V, Huxley R, Wu Y, Prabhakaran D, MacMahon S. The burden of blood pressure-related disease: a neglected priority for global health. Hypertension, 2007; 50: 991–997. [DOI] [PubMed]
- 6).NeupaneD, McLachlan CS, Sharma R, Gyawali B, Khanal V, Mishra SR, et al. Prevalence ofhypertensionin member countries of South Asian Association for Regional Cooperation (SAARC): systematic review and meta- analysis. Medicine (Baltimore), 2014; 93: e74. [DOI] [PMC free article] [PubMed]
- 7).Saeed KM, Rasooly MH, Brown NJ. Prevalenceand predictors of adulthypertension in Kabul, Afghanistan. BMC Public Health, 2014; 14: 386 [DOI] [PMC free article] [PubMed]
- 8).Marques da Silva P, Carvalho D, Nazaré J, Martins L, Aguiar C, Manso MC, et al. Prevalence of microalbuminuria in hypertensive patients with or without type 2 diabetes in Portuguse primary care setting: The RACE (micRoAlbumin sCreening survEy) study. Rev port Cardiol, 2015; 34: 237–246. [DOI] [PubMed]
- 9).Hillege HL, Fidler V, Diercks GF, van Gilst WH,de Zeeuw D,van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation, 2002; 106: 1777–1182. [DOI] [PubMed]
- 10).Schmieder RE, Mann JF, Schumacher H, Gao P, Mancia G,Weber MA, et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol, 2011; 22: 1353–1364. [DOI] [PMC free article] [PubMed]
- 11).Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens, 2013; 31: 1281–1357. [DOI] [PubMed]
- 12).Carretero OA, Oparil S. Essential Hypertension. Part I: Definition and Etiology. Circulation, 2000; 101: 329–335. [DOI] [PubMed]
- 13).Mayfield J. Diagnosis and Classification of Diabetes Mellitus: New Criteria. Am Fam Physician, 1998; 58: 1355–1362, 1369–1370. [PubMed]
- 14).National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation, 2002; 106: 3143–3421. [PubMed]
- 15).Musaiger AO. Overweight and obesity in Eastern Mediterranean region: prevalence and Possible causes. J Obes, 2011; 407237. [DOI] [PMC free article] [PubMed]
- 16).Modesti PA, Bamoshmoosh M, Rapi S, Massetti L, Bianchi S, Al-Hidabi D, et al. Relationship between hypertension, diabetes and proteinuria in rural and urban households in Yemen. J Hum Hypertens, 2013; 27: 572–579. [DOI] [PMC free article] [PubMed]
- 17).Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: update recommendation from adult. The American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc, 2007; 39: 1423–1434. [DOI] [PubMed]
- 18).Habbal R, Sekhri AR, Volpe M; i-Search Investigators. Prevalence of microalbuminuria in hypertensive patients and its associated cardiovascular risk in clinical cardiology: Moroccan results of the global i-SEARCH survey - a sub-analysis of a survey with 21,050 patients in 26 countries worldwide. Cardiovasc J Afr, 2010; 21: 200–205. [DOI] [PMC free article] [PubMed]
- 19).Kozan O, Ozcan EE, Sancaktar O, Kabakcı G; Turkish investigators of the i-SEARCH study. The prevalence of microalbuminuria and relevant cardiovascular risk factors in Turkish hypertensive patients. Turk Kardiyol Dern Ars, 2011; 39: 635–645. [DOI] [PubMed]
- 20).Nabbaale J, Kibirige D, Ssekasanvu E, Sebatta ES, Kayima J, Lwabi P, et al. Microalbuminuria and left ventricular hypertrophy among newly diagnosed black African hypertensive patients: a cross sectional study from a tertiary hospital in Uganda. BMC Res Notes, 2015; 8:198. [DOI] [PMC free article] [PubMed]
- 21).Böhm M, Thoenes M, Danchin N, Reil JC, Volpe M. Overview of the i-SEARCH Global Study : Cardiovascular Risk Factors and Microalbuminuria in Hypertensive Individuals. High Blood Press Cardiovasc Prev, 2008; 15: 217–224. [DOI] [PubMed]
- 22).Hamrah MS, Harun-Or-Rashid M, Hirosawa T, Sakamoto J, Hashemi H, Emamian MH, et al. Smoking and associated factors among the population aged 40–64 in Shahroud, Iran. Asian Pac J Cancer Prev, 2013; 14: 1919–1923. [DOI] [PubMed]
- 23).Pontremoli R, Leoncini G, Viazzi F, Parodi D, Vaccaro V, Falqui V, et al. Role of microalbuminuria in the assessment of cardiovascular in essential hypertension. J Am Soc Nephrol, 2005; 16: S39–S41. [DOI] [PubMed]
- 24).Zhang YP, Zuo XC, Huang ZJ, Kuang ZM, Lu MG, Duan DD, et al. The impact of blood pressure on kindney function in the elderly: a cross-sectional study. Kidney Blood Press Res, 2013; 38: 205–216. [DOI] [PMC free article] [PubMed]
- 25).Al-Saffar HB, Nassir H, Mitchell A, Philipp S. Microalbuminuria in non-diabetic patients with unstable angina/non ST-segment elevation myocardial infarction BMC Res Notes, 2015; 8: 371. [DOI] [PMC free article] [PubMed]
- 26).Damsgaard EM, Frøland A, Jørgensen OD, Mogensen CE. Microalbuminuria as a predictor of increased mortality in eldely people. BMJ, 1990; 300: 297–300. [DOI] [PMC free article] [PubMed]
- 27).Stamm AM, Meinerz G, Silva JC. Systemic hypertension and microalbuminuria. Arq Bras Carilol, 2007; 89: 415–420. [DOI] [PubMed]
- 28).Hitha B, Pappachan JM, Pillai HB, Sujathan P, Ramakrishna CD, Jayaprakash K, et al. Microalbuminuria in patients with essential hypertension and its relationship to target organdamage: an Indian experience. Saudi J Kidney Dis Transpl, 2008; 19: 411–419. [PubMed]
- 29).Polónia J, Carmona J, Mendes E, Pisco L. Prevalence of microalbuminuria in non-diabetic hypertensive patients attended by Portuguese GPs. Rev Port Cardiol, 2007; 26: 637–644. [PubMed]
- 30).Fredrickson SK,Ferro TJ,Schutrumpf AC. Disappearance of microalbuminuria in a patient with type 2 diabetes and the metabolic syndrome in the setting of an intense exercise and dietary program with sustained weight reduction. Diabetes Care, 2004; 27: 1754–1755. [DOI] [PubMed]
- 31).Robinson ES, Fisher ND, Forman JP, Curhan GC. Physical activity and albuminuria. Am J Epedemiol, 2010; 171: 515–521. [DOI] [PMC free article] [PubMed]
- 32).Monster TB, Janssen WM, de Jong PE, de Jong-van den Berg LT; PREVEND Study Group. The impact of antihypertensive drug groups on urinary albumin excretion in a non-diabetic population. Br J Clin Pharmacol, 2002; 53: 31–36. [DOI] [PMC free article] [PubMed]