Abstract
Background
Heavy menstrual bleeding affects as many as one in three women and has negative physical, economic, and psychosocial impacts including activity limitations and reduced quality of life. The goal of treatment is to make menstruation manageable, and options include medical therapy or surgery such as endometrial ablation or hysterectomy. This review examined the evidence of effectiveness and cost-effectiveness of the 52-mg levonorgestrel-releasing intrauterine system (LNG-IUS) as a treatment alternative for idiopathic heavy menstrual bleeding.
Methods
We conducted a systematic review of the clinical and economic evidence comparing LNG-IUS with usual medical therapy, endometrial ablation, or hysterectomy. Medline, EMBASE, Cochrane, and the Centres for Reviews and Dissemination were searched from inception to August 2015. The quality of the evidence was assessed according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We also completed an economic evaluation to determine the cost-effectiveness and budget impact of the LNG-IUS compared with endometrial ablation and with hysterectomy. The economic evaluation was conducted from the perspective the Ontario Ministry of Health and Long-Term Care.
Results
Relevant systematic reviews (n = 18) returned from the literature search were used to identify eligible randomized controlled trials, and 16 trials were included. The LNG-IUS improved quality of life and reduced menstrual blood loss better than usual medical therapy. There was no evidence of a significant difference in these outcomes compared with the improvements offered by endometrial ablation or hysterectomy. Mild hormonal side effects were the most commonly reported. The quality of the evidence varied from very low to moderate across outcomes. Results from the economic evaluation showed the LNG-IUS was less costly (incremental saving of $372 per person) and more effective providing higher quality-adjusted life years (incremental value of 0.05) compared with endometrial ablation. Similarly, the LNG-IUS costs less (incremental saving of $3,138 per person) and yields higher quality-adjusted life-years (incremental value of 0.04) compared with hysterectomy. Publicly funding LNG-IUS as an alternative to endometrial ablation and hysterectomy would result in annual cost savings of $3 million to $9 million and $0.1 million to $23 million, respectively, over the first 5 years.
Conclusions
The 52-mg LNG-IUS is an effective and cost-effective treatment option for idiopathic heavy menstrual bleeding. It improves quality of life and menstrual blood loss, and is well tolerated compared with endometrial ablation, hysterectomy, or usual medical therapies.
BACKGROUND
Heavy Menstrual Bleeding
Heavy menstrual bleeding (menorrhagia) is excessive or prolonged menstrual bleeding, either alone or in combination with other symptoms that affect women of reproductive age.1 This health technology assessment focuses on the management of heavy menstrual bleeding as defined by the Society of Obstetricians and Gynaecologists of Canada (adopting the definition from the UK National Institute for Health and Care Excellence): “excessive menstrual blood loss which interferes with a woman's physical, social, emotional, and/or material quality of life.”2,3 The term “abnormal uterine bleeding” includes heavy menstrual bleeding as well as several other variations to the menstrual cycle and other non-menstrual types of uterine bleeding that are beyond the scope of this review.3
Heavy menstrual bleeding can result from a number of underlying causes including cervical pathology, endometrial polyps, endometrial hyperplasia, cancers, fibroids, or systemic bleeding disorders.3 It can also be idiopathic, meaning it occurs in the absence of any recognizable pelvic or systemic pathological condition; this is sometimes called cyclical dysfunctional uterine bleeding.1 Women with idiopathic heavy menstrual bleeding tend to be younger than women whose heavy bleeding is due to organic causes such as fibroids, polyps, or endometrial hyperplasia.4
Population-based surveys from the mid-1960s and early 1970s estimated that the prevalence of “objective menorrhagia” (heavy menstrual bleeding of > 80 mL of blood loss per cycle) was about 10%.5,6 In contrast, self-reported data suggest prevalence is on the order of 30%.1 Experts we consulted estimate that 15% to 20% of women of reproductive age (15 to 55 years old) in Ontario are affected by heavy menstrual bleeding. The prevalence of heavy menstrual bleeding increases with age and peaks in perimenopause.7 Defining the severity of the condition is a challenge, but a patient's perception of bleeding and its impact on her life is most important in considering treatment options and determining the degree of intervention.3
The pervasive impact of heavy menstrual bleeding is on a woman's quality of life, and its effects are biopsychosocial in nature. Focus groups have identified inconvenience, pain, self-consciousness, and social embarrassment as themes of the impacts of heavy menstrual bleeding on a woman's life.8 Pain associated with bleeding is common and can be localized to the uterus or back, or it can manifest as a general sensation of cramping or heaviness.8 The emotional toll of worry and embarrassment is compounded by the social impact of limitations in activities in general.9 Heavy menstrual bleeding affects interpersonal relationships including sexual function,5 and as many as two-thirds of women can experience mild to moderate depression.10 There is evidence of economic impact in the form of lower earnings among women with excessive menstrual bleeding due to absence from work.11 In the US, heavy menstrual bleeding accounts for an 8% loss in employee wages.7
If excessive bleeding is not treated effectively, approximately two-thirds of women will develop iron-deficiency anemia, which can produce fatigue, weakness, mood swings, weight loss, and impaired cognition.12 Heavy menstrual bleeding is the most common cause of this anemia in developed countries,12 and the anemia limits women's activities and negatively impacts their overall health and well-being.9 Correction of anemia is associated with significant improvements in health-related quality of life in women with heavy menstrual bleeding.13
While in clinical practice a patient's own experience of heavy menstrual bleeding is key to understanding its impact on her life, research often involves quantifying the actual blood loss. Volume of blood loss can be assessed semi-quantitatively via the pictorial blood loss assessment chart (PBAC), self-report (e.g., diary keeping), or most objectively by the alkaline hematin lab test.9 Using the PBAC method of blood loss measurement, women record and score the number and degree of saturation of the sanitary products they use, and the frequency and size of clots, and episodes of flooding over the course of a month.14 The alkaline hematin method requires all used sanitary materials to be sent for laboratory analysis and is the standard for determining objective menorrhagia (blood loss > 80 mL per cycle). Both methods use standardized sanitary products to facilitate comparability between subjects. The alkaline hematin method is less commonly used owing to practicality.
The PBAC score is significantly correlated with objective menorrhagia defined by the alkaline hematin test in women with both normal and heavy menses.15 A PBAC score of more than 100 reflects objective menorrhagia, with a sensitivity of 97% in heavy-bleeding populations16 and 86% in the general female population.14 A comparative study of the two methods found that, after adjustment for known confounders, the PBAC score is an independent predictor of menstrual blood loss of more than 80 mL.15
Treatment Options
The goal of treating heavy menstrual bleeding is to make menstruation manageable for a patient, and treatment options will reflect a woman's values and preferences as well as the unique clinical attributes of her condition. A number of systemic medications (oral or injected) may be prescribed as usual medical therapy to manage heavy menstrual bleeding. Hormonal medications may be beneficial both for reduction of menstrual blood loss and also for regulation of the menstrual cycle. Options may include combined oral contraceptives (estrogen and progestogens) or progestogens alone (oral, injection, or depot injection).1 For women with predictable timing of bleeding, nonsteroidal anti-inflammatory drugs and antifibrinolytic drugs can be used to reduce bleeding.3
Medications that have some evidence of effectiveness include tranexamic acid, mefenamic acid, medroxyprogesterone acetate, combined oral contraceptives, and danazol.17 In situations where other treatment options are contraindicated, gonadotropin-releasing hormone agonists may also be prescribed occasionally to treat heavy menstrual bleeding.3 Table 1 describes these common medications.
Table 1:
Overview of Usual Medical Therapies Available for Heavy Menstrual Bleeding
| Medication | Drug Category | Dose/Regimen | Mechanism of Action | Side Effects |
|---|---|---|---|---|
| Tranexamic acid | Antifibrinolytic | During menstruation only | Inhibits breakdown of blood clots | Mild nausea, diarrhea |
| Mefenamic acid Naproxen | NSAID | During menstruation only | Inhibits prostaglandin production | Headaches, gastrointestinal issues |
| Norethisterone MPA | Progestogen | Days 5–26 of menstrual cycle | Suppresses endometrial growth and activity | Breast tenderness, bloating, headaches, breakthrough bleeding |
| COC | Estrogen and progestogen | 21 or 28 days per cycle | Inhibits growth and development of endometrium | Nausea, headache, breast tenderness, weight change, altered libido, depression |
| Danazol | Androgen | Daily | Causes endometrial atrophy | Weight gain, headache, nausea, tiredness, acne, possible fetal damage |
Abbreviations: COC, combined oral contraceptive; MPA, medroxyprogesterone acetate; NSAID, nonsteroidal anti-inflammatory drug.
Source: Marjoribanks et al, 2010.17
Effective management with medical therapy is a key aim as it enables a woman to avoid more invasive, irreversible surgical treatments such as endometrial ablation or hysterectomy.1 Endometrial ablation is a procedure to either destroy (ablate) or remove (resect) the lining of the uterus (endometrium).18 This can be done via a number of techniques considered as either first generation (techniques developed in the 1980s, such as rollerball and laser ablation,) or second generation (developed in the 1990s, e.g., thermal balloon and microwave ablation).19 First-generation ablation techniques generally require direct visualization of the endometrium via hysteroscope and a high degree of surgical skill on the part of the clinician, whereas second-generation techniques rely less on operator skill and more on the device for safety and efficacy.19 Most ablation techniques are marketed as outpatient procedures.19 Pregnancy can happen after endometrial ablation and has many risks, so women undergoing the procedure also need to use highly effective contraception such as sterilization, which can be done as part of the procedure, or (for their male partner) vasectomy.
Failure of the initial endometrial ablation can result in the need for either repeat ablation (this occurs in 3% to 7% of women who have had the procedure) or hysterectomy.19 Treatment failure may result from the incomplete destruction of the endometrium, which allows endometrial cells to persist or regenerate, causing ongoing bleeding. Excessive destruction involving the cervix may result in hematometra (blood becoming trapped in the uterus), retrograde bleeding through the fallopian tubes, and significant pain.18 These complications often lead to hysterectomy. A history of dysmenorrhea (painful menstruation), tubal ligation, or endometrial ablation performed in an operating room increase the likelihood of post-ablation hysterectomy.20
Hysterectomy is surgery to remove both the uterus and cervix (total hysterectomy) or just the uterus (subtotal or supracervical). Hysterectomy is one of the most common gynecologic procedures in the United States20 and Canada,21 and heavy menstrual bleeding is the main presenting problem in at least half of women who have a hysterectomy before age 60.9 Removal of the uterus is considered the only definitive cure for heavy menstrual bleeding; however, women who retain their cervix can continue to experience cyclical bleeding after surgery.22 With laparoscopic supracervical hysterectomy, as many as 17% to 24% of women may continue to have cyclical menstrual bleeding up to 3 years after surgery.23,24 Laparoscopy is surgery that uses small incisions and the use of a thin lighted tube to enable the surgeon to operate inside the body.
Even when surgery is uncomplicated, hysterectomy is the most invasive procedure for heavy menstrual bleeding and has a considerable recovery period.25 The surgery usually requires general anesthetic and can be performed using various approaches. In descending order of duration of recovery time and invasiveness, the approaches are vaginal, laparoscopic (including robotic surgery), combination laparoscopic-vaginal, or abdominal (open).19,26 The risks with hysterectomy are those associated with surgery and include postoperative infection (e.g., vaginal cuff cellulitis, urinary tract infection, wound infection, respiratory infection), blood clots (e.g., deep vein thrombosis, pulmonary embolism), and blood loss.26 Hysterectomy-specific complications include potential injury to the nearby bladder or ureter, bowel injury, and rarely, nerve injury (e.g., femoral, iliohypogastric, ilioinguinal, or peroneal nerve), or vaginal cuff dehiscence (separation of the vaginal surgical incision) which can lead to in various degrees of evisceration (when the abdominal organs push through the surgical incision).26 The risk of complications depends on several patient factors, such as older age, obesity, and comorbidities, as well as surgical technique.26
Ontario experts consulted for this report indicated that the surgical risks can be greater for women with higher body mass index (BMI), especially obese women, and therefore understanding the effectiveness of nonsurgical treatment options for heavy menstrual bleeding is of particular interest. This report examines the effectiveness and cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), a hormone-releasing device described in the Technology section, below.
Patient Values and Preferences for Treatment
A patient's perception of her heavy menstrual bleeding and how it affects her quality of life is the most important information for the clinical management and the degree of intervention,3 and multiple factors may influence individual treatment preferences. Clarifying a patient's values and preferences is a key part of decision-making in the management of heavy menstrual bleeding and has been shown to result in lower costs.27 Women who want future pregnancies, have irregular menstruation, are unemployed, or have higher levels of anxiety are more likely to opt for conservative management.28 On the other hand, women who express a preference for hysterectomy before getting treatment or who feel inconvenienced because they experience more symptoms of heavy bleeding have a higher likelihood of having a hysterectomy.28
A Dutch cohort study of women who had already chosen LNG-IUS, endometrial ablation, or hysterectomy for the management of idiopathic heavy menstrual bleeding sheds some light on complexities of women's decision-making about treatment.29 Table 2 summarizes the information patients received about their options, the proportion who chose each treatment, and the main reasons for their preferences.
Table 2:
Summary of Most Common Reasons for Selection of Treatment for Heavy Menstrual Bleeding
| Information Provided About Treatment Option | % Choosing | Most Common Reasons for Selection (% Citing Reasona) |
|---|---|---|
Levonorgestrel-releasing intrauterine system
|
16 |
|
Endometrial ablation
|
67 |
|
Hysterectomy
|
17 |
|
Proportions may add up to more than 100% as respondents could select more than one reason.
Source: Bourdez et al, 2004.29
Ontario gynecology experts reported that the treatment received by female relatives, cultural values around menstruation and amenorrhea (no menstruation), and misperceptions or lack of awareness about treatment options influence women's choices for treatment of heavy menstrual bleeding. In particular, the LNG-IUS is a type of intrauterine device (IUD), and misperceptions about IUDs persist despite the fact that most of the common concerns (e.g., pelvic inflammatory disease, difficult or painful insertion, infertility) are not based on evidence.30 These misperceptions by both patients and providers can serve as barriers to women selecting an IUD, whether for contraception or treatment of heavy menstrual bleeding.30 In addition, the chance of amenorrhea with the LNG-IUS is up to 40%, which may be undesirable to some women or in cultures that view menstruation as a natural and necessary cleansing process.30,31 Globally, uptake of IUDs for contraception has been estimated by United Nations data to be nearly 14% of women aged 15 to 49 who are in committed relationships.30 Despite widespread guidance from international and local authorities supporting IUD use in nearly all women, only 2.3% of women aged 15 to 50 in Canada use either copper IUDs or LNG-IUS for contraception.30 Women's perceptions, beliefs, awareness of treatment options, and the experiences and choices of trusted others all contribute to shaping their treatment choices.
Technology
The 52-mg levonorgestrel-releasing intrauterine system (LNG-IUS) is inserted in the uterus and releases up to 20 mcg of levonorgestrel per day (mean 14 mcg/day).32 Levonorgestrel is a progestogen that suppresses the endometrium, reducing the volume and duration of menstrual bleeding within a few months of use. The device is a polyethylene T-frame with a cylindrical drug reservoir. The 52-mg LNG-IUS is designed to be used for up to 5 years, after which the device should be removed or replaced if desired. The device can be inserted by obstetrician/gynecologist and many family physicians, and it can be removed by most physicians.33
During its 15 years of use, the LNG-IUS has been well studied. A great deal of published evidence documents its therapeutic benefits and side effects.34 Some studies have estimated the LNG-IUS to be a good option for managing heavy menstrual bleeding in upwards of 85% of women.35 Research has found that the decrease in menstrual blood loss is usually seen within the first 3 months after the device is inserted,36 and the reduction can be so large37 that it has even lead women to cancel scheduled surgery.36,38 While intermenstrual bleeding (spotting) and mild hormonal side effects are very common during the first few months, they tend to decrease and disappear with time.33 There is evidence for clinical effectiveness in women with different underlying causes such as uterine fibroids,39,40 adenomyosis,41 and heavy menstrual bleeding resulting from anticoagulant medication.42 However, questions remain about whether the LNG-IUS has a role as a high-quality treatment alternative compared with other options, including hysterectomy and endometrial ablation, to manage idiopathic heavy menstrual bleeding.
The LNG-IUS is very safe and minimally invasive, but not risk-free.
Uterine perforation (when the device partially breaks through the outer surface of the uterus or is inadvertently inserted through the uterus into the abdominal cavity) occurs in 1.4 per 1,000 insertions (ranges reported 0.3 to 2.6 per 1,000).33,43,44 The primary risk factor for perforation is breastfeeding45,46 or postpartum insertion (this risk decreases over time but is elevated up to 36 weeks after delivery).33
Expulsion of the device has been reported to occur in as many as 5% of cases,33 though large cohort studies have estimated the rate of device expulsion to be 0% per year for nulliparous women (those who have never given birth) and 0.2% for parous women (who have given birth).47 Historical concerns about increased risk of adverse events in nulliparous women have not been substantiated by further investigation.48
In the long-term, the LNG-IUS is associated with a slightly elevated risk of breast cancer,49 and women with current or recent breast cancer (within 5 years) are contraindicated for any treatment with progestogens.32 At the same time, a protective effect for endometrial, ovarian, pancreatic, and lung cancers was seen in the same large cohort.49
Use of the LNG-IUS is also associated with increased appearance of ovarian cysts; however, a large study found that the cysts did not cause symptoms and spontaneously resolved in 94% of cases following removal of the device.50
Regulatory Information
The Mirena LNG-IUS 52 mg (Bayer, Inc.) is licensed by Health Canada as a drug product for contraception and for the treatment of idiopathic heavy menstrual bleeding for up to 5 years.33 At the time of writing, Mirena was the only LNG-IUS product currently licensed in Canada for both of these indications. A 13.5-mg LNG-IUS is licensed in Canada for up to 3 years, but it is indicated for contraception only. The use of LNG-IUS has also been explored as a treatment for endometriosis, adenomyosis, endometrial hyperplasia, and heavy menstrual bleeding due to small fibroids, and as an adjunct to estrogen replacement therapy.7 However, these all remain off-label uses in Canada.
Context
The Canadian, American, and British guidelines addressing the role of the LNG-IUS for the treatment of heavy menstrual bleeding are summarized in Table 3. The guidance from the UK National Institute for Health and Care Excellence (NICE) on heavy menstrual bleeding explicitly recommends the LNG-IUS as the first-line medical treatment above oral or injectable medical options.2
Table 3:
Existing Guidelines for the Levonorgestrel-Releasing Intrauterine System for Heavy Menstrual Bleeding
| Organization, Guideline | Year | Summary of Recommendations |
|---|---|---|
| SOGC, Endometrial Ablation in the Management of Abnormal Uterine Bleeding51 | 2015 | The LNG-IUS should be discussed prior to any surgical option for women with abnormal uterine bleeding and a normal uterine cavity. |
| SOGC, Menstrual Suppression in Special Circumstances52 | 2014 | The Canadian Consensus Guideline on Continuous and Extended Hormonal Contraception identifies abnormal uterine bleeding as a medical indication for menstrual suppression. Combined or progesterone-only hormonal products (including LNG-IUS) can be used continuously to obtain menstrual suppression. |
| SOGC, Abnormal Uterine Bleeding in Pre-Menopausal Women3 | 2013 | Combined oral contraceptive pills, depot medroxyprogesterone acetate, and LNG-IUS significantly reduce menstrual bleeding and should be used in women with abnormal uterine bleeding who desire effective contraception. |
| University of Texas at Austin, Evaluation and Management of Ovulatory Heavy Menstrual Bleeding (HMB) in Primary Care53,a | 2012 | LNG-IUS should be first-line therapy for heavy menstrual bleeding for women preferring contraception lasting for 5 years. |
| NICE, Heavy Menstrual Bleeding (Clinical Guidance 44)2 | 2007b | If pharmaceutical treatment is appropriate and both nonhormonal and hormonal treatments are acceptable, the LNG-IUS should be considered first, followed by tranexamic acid/NSAIDs/combined oral contraceptives, then norethisterone/injected long-acting progestogens. |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; NICE, National Institute for Health and Care Excellence; NSAID, nonsteroidal anti-inflammatory drug; SOGC, Society of Obstetricians and Gynaecologists of Canada.
From the School of Nursing, Family Nurse Practitioner Program.
Guidance update is in progress as of March 2015 with anticipated publication in August 2016.
Equity Issues
There are two costs associated with the use of the 52-mg LNG-IUS: the cost of the insertion procedure by a physician and the purchase of the product itself from a pharmacy. All Canadian provinces and territories provide coverage for the physician component of the LNG-IUS (exam, insertion, removal), but none provide universal coverage for the drug product itself, which costs about $340 (Ontario Ministry of Health and Long-Term Care, written communication, June 2015). In Ontario, the 52-mg LNG-IUS is publicly funded only for women over 65 years of age or on social assistance or eligible for the Trillium Drug Program. Similarly, three other provinces (Nova Scotia, Newfoundland and Labrador, and Saskatchewan) provide restricted public coverage of the drug for eligible older women, women on social assistance, or through other income-based coverage. The LNG-IUS is covered under Health Canada's Non-Insured Health Benefit for First Nations and Inuit women.54,55
Those women of reproductive age preferring this treatment option and not eligible for coverage under the above described Provincial policies have to pay for the device. Given that this option is fully funded only for seniors and women with significant economic disadvantage, a potential inequity in access to this therapy exists among the majority of women in Ontario and across Canada.
Research Questions
Clinical Evidence Review
What is the effectiveness of the 52-mg levonorgestrel-releasing intrauterine system for idiopathic heavy menstrual bleeding, compared with each of the following treatment options?
Hysterectomy
Endometrial ablation
Usual medical therapy
Economic Evidence Review
What is the evidence from previous research on the cost-effectiveness of the LNG-IUS compared with endometrial ablation, hysterectomy, or nonsurgical intervention in women with heavy menstrual bleeding?
Primary Economic Evaluation
What is the cost-effectiveness, within the context of the Ontario Ministry of Health and Long-Term Care, of the LNG-IUS compared with surgical interventions (hysterectomy and endometrial ablation) in women with heavy menstrual bleeding?
Budget Impact Analysis
What is the potential budget impact in Ontario of publicly funding the LNG-IUS for treatment of heavy menstrual bleeding compared with hysterectomy and endometrial ablation?
CLINICAL EVIDENCE REVIEW
Objective
The objective of the clinical evidence review was to assess the effectiveness of the 52-mg levonorgestrel-releasing intrauterine system (LNG-IUS) for treatment of heavy menstrual bleeding, compared with hysterectomy, endometrial ablation, and usual medical therapy.
Methods
Literature Search
In scoping the state of knowledge on the topic, we discovered a large number of recent, high-quality systematic reviews that, collectively, have broadly and comprehensively examined treatments for heavy menstrual bleeding. We therefore developed a review approach targeting systematic reviews. We applied a study design filter to the search strategy to return systematic reviews, health technology assessments, and meta-analyses of randomized controlled trials (RCTs) comparing LNG-IUS 52-mg to hysterectomy, endometrial ablation, or usual medical therapy.
We performed a literature search on August 17, 2015, using Ovid MEDLINE, Ovid MEDLINE In-Process, Ovid EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database, for studies published from inception to August 17, 2015.
Search strategies were developed by medical librarians using medical subject headings (MeSH). See Appendix 1 for full details, including all search terms.
Literature Screening
A single reviewer screened the abstracts and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. We used DistillerSR to review citations, and reasons for exclusion of full-text articles are reported in a Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram.
Inclusion Criteria
English-language full-text publications
Studies published before August 17, 2015
Systematic reviews, health technology assessments, meta-analyses of randomized controlled trials; if none identified, randomized controlled trials meeting all other inclusion criteria
Women of reproductive age with idiopathic heavy menstrual bleeding
52-mg LNG-IUS that delivers up to 20 mcg levonorgestrel per day, indicated for heavy menstrual bleeding and approved for up to 5 years
-
Comparison with at least one of:
○ total or supracervical (subtotal) hysterectomy via vaginal, laparoscopic, robotic, or abdominal surgical approach
○ endometrial ablation or resection with first- or second-generation techniques
○ usual medical therapy (systemic medications) consisting of nonsteroidal anti-inflammatory drugs, antifibrinolytics, combined hormonal contraceptives, oral progestogens, depot-medroxyprogesterone acetate, danazol, or gonadotropin-releasing hormone agonists
Reporting outcomes of interest
Exclusion Criteria
Animal or in vitro studies
Quasi-randomized studies, observational or therapeutic studies, editorials, case reports, commentaries
Menopausal women or those seeking treatment for perimenopausal menstrual bleeding, or menorrhagia due to structural or pathological causes (e.g., fibroids, cancer, polyps, adenomyosis, endometriosis, systemic bleeding disorders)
Intrauterine systems releasing different doses of levonorgestrel, other progestogens, or other medications, or other 52-mg levonorgestrel products with differing guidance on indications or administration
Noncomparative studies or those using an ineligible or inactive comparator (e.g., placebo, observation)
Not reporting outcomes of interest
Outcomes of Interest
Primary outcome:
Quality of life (overall or health-related as measured by a validated tool)
Secondary outcomes:
Menstrual bleeding (measured by pictorial blood loss assessment chart (PBAC), self-report, or alkaline hematin test)
Satisfaction/acceptability (measured by a validated tool, if evaluated separately from quality of life)
Complications/side effects/adverse events of the intervention and all comparators
Data Extraction
Data on study context, methods, population, intervention, comparator, outcomes, results, and risk of bias items were extracted by a single reviewer based on the information available in the published articles. Data included study eligibility criteria and population characteristics (age, parity, medical history, socioeconomic characteristics, obesity status, etc.); information on the LNG-IUS procedure (location of insertion), surgical approach to hysterectomy or endometrial ablation technique (device, location, administering clinician) and type of medical therapy; and information related to all defined outcomes.
Statistical Analysis
Statistical synthesis was not appropriate due to methodological heterogeneity in the studies we identified; thus, we analyzed the studies in a narrative synthesis.
Quality of Evidence
The quality of the body of evidence for each outcome was examined according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria.56 The overall quality was determined to be high, moderate, low, or very low using a step-wise, structural methodology.
Expert Consultation
We sought expert consultations on the use of the 52-mg LNG-IUS for idiopathic heavy menstrual bleeding. Members consulted included physicians in the specialty areas of gynecology and family medicine. The role of the expert advisors was to provide important contextual information on the use of the LNG-IUS, including expertise on the health condition, patients, diffusion of the technology, or clinical issues that contextualize the research question to Ontario. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Results
Literature Search
The database search yielded 311 citations published up until August 17, 2015. After removing duplicates, we reviewed titles and abstracts to identify potentially relevant articles. We obtained the full texts of these articles for further assessment. Seventeen systematic reviews on LNG-IUS (including two health technology assessments) were identified. We hand-searched the reference lists of the systematic reviews, along with health technology assessment websites and other sources, to identify additional relevant studies. One additional citation (a health technology assessment) was identified, for a total of 18 systematic reviews.
Systematic Reviews Identified
Each of the 18 systematic reviews compared the LNG-IUS with one or more of usual medical therapy, endometrial ablation, and/or hysterectomy. The earliest was published in 2001 and the two most recent in 2015. Table 4 summarizes the scope of the identified systematic reviews from the literature search.
Table 4:
Systematic Reviews with Relevance to the Levonorgestrel-Releasing Intrauterine System for Heavy Menstrual Bleeding
| Author, Year | Objective | Search Dates (Records Screened, N) | Databases Searched | LNG-IUS Comparator(s) |
|---|---|---|---|---|
| Lethaby et al, 201525 | Determine the effectiveness, acceptability, and safety of progesterone or progestogen-releasing IUDs in achieving a reduction in HMB | Up to Jan 2015 (609) | Cochrane Menstrual Disorders and Subfertility Group Specialised Trials Register; CENTRAL, MEDLINE, EMBASE, CINAHL, PsycINFO; MetaRegister of Clinical Trials; US NIH Clinical Trials Register; WHO International Clinical Trials Registry Portal; Web of Knowledge register | Medical therapy EA Hysterectomy |
| Bitzer et al, 201557 | Provide a comprehensive summary of the efficacy and safety of available medical treatments of HMB | Up to Mar 2013 (2,552) | MEDLINE; EMBASE | Medical therapy EA Hysterectomy |
| Uhm and Perriera, 201458 | Focus on the efficacy of hormonal contraceptive methods in the treatment of HMB in reproductive-aged women | Up to Feb 2014 (734) | PubMed | Medical therapy |
| Qiu et al, 201434 | Compare the effects of the LNG-IUS with conventional medical treatment in reducing HMB | Up to Apr 2014 (1,198) | MEDLINE; EMBASE; CENTRAL | Medical therapy |
| Hartmann et al, 201359 | Evaluate interventions and direct comparisons among treatments that are often used and promoted as first-line choices with the goal of clearly describing their effectiveness and potential harms for use in primary care settings | 1980 to Sep 2011/Jun 2012 (1,775) | MEDLINE; CINAHL; EMBASE | Medical therapy Hysterectomy |
| Lethaby et al, 201360 | Investigate the effectiveness of NSAIDs in achieving a reduction in menstrual blood loss in women of reproductive years with HMB | Up to Jul 2012 (NR) | Cochrane Menstrual Disorders and Subfertility Group Specialised Trials Register; CENTRAL, MEDLINE, EMBASE, CINAHL, PsycINFO; trial registers; citation indices; LILACS; PubMed; OpenSIGLE; Google/Google Scholar | Medical therapy |
| Matteson et al, 201261 | Compare hysterectomy and less invasive alternatives for AUB in 7 clinically important domains | Up to Jan 2011 (5,503) | MEDLINE | Hysterectomy |
| Kauntiz and Inki, 201262 | Review the clinical evidence and provide and update on the risk and benefits of using the LNG-IUS in the management of HMB | Up to Apr 2011 (NR) | MEDLINE; EMBASE | Medical therapy EA Hysterectomy |
| Duckitt and Collins, 20129 | Answer the following clinical questions: What are the effects of medical treatments for menorrhagia? What are the effects of surgical treatments for menorrhagia? What are the effects of endometrial thinning before endometrial destruction in treating menorrhagia? | Up to Jun 2011 (NR) | MEDLINE; EMBASE; CDSR; Cochrane Library; DARE; HTA database | Medical therapy EA Hysterectomy |
| Middleton et al, 2010; Bhattacharya et al, 201163,64 | Determine the clinical effectiveness and cost-effectiveness of hysterectomy, 1st- and 2nd-generation EA, and Mirena for the treatment HMB | Up to May 2010 (556) | Cochrane Library; MEDLINE; CINAHL; MetaRegister of Controlled Trials; International Standard Randomised Control Trial Number register | EA Hysterectomy |
| Blumenthal et al, 201165 | Review the literature for economic and HRQOL data associated with the use of the LNG-IUS in the management of HMB | Up to Jul 2010 (NR) | MEDLINE; EMBASE | EA Hysterectomy |
| Marjoribanks et al, 201017 | Compare the effectiveness, safety, and acceptability of surgery versus medical therapy for HMB | Up to Mar 2010 (NR) | Cochrane Menstrual Disorders and Subfertility Group Specialised Trials Register; CENTRAL; MEDLINE; EMBASE; PsycINFO | EA Hysterectomy |
| Kaunitz et al, 200935 | Compare the effects of LNG-IUS and EA in reducing HMB | Up to Jan 2009 (NR) | MEDLINE; EMBASE | EA |
| Lethaby et al, 200866 | Investigate the effectiveness of oral progestogen therapy taken either during the luteal phase or for a longer course of 21 days in achieving a reduction in menstrual blood loss in women of reproductive years with HMB | Up to Apr 2007 (NR) | Cochrane Menstrual Disorders and Subfertility Group Specialised Trials Register; CENTRAL; MEDLINE; EMBASE; | Medical therapy |
| Iavazzo et al, 200867 | Review the role of TBEA as an alternative in treating AUB | 1994 to May 2007 (NR) | PubMed | EA |
| NICE, 20072 | Offer best practice advice on the care of women with HMB | Up to Jun 2006 (NR) | MEDLINE, EMBASE, CINAHL, British Nursing Index, PsycINFO, CENTRAL, Cochrane Library, DARE, NHS EED, NHS CRD | Medical therapy EA Hysterectomy |
| Varma et al, 200668 | Expand on past reviews by incorporating recent advances and performing an up-to-date systematic review focused entirely on LNG-IUS, evaluating the quality of supporting evidence and, where available, present information relating to adverse events, cost-effectiveness, and HRQOL | 1996 to 2005 (NR) | MEDLINE; EMBASE; CENTRAL; CDSR; DARE; National Research Register; MRC Clinical Trials Register; NHS CRD | Medical therapy EA Hysterectomy |
| Stewart et al, 200169 | Determine whether the LNG-IUS (currently licensed for contraceptive use) may reduce menstrual blood loss with few side effects | Up to Mar 1999 (143) | MEDLINE; CINAHL; EMBASE; Grateful Med; BMJ Website Archiving Facility; Cochrane Library; Best Evidence; NHS CRD; Internet search engines | Medical therapy EA |
Abbreviations: AUB, abnormal uterine bleeding; BMJ, British Medical Journal; CENTRAL, The Cochrane Central Register of Controlled Trials; CDSR, Cochrane Database of Systematic Reviews; CINAHL, Cumulative Index of Nursing and Allied Health Literature; CRD, Centre for Reviews and Dissemination; DARE, Database of Abstracts of Reviews of Effects; EA, endometrial ablation; EED, Economic Evaluation Database; EMBASE, Excerpta Medica database; HMB, heavy menstrual bleeding; HRQOL, health-related quality of life; HTA, health technology assessment; IUD, intrauterine device; LILACS, Latin American and Caribbean Health Sciences Literature; LNG-IUS, levonorgestrel-releasing intrauterine system; MRC, Medical Research Council; NICE, National Institute for Health and Care Excellence; NIH, National Institutes of Health; NHS, National Health Service; NR, not reported; NSAID, nonsteroidal anti-inflammatory drug; TBEA, thermal balloon endometrial ablation; WHO, World Health Organization.
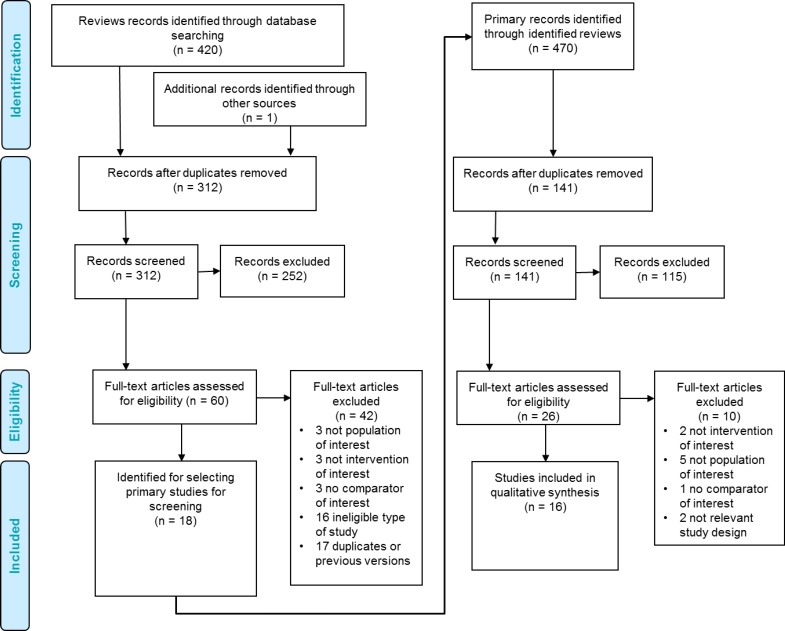
No systematic review met all inclusion criteria for our clinical evidence review. The published systematic reviews varied in their overall objectives and in their definition of the population with heavy menstrual bleeding. While most did include the population of interest, they combined it with mixed, ineligible populations which precluded their authors from making conclusions about idiopathic heavy menstrual bleeding. Thus, we used the 18 systematic reviews to identify eligible RCTs that only included women with idiopathic heavy menstrual bleeding. We obtained the full texts of all RCTs included in all of the systematic reviews to assess their eligibility against our inclusion and exclusion criteria. Figure 1 shows the flow of citation assessment and the reasons articles were excluded.
Figure 1: PRISMA Flow Diagram for Clinical Evidence Review.
Source: Adapted from Moher et al, 2009.70
Randomized Controlled Trials
From the 18 systematic reviews summarized in Table 4, we obtained 26 unique RCTs evaluating the LNG-IUS. We excluded 10 of these studies: two that investigated LNG-IUS with alternative dosage,71,72 three that included populations with heavy menstrual bleeding due to structural or pathological causes,39–41 one that was of a mixed population (heavy menstrual bleeding and endometrial hyperplasia),73 one whose population was women experiencing iatrogenic heavy menstrual bleeding from anticoagulant medication,42 one study without a comparator of interest,74 and two others because they did not use randomized study designs.75,76
Ultimately, 16 RCTs reported in 23 articles met the inclusion criteria for our review.77–99 Where multiple articles reported on the same study and results, we extracted data from the article that reported the most comprehensive and up-to-date data on each outcome. We hand-searched the reference lists of the included studies, along with health technology assessment websites and other sources, and no additional relevant studies were identified.
We also identified two ongoing relevant RCTs.25 One comparing the LNG-IUS to endometrial ablation is expected to close recruitment in December 2015 (NTR2984).100 The other trial is comparing the LNG-IUS with combined oral contraceptives and is expected to be completed in January 2017 (NCT02002260, ClinicalTrials.gov).
Of the 16 included RCTs, two compared the 52-mg LNG-IUS with hysterectomy, nine compared it with endometrial ablation techniques, and five compared it with medical therapies. All included RCTs stated that they excluded women with heavy menstrual bleeding as a result of anatomical, structural, and pathological causes (Table 5).
Table 5:
Included RCTs Evaluating the 52-mg Levonorgestrel-Releasing Intrauterine System for Idiopathic Heavy Menstrual Bleeding
| Author, Year | Country, No. Sites (if > 1) | Recruitment Period | Sample Size (% Lost to Follow-Up) | Heavy Menstrual Bleeding Criteriaa |
|---|---|---|---|---|
| Compared with hysterectomy | ||||
| Sesti et al, 201277 | Italy | Apr 2008 – Sep 2010 | 72 (0) | Score ≥ 100 PBAC |
| Hurskainen et al, 2001, 2004; Heliovaara-Peippo et al, 201378–80 |
Finland, 5 | Oct 1994 – Sep 1997 | 236 (6)b | Referred to gynecologist |
| Compared with endometrial ablation | ||||
| Ghazizadeh et al, 201181 | Iran | NR | 104 (12) | Score ≥ 100 PBAC |
| De Souza et al, 201082,83 | Brazil | Jan 2005 – Mar 2007 | 58 (10) | > 80 mL according to PBAC |
| Shaw et al, 200784 | UK | Nov 2001 – Oct 2003 | 66 (35) | Score ≥ 120 PBACc |
| Tam et al, 200685 | China | NR | 44 (25) | Documented history |
| Busfield et al, 200686 | New Zealand | Mar 1999 – Jul 2001 | 79 (18) | Self-described |
| Malak and Shawki, 200687 | Egypt | NR | 60 (7) | Score ≥ 100 PBAC |
| Barrington et al, 200388 | UK | NR | 50 (12) | NR |
| Kittelsen and Istre, 1998; Istre and Trolle, 2001; Rauramo et al, 200489–91 |
Norway | Mar 1993 – Oct 1995 | 59 (31) | Score 75 PBAC + 60mL |
| Crosignani et al, 199792 | Italy | NR | 70 (1) | Score ≥ 100 PBAC |
| Compared with usual medical treatment | ||||
| Gupta et al, 2013, 201593,94 | UK, 63 | Feb 2005 – Jul 2009 | 571 (26) | RCOG definitiond |
| Shaaban et al, 201195 | Egypt | May 2003 – Mar 2004 | 112 (15) | Self-described |
| Kaunitz et al, 2012, 201096,97 | US, Canada, Brazil, 55 | Jun 2006 – Jun 2008 | 165 (12) | > 80 mL alkaline hematin |
| Reid and Virtanen-Kari, 200598 | England | May 1996 – Dec 1998 | 51 (18) | > 80 mL alkaline hematin |
| Irvine et al, 199899 | UK | NR | 44 (18) | > 80 mL alkaline hematin |
Abbreviations: PBAC, pictorial blood loss assessment chart; NR, not reported; RCOG, Royal College of Obstetrics and Gynaecology guidelines.
A score of > 100 on the PBAC reflects objective menorrhagia determined by the alkaline hematin method (i.e., > 80 mL blood loss per cycle).
Proportion lost to follow-up at 10 years.
Mean PBAC score over 2 cycles.
Guideline defines menorrhagia as heavy cyclical menstrual blood loss over several consecutive cycles without any intermenstrual or postcoital bleeding; study investigators defined several as 3 consecutive cycles.
Methodological Quality of the Included Studies
Complete results of our analysis of the methodological quality of the body of evidence and risk of bias for included studies are presented in Appendix 2.
Results for Quality of Life
Nine studies evaluated quality of life using various validated assessment tools.77,79,82,85–87,92,93,95 The most common assessment tool used for general quality of life was the Short Form 36 (SF-36), comprised of eight multi-item dimensions of quality of life that are scored from 0 to 100 (higher scores indicate less disability). Dimensions include general health, physical functioning, mental health, social functioning, vitality, pain, and physical and emotional role functioning.101 Other generic quality of life assessment tools used were the EuroQol (EQ) EQ-5D and the EQ visual analogue scale (EQ-VAS).102 The EQ-5D is a five-dimension measure of quality of life (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) that asks a respondent to rank each dimension into three states (no problems, some problems, extreme problems).103,104 Alternatively, the EQ-VAS asks respondents to mark on a line their perception of their overall health, from best imaginable to worst imaginable.
One study measured health-related quality of life using the Center for Disease Control and Prevention's Healthy Days Measure (HRQOL-4).95 This instrument consists of four items: self-rated health and the number of days in the past 30 days that the respondent has experienced physically unhealthy days, mentally unhealthy days, and activity limitation days.105,106
Another single study evaluated health-related quality of life with the Psychological General Well-Being Index, which is focused on overall psychological wellness including anxiety, depressed mood, positive well-being, self-control, general health, and vitality, but does not include any evaluation of physical health.107
One study used a disease-specific quality of life measure in addition to general assessments.93 The menorrhagia multi-attribute scale (MMAS) asks women with heavy menstrual bleeding to report their experience with practical difficulties, social life, psychological health, physical health and well-being, work/daily routines, and family life/relationships.108 The MMAS instrument has been validated using the SF-36, EQ-5D, and Sexual Activity Questionnaire and seeks to capture the subjective experience of heavy menstrual bleeding.109
Of the eight RCTs that reported on quality of life outcomes, the majority (75%) found significant improvement in quality of life from baseline, within both the LNG-IUS and comparator groups (medical therapy or surgery).77,79 86,87 93,95 Given that the measures and metrics (i.e., timing of measurements and summary statistics reported) were heterogeneous among these studies, we were unable to conduct a quantitative synthesis of the data. Quality of life results are summarized narratively, by comparator, below.
LNG-IUS Versus Hysterectomy
Two studies compared the effect on quality of life of the LNG-IUS and hysterectomy (Table 6).77,79 Both studies used the SF-36 questionnaire to measure quality of life, and one study79 also used the EQ-5D. The characteristics of the study populations were generally similar between studies with regard to age, body mass index, and parity. Sesti et al77 reported that all participants were Caucasian, and the Hurskainen et al study79 described the educational attainment in the study groups as being similar (LNG: 32% elementary, 44% lower secondary, 24% upper secondary; hysterectomy: 28% elementary, 40% lower secondary, 32% upper secondary).
Table 6:
Quality of Life Studies of Women Receiving LNG-IUS or Hysterectomy
| Author, Year | Hysterectomy Type(s) (na) | LNG-IUS (na) | Age, Years, Mean (SD) | BMI, Mean (SD) | Parity, Mean (SD) | Baseline Menstrual Bleeding, Mean (SD) |
|---|---|---|---|---|---|---|
| Sesti et al, 201277 | Laparoscopic supracervical (36) | Mirena (36) | 41.7–47.5 (5.9–7.4) | 24–24.5 (3.4–3.9) | 1.8–2.0 (0.9–1.3) | PBAC score 911–937 (330–344) |
| Hurskainen et al, 200179 | Vaginal 28%, abdominal 20%, laparoscopic 52% (117) | Mirena (119) | 43 (3.4) | 25.8 (4.8) | 2.1 (1.1) | 128–130 mL (116) |
Abbreviations: BMI, body mass index; LNG-IUS, levonorgestrel-releasing intrauterine system; PBAC, pictorial blood loss assessment chart; SD, standard deviation.
Number of participants randomized to each study arm.
In the Hurskainen et al trial,79 55 women randomized to the LNG-IUS group went on to have hysterectomy during the 10 years after randomization. Most of these subsequent surgeries occurred within the first 5 years after randomization, including 44% (24 women) within the first year and 47% between 12 months and 5 years (26 women). Only 9% (5 hysterectomies) occurred between years 5 and 10 of the study.79 No women in the Sesti et al trial77 discontinued their LNG-IUS treatment.
In both studies, all dimensions of quality of life improved significantly from baseline within both treatment groups. To compare between the groups, the Hurskainen et al study79 reported change in quality of life from baseline, whereas the Sesti et al study77 reported the quality of life scores at the various assessment points. Therefore, we could not combine the data from these studies. Table 7 outlines the measures and dimensions of quality of life where results differed significantly between the treatment groups in each study.
Table 7:
Quality of Life Results in Women After LNG-IUS or Hysterectomy
| Author, Year | QOL Measure | Follow-Up | QOL Results | P |
|---|---|---|---|---|
| Sesti et al, 201277 | SF-36 | 3 mo |
LNG > Hyst
|
< .05 |
| SF-36 | 6 mo |
LNG > Hyst
|
< .05 | |
| SF-36 | 1 y |
LNG > Hyst
|
< .05 | |
| SF-36 | 2 y |
LNG > Hyst
|
< .05 < .05 | |
| Hurskainen et al, 200179 | EQ-5D | 1 y | No differencea | .90 |
| SF-36 | Hyst > LNG • Less paina |
.01 | ||
| EQ-5D | 5 y | No differencea | .60 | |
| SF-36 | No differences | .60–.90 | ||
| EQ-5D | 10 y | No differenceb | .94 | |
| SF-36 | No differences | 0.39–0.91 |
Abbreviations: EQ-5D, EuroQol 5-dimensions; Hyst, hysterectomy; LNG, levonorgestrel-releasing intrauterine system; mo, months; QOL, quality of life; SF-36, 36-item short-form survey; y, years.
Reduction expressed as magnitude of change from baseline pain assessment.
No significant difference in EQ-5D change from baseline, nor change between 5 and 10 years.
Table 8 shows the overall study findings for quality of life, by follow-up time. In the Sesti study,77 which compared LNG-IUS and laparoscopic supracervical hysterectomy, only the dimensions of general health, physical function, and vitality did not show a difference from pre-treatment levels at 1-year follow-up. The differences found in some SF-36 dimensions at each time point favoured greater scores in the LNG-IUS group, with the exception of less pain in the hysterectomy group at 1 year79 and 2 years.77
Table 8:
Quality of Life Improvement by Follow-Up Time: LNG-IUS vs. Hysterectomy
| Author, Year | Follow-Up | QOL Improvement: LNG-IUS vs. Hysterectomy | P |
|---|---|---|---|
| Sesti et al, 201277 | 3 mo |
LNG better
No difference
|
< .05 |
| Sesti et al, 201277 | 6 mo |
LNG better
No difference
|
< .05 |
| Sesti et al, 201277 | 1 y |
LNG better
No difference
|
< .05 |
| Hurskainen et al 200179 | Hysterectomy better
No difference
|
.01 .90 |
|
| Sesti et al, 201277 | 2 y |
LNG better
Hysterectomy better
|
< .05 < .05 |
| Hurskainen et al 200179 | 5 y |
No difference
|
.60–.90 .60 |
| Hurskainen et al 200179 | 10 y |
No difference
|
.39–.91 .94 |
Abbreviations: EQ-5D, EuroQol 5-dimensions; LNG, levonorgestrel-releasing intrauterine system; mo, months; QOL, quality of life; SF-36, 36-item short-form survey; y, year
Emotional role functioning, physical role functioning, and mental health dimensions of SF-36.
Emotional role functioning, physical role functioning, mental health, vitality, and social function dimensions of SF-36.
Pain dimension of SF-36.
Emotional role functioning and mental health dimensions of SF-36.
Overall, in these two RCTs the improvement in quality of life offered by LNG-IUS was either superior or not significantly different than the benefit of hysterectomy.
LNG-IUS Versus Endometrial Ablation
Five studies compared the impact on quality of life of LNG-IUS and endometrial ablation techniques.82,85–87,92 Table 9 summarizes the study characteristics. Ages of study participants were generally similar across RCTs, but few studies reported information on body mass index (BMI)86,92 and parity.82,87,92 One RCT stipulated failure of medical therapy as an eligibility criteria,85 and one recruited women scheduled for hysterectomy.87
Table 9:
Quality of Life Studies of Women Receiving LNG-IUS or Endometrial Ablation
| Author, Year | Ablation Technique (na) | LNG-IUS (na) | Age, Years, Mean (SD) | BMI, Mean (SD) | Parity, Mean (SD) | Baseline Menstrual Bleeding, Mean (SD) |
|---|---|---|---|---|---|---|
| de Souza et al, 201082 | ThermaChoice TBEA (30) | Mirena (28) | 42–43.4 (0.7) | NR | 2.4–2.6 (0.2–0.4) | PBAC score 492–522 (56.8–90.3) |
| Tam et al, 200685 | ThermaChoice TBEAb (22) | Mirena (22) | 44.1–4.7 (2.7–3.5) | NR | NR | PBAC score 460–534 (270–525) |
| Busfield et al, 200686 | ThermaChoice TBEA (41) | Mirena (42) | NR (range 40–49) | 28.9–29.7 (5.4–8.0) | NR | PBAC score 490–502 (419–422) |
| Malak and Shawki, 200687 | Endometrial resection (30) | Mirena (30) | 46.3–7.7 (1.3–1.4) | NR | 1.9–2.4 (0.3–1.9) | PBAC score 316–346 (143–152) |
| Crosignani et al, 199792 | Endometrial resection (35) | Mirena (35) | 43.8–5.4 (3.8) | 24.0–25.3 (3.0–4.4) | 1.6–1.8 (0.9–1.1) | PBAC score 181–204 (59.4–82.9) |
Abbreviations: BMI, body mass index; LNG-IUS, levonorgestrel-releasing intrauterine system; NR, not reported; PBAC, pictorial blood loss assessment chart; SD, standard deviation; TBEA, thermal balloon endometrial ablation.
Number of participants randomized to each study arm.
Preoperative thinning of the endometrium was performed with either danazol or gonadotropin-releasing hormone agonists.
Three studies measured general quality of life using the SF-36,85,86,92 one study used the EQ-VAS,87 and another single study used the Psychological General Well-Being Index.82 All studies measured quality of life at the end of 1 year, with only one evaluating after 2 years86 and another at 5 years.82 The quality of life findings of these studies are summarized in Table 10.
Table 10:
Quality of Life Results in Women After LNG-IUS or Endometrial Ablation
| Author, Year | QOL Measure | Follow-Up | QOL Results | P |
|---|---|---|---|---|
| de Souza et al, 201082 | PGWBI | 5 y | No difference between groups | .247 |
| Tam et al, 200685 | SF-36 | 1 y | EA > LNG
|
.024 .021 |
| Busfield et al, 200686 | SF-36 | 3 mo 1 y 2y |
No differences No differences No differences |
NR |
| Malak and Shawki, 200687 | EQ-VAS | 6 mo 1 y |
No differences No differences |
NR |
| Crosignani et al, 199792 | SF-36 | 1 y | No differences | NR |
Abbreviations: EA, endometrial ablation; EQ-VAS, EuroQol visual analogue scale; LNG, levonorgestrel-releasing intrauterine system; mo, month; NR, not reported; PGWBI, Psychological General Well-Being Index; QOL, quality of life; SF-36, 36-item short-form survey; y, year.
Two studies reported no significant change in quality of life within treatment groups compared with baseline.82,85 Two other studies found significant improvements within treatment groups from baseline.86,87 The fifth study did not report change within groups from baseline.92
Three studies found no significant differences in any of the domains of quality of life between women treated with LNG-IUS compared with those receiving endometrial ablation.86,87,92 Psychological well-being did not differ between the endometrial ablation and LNG-IUS groups, at baseline or at 5 years post-treatment.82 The Tam et al study85 found that participants treated with endometrial ablation had statistically significantly superior general health and mental health status at 1 year post-treatment compared with the LNG-IUS group, and no differences in the other six dimensions of quality of life. The quality of life results across the body of evidence did not appear to differ among studies at low risk of bias86,87 or at higher risk of bias.82,85,92 Table 11 shows the overall study findings for quality of life, by follow-up time.
Table 11:
Quality of Life Improvement by Follow-up Time: LNG-IUS vs. Endometrial Ablation
| Author, Year | Follow-Up | QOL Improvement: LNG vs. EA | P |
|---|---|---|---|
| Busfield et al, 200686 | 3 mo |
No difference
|
NR |
| Malak and Shawki, 200687 | 6 mo |
No difference
|
NR |
| Tam et al, 200685 | 1 y | EA better
No difference
|
.021–.024 |
| Busfield et al, 200686 |
No difference
|
NR | |
| Malak and Shawki, 200687 |
No difference
|
NR | |
| Crosignani et al, 199792 |
No difference
|
||
| Busfield et al, 200686 | 2 y |
No difference
|
NR |
| de Souza et al, 201082 | 5 y |
No difference
|
.247 |
Abbreviations: EA, endometrial ablation;; EQ-VAS, EuroQol visual analogue scale; LNG, levonorgestrel-releasing intrauterine system; mo, months; NR, not reported; PGWBI, Psychological General Well-Being Index; QOL, quality of life; SF-36, 36-item short-form survey; y, year
General health perception and mental health dimensions of SF-36.
Both LNG-IUS and endometrial ablation improved quality of life, and most of the five studies found that the improvement in quality of life offered by LNG-IUS was not significantly different from the benefit of endometrial ablation.
LNG-IUS Versus Usual Medical Therapy
Two studies compared the effect of the LNG-IUS with usual medical therapies on quality of life (Table 12).93,95 The ECLIPSE trial (Effectiveness and Cost-effectiveness of Levonorgestrel-Containing Intrauterine System in Primary Care Against Standard Treatment for Menorrhagia) by Gupta et al93,94 used a menorrhagia-specific assessment tool to measure health-related quality of life. The second study by Shaaban et al95 used the HRQOL-4 tool from the Centers for Disease Control and Prevention to evaluate quality of life.
Table 12:
Quality of Life Studies of Women Receiving LNG-IUS or Usual Medical Therapy
| Author, Year | Medications (na) | LNG-IUS (na) | Age, Years, Mean (SD) | BMI, Mean (SD) | Parity, MD (Range) | Baseline Menstrual Bleeding, Mean (SD) |
|---|---|---|---|---|---|---|
| Gupta et al, 201393 | Mefenamic acid, tranexamic acid, norethindrone, COC, progesterone only pill, MPA injection (286b) | Mirena (285) | 41.8–42.1 (5.0–5.5) | 29.1–29.3 (6.1–6.7) | NR | NR |
| Shaaban et al, 201195 | Microvlar low-dose COC (56) | Mirena (56) | 38.7–39.3 (5.2–6.7) | 29.6–31.1 (5.7–5.9) | 3 (1–6) | PBAC score: 306–323 (97.3–131) 274–300 mL (142–150) |
Abbreviations: BMI, body mass index; COC, combined oral contraceptive; LNG-IUS, levonorgestrel-releasing intrauterine system; MD, median; MPA, medroxyprogesterone acetate; NR, not reported; PBAC, pictorial blood loss assessment chart; SD, standard deviation.
Number of participants randomized to each study arm.
Proportions of intended prescriptions within the medical therapy arm were as follows: 47% mefenamic and tranexamic acid, 17% tranexamic acid alone, 11% mefenamic acid alone, 7% norethindrone, 6% COC, 5% MPA injection, 3% mefenamic acid and tranexamic acid and COC, 4% other combination.93
The ECLIPSE trial permitted participants to change treatments during the trial.93 At 2 years, 64% of women allocated to the LNG-IUS group had not changed treatments, compared with 38% of those assigned to the usual medical therapy group (P < .001). Retention rates of assigned therapy at 5-year follow-up were 47% for LNG-IUS and 15% for usual medical therapy. Of the 228 treatment changes over the duration of the trial, 43% in the medical therapy group switched to LNG-IUS and 39% in the LNG-IUS group changed to usual medical therapy.93
Table 13 summarizes the quality of life findings of the two trials that compared LNG-IUS and usual medical therapy.
Table 13:
Quality of Life Results in Women After LNG-IUS or Usual Medical Therapy
| Author, Year | QOL Measure | Follow-Up | QOL Results | P |
|---|---|---|---|---|
| Gupta et al, 201393 | MMAS | 6 mo 1 ya 2 ya |
LNG > UMT
|
< .001 for all |
| SF-36 | 2 y |
LNG > UMT
|
.05 < .001 .007 .001 <.001 <.001 .03 |
|
| EQ-5D descriptive | 2 y | No difference | .38 | |
| EQ-VAS | No difference | .12 | ||
| MMAS | 5 y | No difference | .90 | |
| SF-36 | 5 y |
LNG > UMT
|
.02 | |
| EQ-5D descriptive | 5 y | No difference | .40 | |
| EQ-5D VAS | No difference | .80 | ||
| Shaaban et al, 201195 | HRQOL-4 | 1 y | COC > LNG
LNG > COC
|
.003 < .001 |
Abbreviations: COC, combined oral contraceptive; EQ-5D, EuroQol 5-dimensions; HRQOL-4, 4-item health-related quality of life survey; LNG, levonorgestrel-releasing intrauterine system; MMAS, menorraghia multi-attribute scale; mo, month; QOL, quality of life; SF-36, 36-item short-form survey; UMT, usual medical therapy; VAS, visual analogue scale; y, year.
Results are the same at each time point.
Mean in COC group of 4.4 +/− 1.7 days compared with mean in the LNG-IUS group of 6.7 +/− 3.1 days, P = .003.
Mean in LNG-IUS group of 1.6 +/− 2.4 days compared with mean in the COC group of 6.7 +/− 2.2 days, P < .001.
In each RCT comparing the LNG-IUS and usual medical therapy, quality of life improved significantly from baseline within each study group. Gupta et al93 found that women receiving the LNG-IUS had better quality of life for 2 years, compared with women receiving usual medical therapies, in all domains of the MMAS (P < .001 for all comparisons) and in all domains of the SF-36 (P < .05 for all comparisons), with the exception of mental health, for which there was no difference (P = .23) at 2 years. At 5 years, the differences favouring the LNG-IUS were no longer statistically significant, save for the general health perception dimension of the SF-36.93 In contrast, Shaaban et al95 found significant differences in only two of the four HRQOL-4 domains; women taking combined oral contraceptives had fewer mentally unhealthy days than those treated with the LNG-IUS (mean 4.4 +/− 1.7 days vs. 6.7 +/− 3.1 days, P = .003), but women using the LNG-IUS had significantly fewer activity limitation days than women treated with oral contraceptives (mean 1.6 +/− 2.4 days vs. 6.7 +/− 2.2 days, P < .001). No difference between study groups was found in the number of physically unhealthy days or self-rated health.95 Because only two RCTs comparing LNG-IUS and usual medical therapy studied quality of life, we could not stratify their results by risk of bias. Table 14 shows the overall study findings for quality of life, by follow-up time.
Table 14:
Quality of Life Improvement by Follow-Up Time: LNG-IUS vs. Usual Medical Therapy
| Author, Year | Follow-Up | QOL Improvement: LNG vs Usual Medical Therapy | P |
|---|---|---|---|
| Gupta et al, 201393 | 6 mo |
LNG better
|
< .001 |
| Gupta et al, 201393 | 1 y |
LNG better
|
< .001 |
| Shaaban et al 201195 |
LNG better
|
< .001 | |
COC better
|
.003 | ||
No difference
|
|||
| Gupta et al, 201393 | 2 y |
LNG better
|
< .001 |
|
< .05 | ||
| Gupta et al, 201393 | 5 y |
LNG better
|
.02 |
No difference
|
|||
|
|||
|
|||
|
Abbreviations: COC, combined oral contraceptive; EQ-5D, EuroQol 5-dimensions; HRQOL-4, 4-item health-related quality of life assessment tool; LNG, levonorgestrel-releasing intrauterine system; MMAS, menorrhagia multi-attribute scale; mo, month; QOL, quality of life; SF-36, 36-item short-form survey; VAS, visual analogue scale; y, year.
General health perception dimension of SF-36.
Overall, the LNG-IUS improved quality of life significantly more than usual medical therapy in the first years of use, although with some variation in the dimensions that improved at various time points. By 5 years, however, there was no evidence of a significant difference in the improvement in quality of life between usual medical therapy and the LNG-IUS.
Summary of Results for Quality of Life
Of the nine RCTs that offer evidence on the impact of the LNG-IUS on quality of life, two did not find improvement from baseline,82,85 and one did not evaluate change from baseline.92 The lack of change within treatment groups may be due to using a measure of quality of life that had no assessment of physical well-being82 and due to inadequate power to detect a difference.85 Of the eight RCTs that evaluated change in quality of life from baseline, the majority (75%) found significant improvement within each treatment group.
Compared with hysterectomy, the LNG-IUS offered superior or similar (i.e., not significantly different) benefit for women's quality of life (GRADE: Moderate). Compared with endometrial ablation, the LNG-IUS offered similar benefit for quality of life (GRADE: Low). Compared with usual medical therapy, the LNG-IUS offered superior or similar benefit for quality of life (GRADE: Low) (Table 15).
Table 15:
GRADE Evidence Profile for Quality of Life Change with LNG-IUS
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Compared with hysterectomy | |||||||
| 2 (RCTs) | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Compared with endometrial ablation | |||||||
| 5 (RCTs) | Serious limitations (−1)b | No serious limitations | No serious limitations | Serious limitations (−1)c | Undetected | None | ⊕⊕ Low |
| Compared with usual medical therapy | |||||||
| 2 (RCTs) | Serious limitations (−1)a | Serious limitations (−1)d | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; RCT, randomized controlled trial.
Lack of blinding. See Appendix 2, Tables A4 to A6, for full risk of bias assessments.
Inadequate allocation concealment, lack of blinding, intention-to-treat analysis was not conducted, and loss to follow-up. See Appendix 2, Tables A4 to A6, for full risk of bias assessment.
Very small sample sizes; loss to follow-up compromised power in most studies.
Results varied from favouring LNG-IUS to no difference depending on quality of life tool used and time point assessed.
Results for Menstrual Blood Loss
Thirteen studies evaluated menstrual blood loss between study groups, and these results are presented by comparator on the following pages.77,81,82,84,86–88,90,92,95,97–99 Of the 12 RCTs that reported change from baseline, all found significant improvement in menstrual bleeding, within each study group, after treatment.77,81,82,84,87,88,90,92,95,97–99 The measures and metrics were heterogeneous, precluding quantitative synthesis; thus, we summarize the results narratively.
LNG-IUS Versus Hysterectomy
The study by Sesti et al77 compared menstrual blood loss, measured by PBAC scores, between the LNG-IUS and laparoscopic supracervical hysterectomy groups at baseline and at 3 months, 6 months, 1 year, and 2 years after treatment. Table 16 summarizes these findings (see Table 6 for characteristics of this RCT).
Table 16:
Menstrual Blood Loss Results for Women Treated with LNG-IUS or Hysterectomy
| Author, Year | Follow-Up | MBL LSH, PBAC Score, Mean (SD) | MBL LNG, PBAC Score, Mean (SD) | P |
|---|---|---|---|---|
| Sesti et al, 201277 | Baseline | 937 (334) | 911 (330) | nsa |
| 3 mo | 52.9 (22.9) | 37.0 (20.1) | .004 | |
| 6 mo | 19.7 (11.6) | 50.4 (13.9) | < .0001 | |
| 1 y | 3.70 (3.0) | 3.50 (16.0) | nsa | |
| 2 y | 3.74 (3.05) | 56.4 (72.8) | < .0001 |
Abbreviations: LNG, levonorgestrel-releasing intrauterine system; LSH, laparoscopic supracervical hysterectomy; MBL, menstrual blood loss; mo, month; ns, not statistically significant; PBAC, pictorial blood loss assessment chart; SD, standard deviation; y, year.
P value not reported.
Both groups experienced a significant decrease in menstrual blood loss compared with pre-treatment (baseline PBAC scores were all greater than 100; P not reported). Bleeding generally tapered to within normal range (PBAC 50–100) and then to spotting (PBAC 0–50) over the 24-month study period. There was no difference between the treatment groups in their baseline menstrual blood loss or at the 1-year assessment (P not reported).77
At 3 months post-treatment, the women treated with the LNG-IUS had significantly less menstrual bleeding compared with those who had received a hysterectomy; however, this significant difference reversed at both 6 months and 2 years post-treatment. At 2 years, there was an apparent but not significant increase in menstrual blood loss in the LNG-IUS group, compared with previous time points.63 The authors could not explain this finding, but speculated that perhaps the local effect of levonorgestrel on the uterus decreased over time.77 At 1 year, no women had amenorrhea, nor did any meet the PBAC criteria for amenorrhea (score of 0) during the study period.77 Although the results varied depending on follow-up time, unsurprisingly the hysterectomy group experienced a significantly greater reduction in menstrual blood loss.
LNG-IUS Versus Endometrial Ablation
Eight studies comparing the LNG-IUS and endometrial ablation reported on the outcome of menstrual blood loss (Table 17).81,82,84,86–88,90,92 The studies reported various metrics of blood loss, with a few reporting comparisons between final mean PBAC scores,81,86,88,92 median change from baseline,84,90 or the difference in proportions of women with increased or decreased menstrual blood loss at differing time points.82
Table 17:
Menstrual Blood Loss Studies of Women Receiving LNG-IUS or Endometrial Ablation
| Author, Year | Ablation Technique (na) | LNG-IUS (na) | Age, Years, Mean (SD) | BMI, Mean (SD) | Parity, Mean (SD) | Baseline PBAC Score |
|---|---|---|---|---|---|---|
| Ghazizadeh et al, 201181 | Transcervical endometrial resection (52) | Mirena (52) | 40.2–41.5 (4.3–4.4) | 26.7–28.3 (3.3–4.2) | NR | M 595–596 (SD 165–185) |
| de Souza et al, 201082 | ThermaChoice TBEA (30) | Mirena (28) | 42–43.4 (0.7) | NR | 2.4–2.6 (0.2–0.4) | M 492–522 (SD 56.8–90.3) |
| Shaw et al, 200784 | MenoTreat TBEA (33) | Mirena (33) | MD 42–43 (range 30–49) | MD 27–28 (range 19–41) | MD 3 (range 1–3) | MD 410–450 (range 126–1800) |
| Busfield et al, 200686 | ThermaChoice TBEA (41) | Mirena (42) | NR (range 40–49) | 28.9–29.7 (5.4–8.0) | NR | M 490–502 (SD 419–422) |
| Malak and Shawki, 200687 | Endometrial resection (30) | Mirena (30) | 46.3–47.7 (1.3–1.4) | NR | 1.9–2.4 (0.3–1.9) | M 316–346 (SD 143–152) |
| Barrington et al, 200388 | ThermaChoice TBEAb (25) | Mirena (25) | NR | NR | NR | M107–122 (SD 74–95) |
| Kittelsen et al, 199890 | Transcervical endometrial resection (30) | Mirena (30) | 41.4–42.1 (3.6–3.8) | NR | MD 2 (range 2–5) | M 404–420 (352–480) |
| Crosignani et al, 199792 | Endometrial resection (35) | Mirena (35) | 43.8–45.4 (3.8) | 24.0–25.3 (3.0–4.4) | 1.6–1.8 (0.9–1.1) | M 181–204 (SD 59.4–82.9) |
Abbreviations: BMI, body mass index; LNG-IUS, levonorgestrel-releasing intrauterine system; M, mean; MD, median; NR, not reported; PBAC, pictorial blood loss assessment chart; SD, standard deviation; TBEA, thermal balloon endometrial ablation.
Number of participants randomized to each study arm.
Preoperative thinning of the endometrium was performed with 3.6 mg goserelin.
Barrington et al88 reported a difference in baseline menstrual bleeding between the LNG-IUS and endometrial ablation groups (P = .025), but at 6 months follow-up there was no difference (P = .6896). Malak and Shawki87 reported a binary blood loss outcome called “treatment success,” defined as a PBAC score greater than 75 at 12 months follow-up (not shown in Table 18). They found that the two treatment groups achieved comparable treatment success: 77% with LNG-IUS and 83% with endometrial ablation (P = .747).87 Results for Malak and Shawki's amenorrhea rates and for amenorrhea and menstrual blood loss as reported by the other seven studies are in Table 18.
Table 18:
Menstrual Blood Loss Results for Women Treated with LNG-IUS or Endometrial Ablation
| Author, Year | Follow-Up | Amenorrhea,% LNG-IUS vs. EA | Menstrual Blood Loss, EA | Menstrual Blood Loss, LNG-IUS | P |
|---|---|---|---|---|---|
| Ghazizadeh et al, 201181 | 1 y | 11 vs. 45a | M final PBAC 526.8 (SD 148) | M final PBAC 560.2 (SD 177.9) | ns |
| de Souza et al, 201082 | 6 mo | NR | Increased MBL in 10%b | Decreased MBL or amenorrhea in 80%b | .035 |
| 1 y | NR | Increased MBL in 10%b | Decreased MBL or amenorrhea in 95%b | .048 | |
| 5 y | 35.3 vs. 0a | Increased MBL in 45.5% | Increased MBL in 0.0% | < .001 | |
| Shaw et al, 200784 | 3 mo | NR | MDc 184 (range 5 to 610) | MDc −172 (range 0 to 729) | ns |
| 6 mo | NR | MDc −81 (range 0 to 440) | MDc −124 (range 0 to 610) | < .05 | |
| 9 mo | NR | MDc −75 (range 0 to 286) | MDc −32 (range 0 to 114) | < .001 | |
| 1 y | 26 vs. 3a | MDc −62 (range 0 to 142) | MDc −26 (range 0 to 68) | < .001 | |
| Malak and Shawki, 200687 | 12 mo | 54 vs. 43 | NR | NR | |
| Busfield et al, 200686 | 3 mo | 6 vs. 16 | M final PBAC 220.8 (SD 438.5) | M final PBAC 125 (SD 198) | .452 |
| 6 mo | 9 vs. 3 | M final PBAC 107.5 (SD 135.4) | M final PBAC 72.1 (SD 118.6) | .080 | |
| 1 y | 20 vs. 7 | M final PBAC 94.7 (SD 112) | M final PBAC 41.1 (SD 86.5) | .002 | |
| 2 y | 35 vs. 5a | M final PBAC 75.4 (SD 91.1) | M final PBAC 20.6 (SD 28.8) | .002 | |
| Barrington et al, 200388 | 6 mo | 14 vs 9 | M final PBAC 61 | M final PBAC 31 | .6896 |
| Kittelsen et al, 199890 | 1 y | 12.5 vs. 25b,d | Diff in MDc −249.5 (range 164 to 261.5) | Diff in MDc −302.5 (range 156 to 311) | .68 |
| 2 y | 25 vs. 33b | Diff in MDc −253 (range 133.5 to 261.5) | Diff in MDc −301 (range 136 to 311) | .34 | |
| 3 y | 32 vs. 41b,d | Diff in MDc −2545 (range 160.5 to 261.5) | Diff in MDc −307 (range 129 to 311) | .86 | |
| Crosignani et al, 199792 | 1 y | 18 vs. 26 | M final PBAC 23.5 (SD 32.6) | M final PBAC 38.8 (SD 37.1) | .015e |
Abbreviations: Diff, difference; EA, endometrial ablation; LNG-IUS, levonorgestrel-releasing intrauterine system; M, mean; MBL, menstrual blood loss; MD, median; mo, month; NR, not reported; ns, not statistically significant; PBAC, pictorial blood loss assessment chart; SD, standard deviation; y, year.
Statistical significance at P < .05.
Change from baseline (M or MD).
Proportions calculated using data reported in article.
P value pertains to comparison of between-group difference in monthly bleeding score at final assessment with PBAC.
Three studies reported a statistically significant difference in the proportion of women who no longer menstruated; two studies found significantly more women with the LNG-IUS reported amenorrhea,84,86 whereas Ghazizadeh and colleagues81 found the opposite: a significantly greater proportion of women treated with endometrial ablation reported amenorrhea. Four studies did not report P values for amenorrhea results.82,88,90,92 Shaw et al84 reported that continued or prolonged spotting was the main reason women discontinued the allocated treatment (low willingness to tolerate side effects). In that study, by 2 years 20.7% of participants who withdrew from using the LNG-IUS and 13.3% who withdrew from endometrial ablation chose hysterectomy.84
Crosignani and his group92 reported a significantly larger reduction in menstrual blood loss from baseline for women treated with endometrial ablation compared with LNG-IUS (89% vs. 79%). Busfield et al86 found no difference at 3 and 6 months but a significantly lower final mean PBAC score in the LNG-IUS group compared with endometrial ablation at both 1 and 2 years follow-up. Similarly, Shaw et al84 found no difference at 3 months; at 6 months women treated with endometrial ablation had a significantly lower PBAC score than women treated with the LNG-IUS; but the opposite was true at 9 months and 1 year (P < .001 for both follow-up points). Three studies found no difference between groups in the change from baseline or in their final PBAC scores (P > .05), though each of these studies reported significant reductions from baseline within each group.81,88,90 Results for the studies' menstrual blood loss comparisons by follow-up time are outlined in Table 19.
Table 19:
Menstrual Blood Loss Improvement by Follow-Up Time: LNG-IUS vs. Endometrial Ablation
| Author, Year | Follow-Up | MBL Comparison: EA vs. LNG | P |
|---|---|---|---|
| Shaw et al, 200784 | 3 mo | No differencea | ns |
| Busfield et al, 200686 | No differenceb | .452 | |
| de Souza et al, 201082 | 6 mo | LNG betterc | .035 |
| Shaw et al, 200784 | EA bettera | < .05 | |
| Busfield et al, 200686 | No differenceb | .080 | |
| Barrington et al, 200388 | No differenceb | .6896 | |
| Shaw et al, 200784 | 9 mo | LNG bettera | < .001 |
| Ghazizadeh et al, 201181 | 1 y | No differenceb | ns |
| de Souza et al, 201082 | LNG betterc | .048 | |
| Shaw et al, 200784 | LNG bettera | < .001 | |
| Busfield et al, 200686 | LNG betterb | .002 | |
| Kittelsen et al, 199890 | No differencea | .68 | |
| Crosignani et al, 199792 | EA betterd | .015 | |
| Busfield et al, 200686 | 2 y | LNG betterb | .002 |
| Kittelsen et al, 199890 | No differencea | .34 | |
| Kittelsen et al, 199890 | 3 y | No differencea | .86 |
| de Souza et al, 201082 | 5 y | LNG betterc | < .001 |
Abbreviations: EA, endometrial ablation; LNG, levonorgestrel-releasing intrauterine system; MBL, menstrual blood loss; mo, month; ns, not statistically significant; PBAC, pictorial blood loss assessment chart; y, year.
Difference between groups in median change in MBL from baseline.
Difference between groups in final PBAC score (mean or median).
Difference in percentage with increased MBL.
Difference in percent reduction in MBL from baseline.
Among the 17 comparisons listed in Table 19, eight showed no difference in the reduction of menstrual blood loss; in seven comparisons, LNG-IUS produced superior reduction in menstrual blood loss; and in two, endometrial ablation was superior. Significant reductions from baseline within both arms were reported, and at most assessments in seven of the eight RCTs, LNG-IUS was either superior or not significantly different in improving menstrual blood loss compared with endometrial ablation.
LNG-IUS Versus Usual Medical Therapy
Four studies compared menstrual blood loss among women treated with either the LNG-IUS or usual medical therapy (Table 20).95,97–99
Table 20:
Menstrual Blood Loss Studies of Women Receiving LNG-IUS or Usual Medical Therapy
| Author, Year | Medication (na) | LNG-IUS (na) | Age, Years, Mean (SD) | BMI, Mean (SD) | Parity, Mean (SD) | Baseline MBL |
|---|---|---|---|---|---|---|
| Shaaban et al, 201195 | Microvlar low-dose COC (56) | Mirena (56) | 38.7–39.3 (5.2–6.7) | 29.6–31.1 (5.7–5.9) | 3 (1–6) | M PBAC score 306–323 (SD 97.3–131) |
| Kaunitz et al, 201097 | MPAb (83) | Mirena (82) | 38.3–39.3 (5.2–5.4) | 27.2–27.4 (3.9–4.6) | 2.5–2.6 (range 1–7) | MD 148–154.2 mL (range 63.4–456) |
| Reid and Virtanen-Kari, 200598 | Mefenamic acidc (26) | Mirena (25) | 38.5–39.4 (4.2–4.4) | NR | NR | MD 121–122 mL (range 81–389) |
| Irvine et al, 199899 | Norethisteroned (22) | Mirena (22) | MD 38.5–39 (range 30–45) | 27.8–28.1e | MD 2 (range 1–5) | MD 105–120 mL (range 82–780) |
Abbreviations: BMI, body mass index; COC, combination oral contraceptive; LNG-IUS, levonorgestrel-releasing intrauterine system; M, mean; MBL, menstrual blood loss; MD, median; MPA, medroxyprogesterone acetate; NR, not reported; PBAC, pictorial blood loss assessment chart; SD, standard deviation.
Number of participants randomized to each study arm.
Oral dose of 10 mg daily for 10 days per cycle, beginning on day 16 of menstrual cycle.
Oral dose 500 mg taken 3 times daily oral for the first 4 days of menstrual cycle.
Oral dose of 5 mg taken 3 times daily for days 5–26 of menstrual cycle.
Calculated based on raw data on height and weight presented in the article.
Only one study investigated the effect of a nonhormonal medical therapy98; thus, we could not compare results between hormonal and nonhormonal medication. The baseline characteristics of the study populations were similar among the studies and within treatment groups in each study. Results for each study's evaluations of menstrual blood loss are in Table 21.
Table 21:
Menstrual Blood Loss Results for Women Treated with LNG-IUS or Usual Medical Therapy
| Author, Year | Follow-Up | Amenorrhea, % LNG-IUS vs. UMT | MBL UMT | MBL LNG-IUS | P |
|---|---|---|---|---|---|
| Shaaban et al, 201195 | 1 y | 12 vs. 0a | M PBAC 118.2 (SD 75) | M PBAC 44.4 (SD 34.9) | < .001 |
| Kaunitz et al, 201097 | 3 cyc | NR | MDb −3.2 mL (range +146.7 to −270.9) MDb 2.2% | MDb −115.1 mL (range +54.4 to −405.8) MDa 83.2% | < .001 < .001 |
| 6 cyc | NR | MDb −17.8 mL (range +78.6 to −271.5) MDb 13.1% | MDb −128.8 mL (range +1,242.2c to −393.6) MDb 94.5% | < .001 < .001 |
|
| Reid and Virtanen-Kari, 200598 | 3 cyc | NR | MD 94 mL (range 29–219 mL) | MD 12 mL (range 0–240 mL) | < .001 |
| 6 cyc | NR | MD 100 mL (range 46–148) | MD 5 mL (range 0–45) | < .001 | |
| Irvine et al, 199899 | 1 mo | NR | MD 46 mL (range 0–213) | MD 16 mL (range 0–62) | .02 |
| 3 mo | 32 vs. 0a | MD 20 mL (range 4–137) | MD 6 mL (range 0–284) | .03 |
Abbreviations: cyc, cycle; LNG-IUS, levonorgestrel-releasing intrauterine system; M, mean; MBL, menstrual blood loss; MD, median; mo, month; NR, not reported; PBAC, pictorial blood loss assessment chart; SD, standard deviation; UMT, usual medical therapy; y, year.
Indicates statistical significance at P < .05.
Change from baseline (M or MD).
One woman had very heavy menstrual blood loss suspected by the authors to have resulted from LNG expulsion.
As with the previous comparisons, the metrics reported for menstrual blood loss in this group of studies were varied and posed a challenge for quantitative synthesis. Two studies reported median change from baseline;97,99 one reported median final blood loss;98 and another, final mean PBAC score.95 The findings are summarized below.
The 1998 study by Irvine and colleagues99 reported a 94% reduction in menstrual blood loss within the LNG-IUS group from baseline (both intention-to-treat and per-protocol analysis) and an 87% reduction in the usual medical therapy (norethisterone) group; these reductions from baseline were not significantly different between groups (P = .56). More recently, Reid and Virtanen-Kari98 reported that PBAC scores decreased by 79% from baseline (at 6 cycles). Shaaban et al95 reported that mean reduction in menstrual blood loss between baseline and 12 months was significantly greater in the LNG-IUS group than in the group taking combined oral contraceptives (86.6% vs. 2.5%, P < .001). The RCT conducted by Kaunitz et al97 found superior relative and absolute reductions from baseline in the LNG-IUS group at both 3 and 6 months (P < .001 for all).
All studies found that the LNG-IUS was superior to usual medical therapy, regardless of follow-up time or medication (Table 22).
Table 22:
Menstrual Blood Loss Improvement by Follow-Up Time: LNG-IUS vs. Usual Medical Therapy
| Author, Year | Follow-Up | MBL Comparison: UMT vs. LNG | P |
|---|---|---|---|
| Irvine et al, 199899 | 1 mo | LNG bettera | .02 |
| Kaunitz et al, 201097 | 3 mo | LNG betterb | < .001 |
| Reid and Virtanen-Kari, 200598 | LNG bettera | < .001 | |
| Irvine et al, 199899 | LNG bettera | .03 | |
| Kaunitz et al, 201097 | 6 mo | LNG betterb | < .001 |
| Reid and Virtanen-Kari, 200598 | LNG bettera | < .001 | |
| Shaaban et al, 201195 | 1 y | LNG bettera | < .001 |
Abbreviations: LNG, levonorgestrel-releasing intrauterine system; MBL, menstrual blood loss; mo, month; PBAC, pictorial blood loss assessment chart; UMT, usual medical therapy; y, year.
Difference between groups in final PBAC score or mL (mean or median).
Difference between groups in median change in MBL from baseline.
Collectively, these RCTs consistently illustrated that up to 1 year, compared with various usual medical therapies, the LNG-IUS provided superior improvement in menstrual blood loss.
Summary of Results for Menstrual Blood Loss
Of the 13 RCTs that demonstrated evidence on menstrual blood loss, only one did not report data on change from baseline.85 Compared with laparoscopic supracervical hysterectomy, the LNG-IUS provided a similar (not significantly different) or inferior improvement in menstrual blood loss (GRADE: Moderate). Compared with endometrial ablation, the LNG-IUS provided a not significantly different or a superior improvement in menstrual blood loss (GRADE: Very low). Compared with usual medical therapy, the LNG-IUS provided superior improvement in menstrual blood loss (GRADE: Low) (Table 23).
Table 23:
GRADE Evidence Profile for Menstrual Blood Loss Change with LNG-IUS
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Compared with hysterectomy | |||||||
| 1 (RCT) | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitationsb | Undetected | None | ⊕⊕⊕ Moderate |
| Compared with endometrial ablation | |||||||
| 8 (RCTs) | Serious limitations (−1)c | Serious limitations (−1)d | No serious limitations | Serious limitations (−1)e | Undetected | None | ⊕ Very low |
| Compared with usual medical therapy | |||||||
| 4 (RCTs) | Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)e | Undetected | None | ⊕⊕ Low |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; RCT, randomized controlled trial.
Lack of blinding. See Appendix 2, Tables A4 to A6, for full risk of bias assessments.
Single small study but achieved 100% completion for full 2-year study period.
Inadequate allocation concealment, lack of blinding, and some concerns about loss to follow-up. See Appendix 2, Tables A4 to A6, for full risk of bias assessments.
Inconsistent findings between studies and within them at various follow-up points with regards to superior benefit or lack of significant difference in menstrual blood loss.
Very small sample sizes; loss to follow-up compromised power in most studies.
Results for Satisfaction and Acceptability
Only six studies evaluated satisfaction with treatment between study groups.80–82,84,92,99 These studies are summarized below, by comparator. Table 24 shows the GRADE evidence profile for this outcome.
Table 24:
GRADE Evidence Profile for Satisfaction with LNG-IUS
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Compared with hysterectomy | |||||||
| 1 (RCT) | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Compared with endometrial ablation | |||||||
| 4 (RCTs) | Serious limitations (−1)b | Serious limitations (−1)c | No serious limitations | Serious limitations (−1)d | Undetected | None | ⊕ Very low |
| Compared with usual medical therapy | |||||||
| 1 (RCT) | Serious limitations (−1)a | No serious limitationse | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; RCT, randomized controlled trial.
Lack of blinding in the study. See Appendix 2, Tables A4 to A6, for full risk of bias assessment.
Inadequate allocation concealment, lack of blinding in all, intention-to-treat analysis not conducted, and substantial loss to follow-up. See Appendix 2, Tables A4 to A6, for full risk of bias assessment.
Mixed findings between studies and within them at various follow-up points with regards to superior benefit or lack of significant difference.
Very small sample sizes and substantial loss to follow-up compromised power in most studies.
Only 1 small study providing evidence could not assess inconsistency.
LNG-IUS Versus Hysterectomy
The Hurskainen et al study80 reported women's satisfaction with the LNG-IUS and with hysterectomy. Satisfaction was assessed via a 5-point Likert scale (from very unsatisfied to very satisfied) by 115 LNG-IUS patients and 114 hysterectomy patients. At 5 years after randomization, 94% of women in the LNG-IUS group and 93% of women in the hysterectomy group were satisfied or very satisfied with their treatments.80 The authors did not report a statistical comparison of these proportions.
LNG-IUS Versus Endometrial Ablation
Satisfaction with endometrial ablation or LNG-IUS was reported in four studies. Ghazizadeh and colleagues81 asked patients to rate their satisfaction with the treatment on a scale from 1 (least satisfied) to 5 (most satisfied). The mean score in the LNG group was 3.08 (standard deviation 1.6) compared with a mean of 2.5 (standard deviation 1.59) in the endometrial ablation group; the difference in these scores was not statistically significant (P = .43).81
Crosignani et al92 assessed patients’ satisfaction with their treatment by asking if they were very satisfied, satisfied, uncertain, or dissatisfied. At 1-year follow-up, the proportion who were satisfied or very satisfied in both groups was high and did not differ by intervention (85% of LNG-IUS and 94% of endometrial ablation (P = .26, odds ratio 0.35, 95% confidence interval 0.60–1.95).92
De Souza et al83 evaluated acceptability and satisfaction in terms of whether patients felt better after treatment, were satisfied with the treatment, would hypothetically choose the treatment again, and noticed great improvements in their physical and emotional well-being after treatment. At 5 years, acceptability and satisfaction with LNG-IUS was superior compared with thermal balloon endometrial ablation (P < .05 for all aspects).83
A fourth RCT asked patients to evaluate their satisfaction with either the LNG-IUS or thermal balloon endometrial ablation. More LNG-IUS patients were dissatisfied at 3 and 6 months, but satisfaction in both groups was similar at 12 and 24 months.84 The authors did not report a statistical comparison of the proportions rating “good” or “very good” perceived treatment effect.
LNG-IUS Versus Usual Medical Therapy
Irvine and colleagues99 asked women who completed the treatment to report if they were well satisfied, moderately well satisfied, moderately satisfied, or poorly satisfied after 3 months. In the LNG-IUS group, 64% were well or moderately well satisfied, while the same was true for 44% in the norethisterone group.99 No statistical comparison of these proportions was reported.
Results for Complications, Side Effects, and Adverse Events
Most of the 16 studies reported some data on complications, side effects, or adverse events; four studies did not.82,85,88,95 These occurrences during the study periods (ranging from 3 months to 10 years) are summarized below, by treatment option.
Complications and Side Effects
No study reported any occurrence of uterine perforation during insertion of the LNG-IUS. No pregnancies were reported during treatment with the LNG-IUS in any of the studies. Total or partial expulsion of the device occurred in 3% to 16% of cases, across the RCTs. Among the four studies reporting device expulsion, 50% to 100% of the women involved successfully had a new LNG-IUS inserted and thus continued treatment.79,85,90,97
Most studies provided only descriptive information about side effects related to the use of LNG-IUS. Although it is unclear exactly how many women experience them, side effects associated with LNG-IUS treatment appeared to occur in as many as 40% of study participants across all RCTs. Side effects were described in the studies as mild, and the LNG-IUS was well tolerated overall.81,87,90
Across all studies, the most common side effects were related to menstrual bleeding (irregular, persistent, heavy, or prolonged in less than 1% to 53% of participants).81,84,85,87,90,94,95,99 Spotting (intermenstrual bleeding) affected the largest proportion of women (10%87 to 53%) across RCTs; the largest percentage (53%) occurred at 3 months follow-up in one study.99 Hormonal side effects were common, experienced by up to two-thirds of participants (e.g., bloating, weight gain, mood changes, breast tenderness).81,87,90,92,98 Genitourinary infections occurred in up to 9 participants in the RCTs97 and pelvic inflammatory disease in 2 participants,90 and all cases were resolved. Pelvic pain occurred in 2% to 10% of study participants87,92,93,97 and abdominal pain in 4% to 32%.81,87,90,97,98 Benign ovarian cysts were reported as mild and uneventful in three studies81,97,98 in 2% to 24% of women treated with LNG-IUS. One study noted that one woman had her LNG-IUS device removed because of a cyst.80
Of the few available comparisons of this outcome by intervention, Crosignani and his group92 found significantly more side effects reported by participants in the LNG-IUS group compared with endometrial ablation (55.9% vs. 25.7%, P = .02), but provided no detail on the type or severity of side effects. In another study, compared with women taking oral norethisterone, women with an LNG-IUS were more likely to experience breast tenderness (P = .0008) and spotting in the first 3 months (statistics not reported).99 However, the two groups showed no difference in pain, headache, acne, nausea, edema, weight gain, decreased libido, sweating, hair loss or greasy hair, or hirsutism (increased body hair).99 Both groups experienced a small but statistically significant weight gain from baseline that was unnoticed by participants (1.1 kg in norethisterone group, 0.6 kg in the LNG-IUS group; no difference between groups).99
Spotting or continued menorrhagia were the primary reasons that women discontinued treatment.81,84,85,93,95,99 One study reported that side effects were more commonly reported in the LNG-IUS group than in the endometrial ablation group, but this was not statistically significant nor cited as a reason for discontinuing treatment.87 In two RCTs, continuation rates were reportedly higher in the LNG-IUS groups compared with usual medical therapy.93,99
Adverse Events
A minority of RCTs reported the occurrence of serious adverse events during the study periods. Several studies reported that no serious adverse events occurred in either group.77,86,97,99
Reid and Virtanen-Kari98 compared the LNG-IUS to mefenamic acid and reported two serious adverse events in the LNG-IUS group that were successfully resolved. The first event involved numbness in the arm, light-headedness, and pelvic pain that were due to pre-existing hypertension and resolved with treatment of the hypertension. In the same woman, the device was found in the endocervical canal and was subsequently removed. The second adverse event in the LNG-IUS group was the development of chlamydial endometritis, which was successfully treated, but the LNG-IUS was still ultimately expelled.98 As reported by Gupta et al93 in another study comparing the LNG-IUS to multiple medications, the rate of serious adverse events among women treated with the LNG-IUS was not significantly different from the usual medical therapy group (49 events in 46 of 285 women vs. 58 in 51 of 286 women, respectively; P = .59).
Kittelsen et al90 reported a number of significant adverse events in their study comparing the LNG-IUS and endometrial ablation. In the LNG-IUS group, there were three diagnoses of endometritis and two of pelvic inflammatory disease. In the endometrial ablation group, the diagnoses included one woman with hypertension who developed a stroke (considered unrelated), three cases of pelvic inflammatory disease, one woman with adenomyosis, one with myometritis, and two with abnormal Pap tests. No other details were provided.90
The studies comparing hysterectomy and the LNG-IUS provided little information on adverse events or complications. Sesti et al77 reported no early postoperative complications requiring readmission, blood transfusion, or repeat surgery in either group. Hurksainen and colleagues79 reported that the complication rate from hysterectomy was high in their study compared with other registry studies, but similar to cohort studies.
Discussion
The evidence in this review supports the 52-mg LNG-IUS as an effective treatment option for idiopathic heavy menstrual bleeding. The majority of RCTs that we included found significant improvement in quality of life and menstrual blood loss from baseline within the LNG-IUS group and the comparator treatment groups (medical therapy, endometrial ablation, and hysterectomy), irrespective of which treatments were being compared. Essentially, these studies often showed no significant differences in benefit between treatment options.
Our results are consistent with previous reviews comparing these treatment options (Table 4). Though our review focused on women with idiopathic heavy menstrual bleeding, our findings were similar to other systematic reviews that included this population along with women whose menorrhagia was related to uterine fibroids, adenomyosis, endometriosis, endometrial hyperplasia, or treatment for other conditions. Thus, our findings are likely generalizable to other populations with heavy menstrual bleeding from a variety of causes.
Also consistent with previous reviews, we were unable to perform quantitative synthesis of this body of evidence due to the significant methodological and data heterogeneity among the studies. Challenges included variation in measuring quality of life outcomes: different approaches to measurement, unknown or undefined minimum clinically important differences in scores, issues with interpretability of dimension-specific and aggregate scores, and different metrics reported. Measurement challenges across studies were also present for the outcome of menstrual blood loss.
Sample sizes tended to be small overall, and a priori power calculations were not consistently reported. Most studies sought menstrual blood loss as the primary outcome, whereas only three studies were designed and oriented to evaluate quality of life as a primary or secondary endpoint.79,82,93 An advantage was that the follow-up horizons for many studies extended into 2, 3, 5, or even 10 years. This allowed us to see effects beyond the first three to six cycles where spotting and side effects are known to occur, and to better understand treatment effects over the 5-year lifespan of the device, and beyond.
According to local experts, the populations captured in this review are generally representative of those seen in Ontario practice. A possible difference is that some Ontario patients (e.g., rural residents110) may have a higher body mass index than patients in the included RCTs. We had planned to examine results by obesity status, but these subanalyses could not be conducted, so there is no evidence to support or refute a difference in benefits or side effects in using the LNG-IUS in a population with higher BMI.
In the clinical management of heavy menstrual bleeding, it is essential to understand patients’ individual values and preferences, as they will invariably drive the selection of treatment. Similarly, it is important to recognize that the perceived effectiveness of treatment partly depends on a patient's tolerance for side effects and the magnitude of improvement she will accept. These factors in turn may be reflected in a patient's preference for a more invasive or less invasive treatment option. These factors also likely play a role in a paradoxical result sometimes observed with the LNG-IUS: objective outcomes varied (e.g., some women saw less improvement in menstrual blood loss) while at the same time most participants were generally satisfied with this treatment option.
In addition to the influence of patient preference, treatment selection is also qualified by each patient's eligibility for the various treatment options, although subpopulations with differing eligibility were not represented in the RCTs included in this review. For instance, women under 40 years of age, smokers aged 35 or older, and morbidly obese women (BMI > 40) are typically ineligible, respectively, for endometrial ablation, combined oral contraceptives, and hysterectomy. Such relative and absolute contraindications further emphasize the importance of the LNG-IUS as a treatment option for heavy menstrual bleeding.
Conclusions
Based on very low to moderate quality of evidence from 16 randomized controlled trials, the 52-mg LNG-IUS appears to be an effective treatment option for heavy menstrual bleeding of unknown cause (idiopathic menorrhagia) to improve quality of life and menstrual blood loss, and is well tolerated compared with endometrial ablation, hysterectomy, or usual medical therapies.
We found limited evidence of a significant difference in quality of life outcomes for women treated with the LNG-IUS compared with endometrial ablation techniques or hysterectomy. The vast majority of quality of life scores were superior among women treated with the LNG-IUS compared with usual medical therapies.
The improvement in menstrual blood loss was not significantly different between the LNG-IUS and surgical treatments (endometrial ablation or hysterectomy), but there was evidence that the LNG-IUS was superior in improving menstrual blood loss compared with usual medical therapy.
The most common side effects of the LNG-IUS were mild hormonal effects related to taking progestogens.
ECONOMIC EVIDENCE REVIEW
Objectives
The primary objective of this study was to review the published literature on the cost-effectiveness of the 52-mg levonorgestrel-releasing intrauterine system (LNG-IUS) compared with endometrial ablation, hysterectomy, or nonsurgical intervention in patients with heavy menstrual bleeding.
Methods
Sources
We performed an economic literature search on August 18, 2015, using Ovid MEDLINE, Ovid MEDLINE In-Process, Ovid EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database, for studies published from 1946 to August 18, 2015. We also reviewed reference lists of included economic literature for any additional relevant studies not identified through the systematic search. Appendix 3 provides details of the search strategy.
Literature Screening
We based our search terms on those used in the clinical evidence review of this report and applied economic filters to the search results. A single reviewer reviewed titles and abstracts and, for those studies meeting the inclusion and exclusion criteria, we obtained full-text articles.
Inclusion Criteria
English-language full-text publications
Studies published between 1946 and August 18, 2015
Studies in patients with heavy menstrual bleeding
Studies reporting on LNG-IUS compared with endometrial ablation, hysterectomy, nonsteroidal anti-inflammatory drugs, oral contraceptive pills, progestin therapy, gonadotropin-releasing hormone agonists, danazol, and/or tranexamic acid
Cost-utility, cost-effectiveness, or cost-benefit analyses
Exclusion Criteria
Menopausal women or those seeking treatment for perimenopausal menstrual bleeding, or menorrhagia due to structural or pathological causes (e.g., fibroids, cancer, polyps, adenomyosis, endometriosis, systemic bleeding disorders)
Intrauterine systems releasing different doses of levonorgestrel, other progestogens, or other medications, or other 52-mg levonorgestrel products with differing guidance on indications or administration
Outcomes of Interest
Costs, cost per quality-adjusted life-year, cost per clinical effect
Data Extraction
We extracted relevant data on the following:
source (i.e., name, location, year)
population and comparator
interventions
outcomes (i.e., health outcomes, costs, cost-effectiveness)
We contacted authors of the studies to provide unpublished data where required.
Study Applicability Appraisal
We determined the usefulness of each identified study for decision-making by applying a modified methodology checklist for economic evaluations developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom. The original checklist is used to inform development of clinical guidelines by NICE.111 An example of the modified methodology checklist can be found in Appendix 4. We modified the wording of the questions to remove references to guidelines and to make it Ontario specific. The original NICE checklist was separated into two sections: an applicability section and a methodological quality section. We used only the first section for our review. From this checklist, studies are deemed directly applicable, partially applicable, or not applicable to the research question.
Limitations
The literature review was limited to a single reviewer.
Results
Literature Search
The database search yielded 373 citations published between 1946 and August 18, 2015 (with duplicates removed). We excluded a total of 359 articles based on information in the title and abstract. We then obtained the full texts of 14 potentially relevant articles for further assessment. Figure 2 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 2: PRISMA Flow Diagram for Economic Evidence Review.
aDysfunctional uterine bleeding = Blumenthal 2006112; preliminary reports = Hurskaienen 2001, 200479,80; summary paper = Hurskaienen 2001113; repeat = Roberts 2011.114
Source: Adapted from Moher et al, 2009.70
Fourteen studies met the inclusion criteria. We hand-searched the reference lists of the included studies but did not find any more studies. After a full-text review of all articles, five studies were excluded, resulting in a total of nine studies included in the critical review.
Study Applicability
After review of the nine studies using the quality appraisal checklist, we found that none of the studies evaluated the LNG-IUS from the perspective of the Ontario or Canadian public health care payer. As a result, all studies were deemed partially applicable. The complete results of the quality appraisal checklist applied to all of the included full-text articles can be found in Appendix 5, Table A7.
Two studies evaluated the LNG-IUS against both surgical and nonsurgical treatments,115,116 four had surgical treatments as comparators,78,117–119 and three used nonsurgical comparators.120–122 Table 25 presents a summary of the study characteristics and results for articles comparing the LNG-IUS to both surgical and nonsurgical treatments. Studies comparing the LNG-IUS to surgical treatments are summarized in Table 26, and Table 27 describes articles on the LNG-IUS versus nonsurgical comparators.
Table 25:
Results of Economic Literature Review—Summary, LNG-IUS vs. Surgical and Nonsurgical Comparators (Cost-Utility Analyses)
| Results | ||||||
|---|---|---|---|---|---|---|
| Name, Year, Location | Study Design and Perspective | Population | Interventions | Health Outcomes | Costs | Cost-Effectiveness |
| Ganz et al, 2013, United States115 | Markov model United States public health care payer perspective 5-year model | 30-year-old women with menorrhagia also seeking contraception |
|
LNG-IUS = 3.78 QALYs per person Combined oral contraceptive = 3.71 QALYs per person Oral progestin = 3.67 QALYs per person Tranexamic acid = 3.72 QALYs per person EA = 3.79 QALYs per person Hysterectomy = 3.88 QALYs per person 3% discount rate |
LNG-IUS = $1,137 per person Combined oral contraceptive = $1,804 (branded); $1,196 (generic) per person Oral progestin = $1,583 per person Tranexamic acid = $3,065 per person EA = $2,612 per person Hysterectomy = $6,250 per person 3% discount rate |
LNG-IUS dominated all nonsurgical treatments Hysterectomy = $49,614/QALY compared with LNG-IUS EA = $122,278/QALY compared with LNG-IUS |
| You et al, 2006, China116 | Markov model Chinese public health care payer perspective 5-year model |
Women of reproductive age (≤ 40 years) with menorrhagia |
|
LNG-IUS = 4.625 QALYs per person EA = 4.624 QALYs per person Hysterectomy = 4.725 QALYs per person Oral medical therapy = 4.575 QALYs per person |
LNG-IUS = $4,528 per person EA = $6,185 per person Hysterectomy = $6,878 per person Oral medial therapy = $5,508 per person 3% discount rate |
LNG-IUS dominated EA and oral medical treatment Hysterectomy = $23,500/QALY |
Abbreviations: EA, endometrial ablation; LNG-IUS, levonorgestrel-releasing intrauterine system; QALY, quality-adjusted life-year.
Table 26:
Results of Economic Literature Review—Summary, LNG-IUS vs. Surgical Comparators (Cost-Utility Analyses)
| Results | ||||||
|---|---|---|---|---|---|---|
| Name, Year, Location | Study Design and Perspective | Population | Interventions | Health Outcomes | Costs | Cost-Effectiveness |
| Bhattacharya et al, 2011, United Kingdom117 | Markov model UK National Health Service perspective 10-year model |
42-year-old women with menorrhagia |
|
LNG-IUS = 68,566 QALYs (total cohort) First-generation EA = 63,745 QALYs (total cohort) Second-generation EA = 69,678 QALYs (total cohort) Hysterectomy = 73,332 QALYs (total cohort) 3.5% discount rate |
LNG-IUS = £16 million (total cohort) First-generation EA = £24 million (total cohort) Second-generation EA = £19 million (total cohort) Hysterectomy = £23 million (total cohort) UK pounds, 2008 rate 3.5% discount rate |
LNG-IUS = 1,440/QALY First-generation EA = Dominated Second-generation EA = 970/QALY (Hysterectomy comparator) |
| Heliovaara-Peippo et al, 2013, Finland78 | RCT piggy-back Finland hospital and patient perspective 10-year follow-up |
Women aged 35–49 years with menorrhagia, had completed childbearing |
|
LNG-IUS = 0.45 QALYs per person; mean −0.02 change in utility at 10 years compared with baseline Hysterectomy = 0.51 QALYs; per person mean −0.01 change in utility at 10 years compared with baseline |
LNG-IUS = $2,291 direct costs over 10 years per person ($1,133 indirect costs) Hysterectomy = $3,036 direct costs over 10 years per person ($1,900 indirect costs) 3% discount rate |
LNG-IUS = $7,607/QALY Hysterectomy = $9,680/QALY (as reported by author) |
| Clegg et al, 2007, United Kingdom118 | Markov model UK National Health Service perspective 5-year model |
43-year-old women with menorrhagia |
|
LNG-IUS followed by EA = 4.14 QALYs per person LNG-IUS followed by hysterectomy = 4.12 QALYs per person Thermal balloon EA = 4.13 QALYs per person Microwave EA = 4.13 QALYs per person Hysterectomy = 4.01 QALYs per person 3.5% discount rate |
LNG-IUS followed by EA = £828 per person LNG-IUS followed by hysterectomy = £1,355 per person Thermal balloon EA = £1,679 per person Microwave EA = £1,812 per person Hysterectomy = £2,983 per person 3.5% discount rate |
LNG-IUS followed by EA dominated LNG-IUS followed by hysterectomy, microwave EA, thermal balloon EA, and hysterectomy EA dominated hysterectomy Microwave EA = £66,800/QALY vs. LNG-IUS Thermal balloon EA = £47,500/QALY vs. LNG-IUS |
| Brown et al, 2006, New Zealand119 | Decision model Uncertain perspective (possibly societal) 2-year model |
Women with menorrhagia |
|
LNG-IUS = 15-point increase in SF-36 score per person Thermal balloon EA = 12-point increase in SF-36 score per person |
LNG-IUS = $1,242 over 2 years per person Thermal balloon EA = $2,419 over 2 years per person |
Results suggest that LNG-IUS results in slight increase in quality of life and is less costly compared with EA |
Abbreviations: EA, endometrial ablation; LNG-IUS, levonorgestrel-releasing intrauterine system; QALY, quality-adjusted life-year; SF 36, 36-item short form survey.
Table 27:
Results of Economic Literature Review—Summary, LNG-IUS vs. Nonsurgical Comparators (Cost-Utility Analyses)
| Results | ||||||
|---|---|---|---|---|---|---|
| Name, Year, Location | Study Design and Perspective | Population | Interventions | Health Outcomes | Costs | Cost-Effectiveness |
| Calaf et al, 2015, Spain120 | Markov model Spanish national health system perspective 5-year model |
Women of reproductive age with menorrhagia, wishing to retain fertility |
|
LNG-IUS = 49.57 QALMs per person Estradiol valerate/ dienogest multiphase oral contraceptive = 47.83 QALMs per person Combined oral contraceptives = 46.24 QALMs per person Progestins = 44.18 QALMs per person |
LNG-IUS = €205 over 6 months per person Estradiol valerate/ dienogest multiphase oral contraceptive = €325 over 6 months per person Combined oral contraceptives = €416 over 6 months per person Progestins = €796 over 6 months per person |
LNG-IUS dominates all other treatment options |
| Sanghera et al, 2014, United Kingdom121 | Markov model UK National Health Services perspective 2-year model |
Women aged 25 to 50 years with menorrhagia |
|
LNG-IUS = 1.580 QALYs per person Usual medical treatment = 1.513 QALYs per person 3.5% discount rate |
LNG-IUS = £430 per person Usual medical treatment = £330 per person 3.5% discount rate |
LNG-IUS = £1,600/QALY |
| Lete et al, 2011, Spain122 | Markov model Spanish national health system perspective 5-year model |
Women of reproductive age with menorrhagia, wishing to retain fertility |
|
LNG-IUS = 50.53 QALMs per person Combination oral contraceptive = 47.86 QALMs per person Progestogens = 45.59 QALMs per person 3% discount rate |
LNG-IUS = €3,099 per person Combination oral contraceptive = €3,409 per person Progestogens = €3,677 per person 3% discount rate |
LNG-IUS dominated both combination oral contraceptive and progestogens |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; QALM, quality-adjusted life-month; QALY, quality-adjusted life-year.
Methodological Quality of the Included Studies
Most of the studies limited the model timeline to 5 years,115,116,118,120,122 while four had a timeline that was longer (10 years)78,117 or shorter (2 years).119,121 Seven studies used Markov models to determine the cost-effectiveness of the LNG-IUS.115–118,120–122 The remaining two studies were economic evaluations piggy-backed to a randomized controlled trial (RCT).78,119 All studies included almost all important outcomes related to treatment and heavy menstrual bleeding. Four of the studies were either sponsored by an LNG-IUS manufacturer or had manufacturer employees as study co-authors.115,118,120,122
Discussion
In terms of surgical comparators, the LNG-IUS was consistently less costly, but the results for quality-adjusted life-years (QALYs) were mixed. When the LNG-IUS was compared with hysterectomy, LNG-IUS was less costly than hysterectomy in all studies.78,115–119 In three of the studies, the LNG-IUS resulted in lower total QALYs than hysterectomy.115–117 One study showed more total QALYs for LNG-IUS than hysterectomy.118 Another study showed no statistical differences in the change in QALYs for treatment with the LNG-IUS versus hysterectomy over 10 years of follow-up.78 Of the four studies that compared the LNG-IUS to endometrial ablation, all studies observed that the LNG-IUS was less costly.115–118 In one study, the LNG-IUS had more total QALYs compared with first-generation endometrial ablation techniques but lower total QALYs compared with second-generation endometrial ablation.117 In one study that compared the LNG-IUS to thermal balloon and microwave ablation, LNG-IUS had greater total QALYs.118 Another study with thermal balloon ablation as comparator found that the LNG-IUS resulted in a greater increase in quality of life, based on Short Form-36 scores.119
For studies that compared the LNG-IUS to medical therapy, LNG-IUS was less costly and had better total QALYs or quality-adjusted life-months in three of the four studies.115,116,120,122 In one study, LNG-IUS had better total QALYs but was more costly.121 The incremental cost-effectiveness ratio observed in this study was £1,600 per QALY.
Conclusions
Overall, the results of published economic evaluations comparing the LNG-IUS to medical therapy suggest that the LNG-IUS is cost-effective: LNG-IUS is either less costly and has better outcomes or it has a lower cost per QALY. Based on studies comparing the LNG-IUS to surgical treatment options, LNG-IUS is less costly. However, the evidence is mixed as to whether the LNG-IUS results in lower or higher QALYs compared with surgical options. Given this uncertainty in the evidence, we conducted an economic evaluation comparing the LNG-IUS to endometrial ablation and hysterectomy.
PRIMARY ECONOMIC EVALUATION
Published economic evaluations identified in our literature review have compared the 52-mg levonorgestrel-releasing intrauterine system (LNG-IUS) with surgical and medical therapy for heavy menstrual bleeding (menorrhagia). Studies comparing the LNG-IUS with medical therapy have consistently shown LNG-IUS to be the more cost-effective option. Results for LNG-IUS versus surgical treatments have been inconsistent: while the LNG-IUS is invariably shown to be the least costly intervention, the quality of life outcomes have been mixed. As a result, we conducted an economic evaluation comparing the LNG-IUS with surgical treatments.
Objectives
The objective of this study was to assess the cost-effectiveness, from the perspective of the Ontario Ministry of Health and Long-Term Care, of the LNG-IUS compared with hysterectomy and endometrial ablation in patients with heavy menstrual bleeding.
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards Statement.123
Type of Analysis
Given the availability of utilities (measures of patients’ preferences) related to treatment options for heavy menstrual bleeding and the uncertainty regarding the total quality-adjusted life-years (QALYs) associated with the LNG-IUS compared with surgical options, we developed a cost-utility analysis.
Target Population
The model population was women with heavy menstrual bleeding who were eligible for all three treatments being evaluated: LNG-IUS, endometrial ablation, or hysterectomy. The age of the women in the model cohort was 42 years, similar to the age in three economic evaluations studying the same interventions.78,117,118
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health and Long-Term Care.
Interventions
We evaluated the LNG-IUS compared with endometrial ablation and with hysterectomy.
Discounting and Time Horizon
We applied an annual discount rate of 5% to both costs and QALYs. With 51 years being the mean age of menopause in Canada, the total time horizon for our base case was 9 years.
Model Structure
For this analysis, we constructed a health-state transition model that followed a cohort of women with heavy menstrual bleeding (Figures 3 to 5). The components of the model were based on the health states commonly observed in prior studies. The model begins when a woman is cleared by a physician for initial treatment with either the LNG-IUS, endometrial ablation, or hysterectomy. Women receiving hysterectomy (Figure 5) or endometrial ablation (Figure 4) treatment will experience a wait time just prior to surgery. After receiving treatment, women can transition between the following states: well, menorrhagia, pregnant, and adverse event.
Figure 3: Model Structure (LNG-IUS).
Abbreviations: GI, gastrointestinal; LNG-IUS, levonorgestrel-releasing intrauterine system.
Figure 5: Model Structure (Hysterectomy).
Abbreviations: GI, gastrointestinal.
Figure 4: Model Structure (Endometrial Ablation).
Women whose initial treatment with the LNG-IUS fails can switch to endometrial ablation (Figure 4) or hysterectomy (Figure 5). For those who switch to endometrial ablation, another failure would lead to hysterectomy. Where endometrial ablation is the initial treatment but fails, women can switch to a repeat endometrial ablation or to hysterectomy. After a second failed endometrial ablation, the only other treatment option is hysterectomy. The cycle length for this model is one month, to adequately capture all important events that a woman may experience.
The different health states in the model are described below.
Well: Women in this state are free of menorrhagia symptoms and are content with their current treatment. They may experience treatment-related adverse events. Women currently on the LNG-IUS or endometrial ablation may experience treatment failure (recurrent menorrhagia) that will result in a treatment switch and proceed to the endometrial ablation or hysterectomy model.
Pregnant: For women being treated with the LNG-IUS or endometrial ablation, there is a small probability of pregnancy. The model assumes that women will not be able to carry a pregnancy to full term and will abort after the first trimester. It is also assumed that after pregnancy the woman will continue to experience menorrhagia and will seek an alternative treatment.
Menorrhagia resolved; no further treatment: Some women initially treated with the LNG-IUS will discontinue treatment and no longer experience menorrhagia.
Menorrhagia; endometrial ablation wait time: Wait times for endometrial ablation in Ontario are approximately 69 days.124 Women will experience menorrhagia while in this state, where they will remain for two cycles.
Menorrhagia; hysterectomy wait time: Current wait times for hysterectomy in Ontario are approximately 86 days, according to public reporting by the Ministry of Health and Long-Term Care.124 In our analysis, women receiving hysterectomy enter the hysterectomy wait-time state for three cycles, during which time they continue to experience menorrhagia symptoms.
Hysterectomy convalescence: For women receiving a hysterectomy, the model assumes a one-month convalescence after surgery. This length of time falls between expected recovery times for laparoscopic, vaginal, and abdominal hysterectomy.125,126
Adverse events: Women may experience adverse events related to their current treatment and transition to the adverse event state for one cycle. We assumed that adverse events will last up to one month and that the woman will return to the same treatment afterwards. The adverse events included in this model were identified in our clinical evidence review (Table 28).
Table 28:
Treatment-Related Adverse Events Included in the Economic Model
| Treatment | Adverse Event |
|---|---|
| LNG-IUS | Device reinsertion |
| Gastrointestinal obstruction | |
| Lower abdominal pain | |
| Pelvic pain | |
| Pelvic inflammatory disease | |
| Endometrial ablation | Hematometra |
| Pelvic pain | |
| Hysterectomy | Gastrointestinal obstruction |
| Lower abdominal pain | |
| Secondary hemorrhage | |
| Urinary retention | |
| Surgical infection |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Death: At any point during the model timeline, a woman has a probability of death.
Model Parameters
We used a number of input parameters to populate the model. These inputs—clinical outcomes, utilities, and costs—are explained below.
Clinical Outcomes
Adverse Events
The probability of experiencing a treatment-related adverse event over time was extracted from the studies identified in our clinical evidence review. Because all extracted data reflected a time frame greater than the cycle length of our model, we converted these data to monthly probabilities (Table 29). Full calculations for our conversion of study data to monthly probabilities are presented in Appendix 6, Tables A8.
Table 29:
Adverse Event Inputs Used in the Economic Model
| Model Parameters | Monthly Probability | Duration of Risk in the Model | Reference |
|---|---|---|---|
| LNG-IUS | |||
| Device reinsertion | 0.0037 | 1 year | Hurskainen et al, 200179 |
| Gastrointestinal obstruction | 0.0009 | 9 years | Heliovaara-Peippo et al, 201378 |
| Lower abdominal pain | 0.0015 | 9 years | Malak and Shawki, 200687 |
| Pelvic pain | 0.0009 | 9 years | Malak and Shawki, 200687 |
| Pelvic inflammatory disease | 0.0004 (0.0003) | Year 1 (Years 2–9) | Hurskainen et al, 200179 |
| Endometrial ablation | |||
| Hematometra | 0.0003 | 9 years | Malak and Shawki, 200687 |
| Pelvic pain | 0.0012 | 9 years | Malak and Shawki, 200687 |
| Hysterectomy | |||
| Gastrointestinal obstruction | 0.0014 | 9 years | Heliovaara-Peippo et al, 201378 |
| Lower abdominal pain | 0.00007 | 9 years | Heliovaara-Peippo et al, 201378 |
| Secondary hemorrhage | 0.0002 | 9 years | Heliovaara-Peippo et al, 201378 |
| Urinary retention | 0.0002 | 9 years | Heliovaara-Peippo et al, 201378 |
| Surgical infection | 0.0031 | 1 year | Hurskainen et al, 200179 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Treatment Switch
The probability of switching treatment over time was extracted from the studies identified in the clinical review, and data were converted to monthly probability. Calculations are presented in Appendix 6, Tables A9 and A10. Model inputs are presented in Table 30.
Table 30:
Treatment Switch Inputs Used in the Economic Model
| Model Parameters | Monthly Probability | Reference |
|---|---|---|
| LNG-IUS to endometrial ablation | ||
| 0–6 months | 0.0053a | Heliovaara-Peippo et al, 201378 |
| 7–12 months | 0.0112a | Heliovaara-Peippo et al, 201378 |
| 2–5 years | 0.0031a | Heliovaara-Peippo et al, 201378 |
| 6–10 years | 0.0007a | Heliovaara-Peippo et al, 201378 |
| LNG-IUS to hysterectomy | ||
| 0–6 months | 0.0080a | Heliovaara-Peippo et al, 201378 |
| 7–12 months | 0.0168a | Heliovaara-Peippo et al, 201378 |
| 2–5 years | 0.0047a | Heliovaara-Peippo et al, 201378 |
| 6–10 years | 0.0011a | Heliovaara-Peippo et al, 201378 |
| Endometrial ablation to hysterectomy | ||
| Year 1 | 0.0087 | Silva-Filho et al, 201383 |
| Year 2 | 0.0031 | Silva-Filho et al, 201383 |
| Year 3 | 0.0066 | Silva-Filho et al, 201383 |
| Year 4 | 0 | Silva-Filho et al, 201383 |
| Year 5 | 0 | Silva-Filho et al, 201383 |
| Years 6–10 | 0 | Assumption |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Assuming that 40% of women switching from LNG-IUS will receive endometrial ablation and 60% will receive hysterectomy.
Physician Follow-Up After Intervention
A randomized controlled trial comparing the LNG-IUS and endometrial ablation over 2 years included data on the total number of family physician or general practitioner visits related to each treatment for menorrhagia.119 For our analysis, we assumed that hysterectomy requires 0.56 times as many general practitioner visits as the LNG-IUS, corresponding to the difference in physician-related costs observed in US administrative data.115 During the waiting period for hysterectomy and endometrial ablation, when women would continue to experience menorrhagia, our model assumed that physician visits were twice the number observed for women successfully treated with the LNG-IUS.
Table 31:
Physician Post-Treatment Follow-Up Inputs Used in the Economic Model
| Model Parameters | Monthly Visits,a n | Reference |
|---|---|---|
| LNG-IUS | 0.05 | Brown et al, 2006119 |
| Endometrial ablation | 0.08 | Brown et al, 2006119 |
| Hysterectomy | 0.028 | Ganz et al, 2013115 |
| Menorrhagia | 0.10 | Assumption |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Visits to a general practitioner or family physician after treatment for menorraghia.
Pregnancy
The risk of pregnancy with LNG-IUS treatment was extracted from a published literature review of contraceptive methods.127 In that study, the observed rate of pregnancy among women using the LNG-IUS was 0.006 per year (monthly probability 0.0005). Likewise, the risk of pregnancy after endometrial ablation was observed to be 0.007 per year, or 0.0006 per month.128
The distribution of pregnancy outcomes during treatment with the LNG-IUS and following endometrial ablation was based on observations from Blumenthal and colleagues1 and Yin,112,129 respectively. We assumed that half of the reported spontaneous abortions were complete and half were incomplete. In the literature, a proportion of women carried the pregnancy to term, but those studies were based on younger cohorts. Our model uses an older cohort so we assumed that women who did not have a spontaneous abortion or ectopic pregnancy had an induced abortion. The distribution of pregnancy outcomes in our model is presented in Appendix 6, Table A11, and calculations for the monthly probabilities are shown in Table A12.
Other Clinical Outcomes
We incorporated into the model the probability that menorrhagia would resolve after a woman discontinued the LNG-IUS, using data from Heliovaara-Peippo and colleagues.78 We included mortality (10-year risk of death) using age-adjusted data from Ontario life tables.130 These model inputs are presented in Table 32. Full calculations are presented in Appendix 6, Table A12.
Table 32:
Other Clinical Outcome Inputs Used in the Economic Model
| Model Parameters | Monthly Probability | Duration of Risk in the Model, years | Reference |
|---|---|---|---|
| Menorrhagia resolved; no further treatment | 0.0014 | 10 | Heliovaara-Peippo et al, 201378 |
| LNG-IUS risk for pregnancy | 0.0005 | 10 | Mansour 2010127 |
| Mortality | 0.00008–0.00022 | 10 | Life tables 2009–2011130 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Intervention Utilities
Utility values were based on a study in the United Kingdom that used time trade-off methodology to elicit preferences in a cohort of 60 women with menorrhagia.130 The utility values extracted from that study included menorrhagia, perimenopause following recovery after abdominal hysterectomy, and the convalescence period after abdominal hysterectomy. For our model, we assumed that the utility values after recovery from hysterectomy observed in the literature were similar to the utilities experienced in the well state in our model, regardless of the intervention. Utility values for women experiencing a treatment-related adverse event were not available in the literature, and we assumed that utility values for these women would fall between the values for the well and menorrhagia states. We tested this assumption in a sensitivity analysis. Table 33 shows the utility values incorporated in the model.
Table 33:
Utilities Used in the Economic Model
| Health State | Mean Utility (Standard Error) | Reference |
|---|---|---|
| Well | 0.86 (0.03) | Sculpher, 1998131 |
| Intervention adverse event | 0.68 (0.03) | Assumption |
| Pregnant | 0.86 (0.03) | Sculpher, 1998131 |
| Menorrhagia resolved; no further treatment | 0.86 (0.03) | Sculpher, 1998131 |
| Well; hysterectomy convalescence | 0.74 (0.05) | Sculpher, 1998131 |
| Menorrhagia; hysterectomy wait time | 0.5 (0.04) | Sculpher, 1998131 |
| Menorrhagia; one month transition between treatments | 0.5 (0.04) | Sculpher, 1998131 |
Cost Parameters
All costs included in our study originated from the Ontario Schedule of Benefits for Physician Services,132 the Ontario Drug Benefit Formulary,133 and Ontario administrative data. A cohort of women who received the LNG-IUS was identified through the provincial physician billing database. The number of women receiving endometrial ablation or hysterectomy for heavy menstrual bleeding was calculated through the inpatient and outpatient hospital care databases. Treatment-related adverse events were likewise identified through inpatient hospital care databases. The diagnosis and procedure codes used to search the administrative data are presented in Appendix 6, Tables A13 and A14.
LNG-IUS
Initial treatment. Cost of initial treatment includes physician services for the procedure as well as the cost of the technology. According to Ontario administrative data, approximately 88% of LNG-IUS insertions were administered by a gynecologist for menorrhagia. Therefore, our model included a consult with a gynecologist in 88% of treatments. The model repeated this cost at 5 years, the lifespan of this technology.134 Unit cost inputs for initial LNG-IUS treatment are presented in Table 34.
Table 34:
LNG-IUS Treatment Costs Used in the Economic Model
| Variable | Unit Cost, $ | Reference |
|---|---|---|
| Gynecologist consultation | 101.70 | Ontario Schedule of Benefits for Physician Services132 (code A205) |
| LNG-IUS initial procedure | 337.90 | Ontario Drug Benefit Formulary133 |
| LNG-IUS procedure physician services | 36.65 | Ontario Schedule of Benefits for Physician Services132 (code G378) |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Physician follow-up. Physician follow-up costs were calculated by multiplying the mean number of visits per month by the cost per physician service. In our analysis, we assumed that all physician visits were to a family physician. Table 35 shows the unit cost.
Table 35:
Physician Follow-Up Costs Used in the Economic Model
| Variable | Unit Cost, $ | Reference |
|---|---|---|
| Family physician | 38.35 | Ontario Schedule of Benefits for Physician Services132 (code A004) |
Endometrial Ablation
Initial treatment. The cost of initial treatment with endometrial ablation includes a concurrent tubal occlusion procedure assumed to occur in 30% of all women receiving this treatment for heavy menstrual bleeding. This was estimated assuming 35% sterilization rates, with 5% coming from vasectomies (Expert opinion, written communication, November 17, 2015). The cost for physician services includes the surgeon, assistant surgeon, and anesthesiologist. If the woman receiving endometrial ablation is switching from the LNG-IUS to endometrial ablation, the model included an additional cost for the removal of the device. Table 36 shows the costs for initial treatment with endometrial ablation.
Table 36:
Endometrial Ablation Treatment Costs Used in the Economic Model
| Variable | Unit Cost, $ (Standard Deviation) | Reference |
|---|---|---|
| Gynecologist consultation | 101.70 | Ontario Schedule of Benefits for Physician Services132 (code A205) |
| Endometrial ablation initial procedure | 1,137.00a (425) | Administrative datab |
| Endometrial ablation procedure physician services | 309.71 | Ontario Schedule of Benefits for Physician Services132 (code S772) |
| Concurrent tubal occlusion procedure | 1,110.00a (114) | Administrative datab |
| Tubal occlusion procedure physician services | 318.00 | Ontario Schedule of Benefits for Physician Services132 (code S741) |
| LNG-IUS removal physician services (only if switching from LNG-IUS) | 47.45 | Ontario Schedule of Benefits for Physician Services132 (code A203) |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Mean cost.
Data provided by Ontario IntelliHEALTH.
Physician follow-up. For endometrial ablation, these costs were the same as described for LNG-IUS treatment (Table 35).
Hysterectomy
Initial treatment. The cost of initial treatment for hysterectomy includes hospital and physician procedure costs. Physician costs include the fees of the surgeon, assistant surgeon, and anesthesiologist. For women receiving hysterectomy after failure of previous treatment with either LNG-IUS or endometrial ablation, the model included an additional cost for a preoperative physician visit.
Physician follow-up. For hysterectomy, these costs were the same as described for LNG-IUS treatment (Table 35).
Table 37:
Hysterectomy Treatment Costs Used in the Economic Model
| Variable | Unit Cost, $ (Standard Deviation) | Reference |
|---|---|---|
| Gynecologist consultation | 101.70 | Ontario Schedule of Benefits for Physician Services132 (code A205) |
| Hysterectomy initial procedure | 4,573.00a (1,575) | Administrative datab |
| Hysterectomy procedure physician services | 640.31 | Ontario Schedule of Benefits for Physician Services132 (code S757 or S816) |
| Hysterectomy preoperative physician services (only if switching from another treatment) | 101.70 | Ontario Schedule of Benefits for Physician Services132 (code A205) |
Mean cost.
Data provided by Ontario IntelliHEALTH.
Treatment-Related Adverse Events
Costs for adverse events were based on the mean inpatient or outpatient hospital length of stay observed in administrative data. Costs included the hospital stay and physician services. These costs are summarized in Table 38, and a further breakdown is provided in Appendix 6, Table A14.
Table 38:
Treatment-Related Adverse Event Costs Used in the Economic Model
| Variable | Unit Cost, $ (Standard Deviation) | Reference |
|---|---|---|
| LNG-IUS–related adverse events | ||
| Reinsertion | ||
| Procedure | 338 | Ontario Drug Benefit Formulary133 |
| Physician | 36.65 | Ontario Schedule of Benefits for Physician Services132 (code G378 and E542) |
| Gastrointestinal obstruction | ||
| Hospital | 4,156 (2,615) | Administrative dataa |
| Physician | 249 | Ontario Schedule of Benefits for Physician Services132 |
| Lower abdominal pain | ||
| Hospital | 385 (222) | Administrative dataa |
| Physician | 77.20 | Ontario Schedule of Benefits for Physician Services132 |
| Pelvic pain | ||
| Hospital | 4,211 (1,063) | Administrative dataa |
| Physician | 218 | Ontario Schedule of Benefits for Physician Services132 |
| Pelvic inflammatory disease | ||
| Hospital | 503 (704) | Administrative datab |
| Physician | 77 | Ontario Schedule of Benefits for Physician Services132 |
| Endometrial ablation–related adverse events | ||
| Hematometra | ||
| Hospital | 2,450 (1,638) | Administrative dataa |
| Physician | 308 | Ontario Schedule of Benefits for Physician Services132 |
| Pelvic pain | ||
| Hospital | 1,372 (1,814) | Administrative dataa |
| Physician | 159 | Ontario Schedule of Benefits for Physician Services132 |
| Hysterectomy-related adverse events | ||
| Gastrointestinal obstruction | ||
| Hospital | 3,823 (2,269) | Administrative dataa |
| Physician | 367 | Ontario Schedule of Benefits for Physician Services132 |
| Lower abdominal pain | ||
| Hospital | 424 (374) | Administrative dataa |
| Physician | 77.20 | Ontario Schedule of Benefits for Physician Services132 |
| Secondary hemorrhage | ||
| Hospital | 1,878 (646) | Administrative dataa |
| Physician | 159 | Ontario Schedule of Benefits for Physician Services132 |
| Urinary retention | ||
| Hospital | 510 (706) | Administrative dataa |
| Physician | 77.20 | Ontario Schedule of Benefits for Physician Services132 |
| Surgical infection | ||
| Hospital | 1,195 (3,331) | Administrative dataa |
| Physician | 77.20 | Ontario Schedule of Benefits for Physician Services132 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Data provided by Ontario IntelliHEALTH.
Pregnancy-related costs
Pregnancy costs consist of physician services. No additional costs were included in our analysis. Unit costs are presented in Table 39.
Table 39:
Physician Costs Related to Pregnancy Outcomes Used in the Economic Model
| Variable | Unit Cost, $ | Reference |
|---|---|---|
| Spontaneous complete abortion | 161.15 | Ontario Schedule of Benefits for Physician Services132 (code A004) |
| Spontaneous incomplete abortion | 183.06 | Ontario Schedule of Benefits for Physician Services132 (code A206) |
| Induced abortion | 202.46 | Ontario Schedule of Benefits for Physician Services132 (code A004) |
| Ectopic abortion | 207.80 | Ontario Schedule of Benefits for Physician Services132 (code A004) |
Analysis
In the base case analysis, we applied actual values or mean values as the model inputs. This method provides the best estimate of the cost-effectiveness of the LNG-IUS, but it does not consider the uncertainty of various inputs to the model or the possibility of other clinical scenarios. We present the results as the incremental costs (the difference in costs) and incremental QALYs of LNG-IUS compared with hysterectomy and endometrial ablation.
While the base case analysis provided the best estimates of the cost-effectiveness of the LNG-IUS, we performed sensitivity analyses to address the uncertainty of model inputs and clinical scenarios. We assessed variability and uncertainty in the model through one-way and probabilistic sensitivity analyses. To determine the impact of simultaneously varying numerous variables within the assigned distributions, we conducted a probabilistic sensitivity analysis by running 1,000 simulations of the model. Results of the probabilistic sensitivity analysis are presented on a cost-effectiveness plane and, if necessary, a cost-effectiveness acceptability curve. We assigned a beta distribution for utility values. For cost inputs where standard deviation or confidence intervals were presented, a gamma distribution was assigned. We conducted one-way sensitivity analyses by varying specific model variables and examining the impact on the results. The variables and ranges are presented in Table 40.
Table 40:
Variables Varied in One-Way Sensitivity Analyses
| Variable | Range | Reference |
|---|---|---|
| Adverse events and switch rates | Clinical inputs from administrative data | Administrative dataa |
| Mean age when initial treatment in administered | 38 to 46 years | Assumption |
| Pregnancy | Exclude from model | Assumption |
| Frequency of physician follow-up after endometrial ablation | 0.028 to 0.05 | Same as LNG-IUS to hysterectomy |
| Women experiencing repeat LNG-IUS | All switch to hysterectomy | Assumption |
| Women experiencing repeat endometrial ablation | All switch to hysterectomy | Assumption |
| Women experiencing repeat LNG-IUS or endometrial ablation | All switch to hysterectomy | Assumption |
| Treatment-related adverse event health state | 0.5 to 0.86 | Sculpher, 1998131 |
| Menorrhagia utility | 0.76 | Heliovaara-Peippo et al, 201378 |
| Physician follow-up | Exclude from model | Assumption |
| Discount rate | 3% | Assumption |
| Hysterectomy wait time | Exclude prior to initial treatment | Assumption |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Data provided by Ontario IntelliHEALTH.
Main Assumptions
The major assumptions for this model are:
A woman's quality of life after successful treatment is the same regardless of treatment
Quality of life after treatment-related adverse events is worse than with successful treatment but better than with continued menorrhagia
Treatment-related adverse events do not last more than a month
Women who become pregnant do not carry the pregnancy to term
First-generation and second-generation endometrial ablation are combined into one treatment arm
Women who initially receive LNG-IUS and switch to endometrial ablation will only be offered hysterectomy if endometrial ablation fails
Women who initially receive endometrial ablation may receive a second endometrial ablation if treatment fails, but not a third.
There are no post-hysterectomy complications that require a repeat procedure
Generalizability
The findings of this economic analysis cannot be generalized to all patients with heavy menstrual bleeding. They may, however, be used to guide decision-making about the specific patient populations in Ontario addressed in the studies evaluated by Health Quality Ontario.
Expert Consultation
Throughout the development of this model, we solicited expert consultation from physicians in the specialty areas of obstetrics and gynecology. The role of the expert advisors was to review the structure and inputs of the economic model to confirm that the information we used reasonably reflects the clinical setting. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Results
Base Case Analysis
The base case results for our analysis are presented in Table 41. The LNG-IUS costs less and has higher quality-adjusted life-years (QALYs) than both endometrial ablation and hysterectomy.
Table 41:
Base Case Analysis Results
| Strategy | Average Total Costs, $ | Incremental Cost, $ | Average Total QALYs | Incremental QALYs | Incremental Cost-Effectiveness Ratio |
|---|---|---|---|---|---|
| LNG-IUS | 3,142 | 6.32 | |||
| Hysterectomy | 6,280 | −3,138a | 6.28 | 0.04b | LNG-IUS dominates |
| Endometrial ablation | 3,514 | −372c | 6.27 | 0.05d | LNG-IUS dominates |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; QALY, quality-adjusted life-year.
Incremental costs = average costs of LNG-IUS – average costs of hysterectomy.
Incremental effects = average effects of LNG-IUS - average effects of hysterectomy.
Incremental costs = average costs of LNG-IUS - average costs of endometrial ablation.
Incremental effects = average effects of LNG-IUS - average effects of endometrial ablation.
Sensitivity Analysis
The incremental cost and incremental QALYs calculated for each simulation of the probabilistic sensitivity analysis for LNG-IUS versus hysterectomy and LNG-IUS versus endometrial ablation are illustrated in Figures 6 and 7, respectively. In the comparison of the LNG-IUS and hysterectomy, incremental costs ranged from −$14,008 to $1,609, while incremental QALYs ranged from 0.05 to 0.15. When LNG-IUS was compared with endometrial ablation, incremental cost ranged from −$2,630 to $1,524 and incremental QALYs ranged from 0.03 to 0.07. We did not develop cost-effectiveness acceptability curves because almost all simulations in the probabilistic sensitivity analysis resulted in a dominant situation for LNG-IUS (lower incremental cost and higher incremental QALYs). The results of the one-way sensitivity analysis are presented in Tables 42 and 43.
Figure 6: Incremental Cost and QALYs of LNG-IUS Compared With Hysterectomy.
Abbreviations: ICER, incremental cost-effectiveness ratio; LNG-IUS, levonorgestrel-releasing intrauterine system; QALY, quality-adjusted life-year.
Figure 7: Incremental Cost and QALYs of LNG-IUS Compared With Endometrial Ablation.
Abbreviations: ICER, incremental cost-effectiveness ratio; LNG-IUS, levonorgestrel-releasing intrauterine system; QALY, quality-adjusted life-year.
Table 42:
One-Way Sensitivity Analysis Results, LNG-IUS vs. Hysterectomy
| Scenario | Incremental Cost, $a | Incremental Effectb | Result |
|---|---|---|---|
| Administrative data inputs for adverse events and switch rates | −4,004 | 0.056 | LNG-IUS dominates |
| Model excludes pregnancy | −3,237 | 0.041 | LNG-IUS dominates |
| 5-year model | −3,367 | 0.041 | LNG-IUS dominates |
| 13-year model | −3,036 | 0.037 | LNG-IUS dominates |
| Endometrial ablation physician follow-up same as hysterectomy | −3,153 | 0.038 | LNG-IUS dominates |
| Endometrial ablation physician follow-up same as LNG-IUS | −3,138 | 0.038 | LNG-IUS dominates |
| All LNG-IUS switches to hysterectomy with recurrence of menorrhagia | −2,792 | 0.037 | LNG-IUS dominates |
| All endometrial ablation switches to hysterectomy with recurrence of menorrhagia | −3,138 | 0.038 | LNG-IUS dominates |
| All LNG-IUS and endometrial ablation switches to hysterectomy with recurrence of menorrhagia | −2,792 | 0.038 | LNG-IUS dominates |
| Treatment-related adverse event quality of life is the same as menorrhagia state | −3,138 | 0.039 | LNG-IUS dominates |
| Treatment-related adverse event quality of life is the same as well state | −3,126 | 0.037 | LNG-IUS dominates |
| Menorrhagia utilities at 0.76 | −3,138 | 0.076 | LNG-IUS dominates |
| Exclude physician follow-up | −3,213 | 0.038 | LNG-IUS dominates |
| 3% discount rate | −3,052 | 0.036 | LNG-IUS dominates |
| Exclude initial hysterectomy wait time | −3,161 | -0.052 | Lower cost and lower QALYs for LNG-IUS |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; QALY, quality-adjusted life year
Incremental costs = average costs of LNG-IUS – average costs of hysterectomy.
Incremental effects = average effects of LNG-IUS – average effects of hysterectomy.
Table 43:
One-Way Sensitivity Analysis Results, LNG-IUS vs. Endometrial Ablation
| Scenario | Incremental Cost, $a | Incremental Effectb | Result |
|---|---|---|---|
| Administrative data inputs for adverse events and switch rates | −1,067 | 0.056 | LNG-IUS dominates |
| Model excludes pregnancy | −318 | 0.045 | LNG-IUS dominates |
| 5-year model | −574 | 0.049 | LNG-IUS dominates |
| 13-year model | −136 | 0.045 | LNG-IUS dominates |
| Endometrial ablation physician follow-up same as hysterectomy | −244 | 0.046 | LNG-IUS dominates |
| Endometrial ablation physician follow-up same as LNG-IUS | −372 | 0.046 | LNG-IUS dominates |
| All LNG-IUS switches to hysterectomy with recurrence of menorrhagia | −46 | 0.045 | LNG-IUS dominates |
| All endometrial ablation switches to hysterectomy with recurrence of menorrhagia | −496 | 0.046 | LNG-IUS dominates |
| All LNG-IUS and endometrial ablation switches to hysterectomy with recurrence of menorrhagia | −42 | 0.043 | LNG-IUS dominates |
| Treatment-related adverse event quality of life is the same as menorrhagia state | −364 | 0.042 | LNG-IUS dominates |
| Treatment-related adverse event quality of life is the same as well state | −372 | 0.050 | LNG-IUS dominates |
| Menorrhagia utilities at 0.76 | −365 | 0.049 | LNG-IUS dominates |
| Exclude physician follow-up | −302 | 0.046 | LNG-IUS dominates |
| 3% discount rate | −279 | 0.045 | LNG-IUS dominates |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Incremental costs = average costs of LNG-IUS – average costs of endometrial ablation.
Incremental effects = average effects of LNG-IUS – average effects of endometrial ablation.
Discussion
In our primary economic evaluation comparing the LNG-IUS with hysterectomy for treatment of heavy menstrual bleeding, we observed that LNG-IUS was less costly and had better QALYs. This observation remained consistent in the one-way sensitivity analyses except when surgical wait time was excluded from the model. In that case, the LNG-IUS was still less costly but had lower QALYs than hysterectomy. The probabilistic sensitivity analysis confirmed that the overall results remained consistent (lower cost and higher QALYs for LNG-IUS) even when the uncertainty in the model inputs was considered.
Compared with endometrial ablation and hysterectomy, the LNG-IUS was less costly and had higher QALYs, and this was consistently observed in more than 99% of all simulation results in the sensitivity analyses. In some cases, costs were higher for LNG-IUS, but all simulations resulted in LNG-IUS costs below the commonly used threshold of $50,000 per QALY.
The results of our analysis are within range of past economic evaluations comparing LNG-IUS and hysterectomy for treatment of heavy menstrual bleeding. The incremental cost savings of LNG-IUS fall between the findings of past studies, where the cost difference ranged between −$745 and −$5,113.78,115 The lower QALYs for LNG-IUS versus hysterectomy when we excluded the wait time for surgery were similar to most previous studies. This is to be expected given that past studies did not consider surgical wait times in their analyses.
Our base case comparing the LNG-IUS and endometrial ablation resulted in incremental cost savings for LNG-IUS that were lower than past studies (−$1,475 to −$1,657).115,116 This may be because the model duration in prior studies was only 5 years, whereas we used a 9-year time horizon. When we limited our model to 5 years, the incremental cost savings for LNG-IUS were closer to past studies. Unlike the mixed results on the incremental QALYs of LNG-IUS versus endometrial ablation from past studies, our results showed an increase in total QALYs for LNG-IUS compared with endometrial ablation.
Our analysis has numerous strengths. The longer timeline allowed our analysis to include all clinical impacts that women with heavy menstrual bleeding typically experience, across the duration of the condition. Using monthly cycles allowed us to model changes in treatment and in rates of treatment-related adverse events that were similar to changes experienced by patients. The probabilities of treatment-related adverse events included in our model were based on an extensive clinical review of the published literature. Where model inputs were not available from published studies, we used Ontario administrative data. This minimized the use of assumptions in the analysis and allowed us to incorporate Ontario-specific data, another strength.
There were also several limitations in our analysis. First, no data were available to provide values for the utility of adverse events related to treatment for heavy menstrual bleeding. As a result, we assumed that this utility was between the values for the well state and the heavy menstrual bleeding state. However, given the small probability of adverse events related to the treatments we modelled, changes to this utility would only result in small changes to the total QALYs for any of the treatments. Second, utility values were based on data collected more than 20 years ago and quality of life data were for women receiving hysterectomy to treat heavy menstrual bleeding. We assumed that these utility values would be similar for women receiving the LNG-IUS or endometrial ablation today. Third, no published studies have compared physician follow-up times for all three treatment options. Our study relied on data from two different studies with the assumption that the two study cohorts were similar to each other and to our population. Fourth, there was no information on the average wait time for receiving the LNG-IUS. For our analysis, we assumed that the wait would be less than a month. A longer wait would reduce the total QALYs for LNG-IUS and shrink the incremental QALYs of that treatment versus hysterectomy or endometrial ablation. Fifth, no information was available on the possibility that some women might discontinue using the LNG-IUS and not switch to another treatment, even though their heavy menstrual bleeding continued. If a proportion of women would make that choice, this would mean our total QALYs for LNG-IUS are an overestimate, as we assumed that all women experiencing treatment failure with LNG-IUS would opt for endometrial ablation or hysterectomy. Sixth, we used a generic diagnosis code of “menstrual problem” to search the administrative data for women receiving the LNG-IUS for heavy menstrual bleeding. We assumed that approximately 50% of this cohort had menorrhagia but the actual size of the cohort could be different. Further, many women who received the LNG-IUS did not have a reported diagnosis code. As a result, we may have underestimated the size of our cohort. Finally, this economic evaluation does not consider patient preference for initial treatment. The underlying assumption with our model was that women were cleared for all treatment options and did not have a preference for any specific treatment.
Conclusions
The results of our analysis show that the LNG-IUS is less costly and has better QALY outcomes compared with both hysterectomy and endometrial ablation. These observations were consistent even when uncertainty in model inputs and parameters was considered.
BUDGET IMPACT ANALYSIS
We conducted a budget impact analysis from the perspective of the Ontario Ministry of Health and Long-Term Care to determine the estimated cost burden over the next 5 years of funding the 52-mg levonorgestrel-releasing intrauterine system (LNG-IUS) for the treatment of heavy menstrual bleeding (menorrhagia). All costs are reported in 2015 Canadian dollars. A 5-year time frame was selected because it corresponds to the lifetime of the LNG-IUS device.
Objectives
The objective of this study was to assess the budget impact, within the context of the Ontario Ministry of Health and Long-Term Care, of publicly funding the LNG-IUS compared with hysterectomy and endometrial ablation for treatment of heavy menstrual bleeding,
Methods
Target Population
Three distinct hypothetical populations were incorporated into this analysis. The first group consists of women who are prescribed the LNG-IUS for heavy menstrual bleeding and would pay for the device out-of-pocket or be reimbursed by private health insurance if LNG-IUS were not publicly funded. (If LNG-IUS were to be publicly funded, the only additional cost from the public health care payer perspective would be the cost to reimburse the device.) We estimated the number of new cases each year by extrapolating from the volume of new LNG-IUS cases between 2006 and 2012 identified through administrative data.
The second group in the analysis is women seeking a replacement LNG-IUS when the device has reached the end of its lifetime (approximately 5 years). We approximated the size of this group by identifying women who received an initial treatment of LNG-IUS for heavy menstrual bleeding 5 years prior to the most recent administrative data. To exclude women who switch to an alternative treatment or stop treatment altogether, we calculated that 70% of women will continue with LNG-IUS at 5 years, based on the primary economic evaluation in this report. We then multiplied the number of new cases of LNG-IUS treatment by 70% to estimate the number of women requiring an LNG-IUS replacement.
The third group is women who are prescribed LNG-IUS for menorrhagia but would instead receive hysterectomy or endometrial ablation if LNG-IUS were not publicly funded. In this cohort, we considered both the cost of the device and downstream costs in six scenarios: four comparing LNG-IUS and hysterectomy and two comparing LNG-IUS and endometrial ablation.
For the first (upper limit) hysterectomy scenario, we assumed that all women who had a hysterectomy for heavy menstrual bleeding will instead receive LNG-IUS as their initial treatment. In a second scenario, we assumed that 88% of those who would have had a hysterectomy if LNG-IUS were not publicly funded will accept LNG-IUS if it is offered. This assumption corresponded to an observation that 12% of women who had a hysterectomy chose that treatment over LNG-IUS.29 A third scenario considered women who will have a hysterectomy because they do not have private health insurance covering LNG-IUS and cannot afford the cost of the device. In this scenario, we assumed that all women with unsupportive pathology for hysterectomy (meaning they may not have needed the surgery as their initial treatment for heavy menstrual bleeding) did not have private health insurance. The proportion of women with unsupportive pathology was 12%, as reported in a US cohort.135 For a fourth (lower limit) scenario, we assumed that 50% of the cohort with unsupportive pathology will be treated by hysterectomy instead of LNG-IUS because they could not afford the LNG-IUS device if they had to pay for it.
In our analyses comparing LNG-IUS and endometrial ablation, in the first (upper limit) scenario we assumed that all women who would have been treated with endometrial ablation will receive LNG-IUS instead. As a second (lower limit) scenario, we assumed that 78% of women who would have received endometrial ablation will choose LNG-IUS if it is offered initially. This assumption is based on a study finding that about 22% of women receiving endometrial ablation for heavy menstrual bleeding preferred that treatment over LNG-IUS.29
Table 44 presents the total estimated volumes of treatment procedures over 5 years for all scenarios. The total volumes were calculated by summing the volumes for the three treatment groups in each scenario (see Appendix 8, Tables A18 to 22 for more detail).
Table 44:
Target Population Scenarios to Compare LNG-IUS and Surgical Treatments
| Number of Women Receiving LNG-IUS | |||||
|---|---|---|---|---|---|
| Strategy | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
| LNG-IUS compared with hysterectomy | |||||
| Scenario 1 (upper limit): Women who would have received hysterectomy if LNG-IUS were not publicly funded (includes all women receiving hysterectomy for heavy menstrual bleeding) | 6,503 | 6,493 | 6,487 | 6,514 | 6,493 |
| Scenario 2: Women who would have received hysterectomy if LNG-IUS were not publicly funded (assumes 12% of women offered LNG-IUS prefer hysterectomy) | 5,479 | 5,516 | 5,559 | 5,633 | 5,660 |
| Scenario 3: Women who would have received hysterectomy if LNG-IUS were not publicly funded (assumes all with unsupportive pathology for hysterectomy did not have private health insurance) | 1,860 | 2,023 | 2,191 | 2,392 | 2,543 |
| Scenario 4 (lower limit): Women who would have received hysterectomy if LNG-IUS were not publicly funded (assumes 50% with unsupportive pathology for hysterectomy did not have private health insurance) | 1,574 | 1,747 | 1,925 | 2,135 | 2,297 |
| LNG-IUS compared with endometrial ablation | |||||
| Scenario 1 (upper limit): Women who have received endometrial ablation if LNG-IUS were not publicly funded (includes all women receiving endometrial ablation) | 7,469 | 7,693 | 7,922 | 8,184 | 8,397 |
| Scenario 2 (lower limit): Women who would have received endometrial ablation if LNG-IUS were not publicly funded (assumes 22% of women offered LNG-IUS prefer endometrial ablation) | 6,109 | 6,324 | 6,544 | 6,797 | 7,001 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Resource and Costs
The cost of the LNG-IUS for women who will privately cover the cost of the device if it is not publicly funded is the cost of the device as reported in the Ontario Drug Benefit Formulary ($338).133 Costs for women who will have a hysterectomy or endometrial ablation if LNG-IUS is not publicly funded are based on the annual incremental cost and cost savings associated with the LNG-IUS compared with the surgical treatments for the first 5 years, as reported in our primary economic evaluation. These costs include the initial treatment, physician follow-up, and costs of treatment-related adverse events. Total annual costs for each treatment group are presented in Table 45.
Table 45:
Estimated Per-Patient Costs for Calculating Budget Impact of Publicly Funding LNG-IUS
| Cost to Publicly Fund LNG-IUS, $ | |||||
|---|---|---|---|---|---|
| Strategy | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
| For women who will privately pay for the device if LNG-IUS is not publicly funded | 338 | 0 | 0 | 0 | 0 |
| For women who will receive hysterectomy if LNG-IUS is not publicly fundeda | −4,507 | 389 | 213 | 355 | 326 |
| For women who will receive endometrial ablation if LNG-IUS is not publicly fundedb | −1,449 | 104 | −33 | 260 | 376 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Cost of LNG-IUS relative to cost of hysterectomy.
Cost of LNG-IUS relative to cost of endometrial ablation.
Analysis
To obtain the total cost of providing the LNG-IUS to women who will choose this option whether or not it is publicly funded, we multiplied the unit cost of LNG-IUS by the estimated number of women who will either pay for the device out-of-pocket or have it reimbursed by private insurance.
For the total cost of new cases (i.e., for women who can be expected to choose the LNG-IUS over surgical options if it is publicly funded), we multiplied the annual incremental cost of the LNG-IUS compared with hysterectomy by the volumes for each of the four scenarios described above, resulting in four separate analyses. Likewise, the annual incremental cost of the LNG-IUS compared with endometrial ablation was multiplied by the total volumes for each the two endometrial ablation scenarios.
Finally, the total budget impact was calculated by summing the total cost of providing the LNG-IUS to women who will choose that option regardless of public funding and the total cost of treating the additional women who will newly choose the LNG-IUS over surgical treatment (i.e. hysterectomy and endometrial ablation) as a result of public funding.
In a supplementary analysis, we calculated the new cost to government of the estimated increase in use of the LNG-IUS to treat heavy menstrual bleeding if this treatment becomes publicly funded. In this analysis, only the cost of the LNG-IUS device was included; we multiplied the total annual volumes for all scenarios by the cost of the device, as reported in the Ontario Drug Benefit Formulary.133
Results
Table 46 presents the results of the budget impact analysis for all of the scenarios comparing the cost of the LNG-IUS versus hysterectomy and endometrial ablation. Detailed calculations for each scenario are presented in Appendix 8, Tables A23 to A28. Results show a general trend towards cost savings each year for LNG-IUS versus hysterectomy and endometrial ablation.
Table 46:
Results of Budget Impact Analysis of Publicly Funding LNG-IUS vs. Surgical Treatments for Heavy Menstrual Bleeding
| $ million | |||||
|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
| LNG-IUS vs. hysterectomy | |||||
| Scenario 1 (upper limit) | −23.1 | −20.1 | −18.1 | −15.4 | −13.0 |
| Scenario 2 | −18.5 | −16.1 | −14.5 | −12.4 | −10.5 |
| Scenario 3 | −2.1 | −1.8 | −1.5 | −1.1 | −0.8 |
| Scenario 4 (lower limit) | −0.8 | −0.6 | 0.5 | 0.2 | −0.1 |
| LNG-IUS vs. endometrial ablation | |||||
| Scenario 1 (upper limit) | −8.5 | −7.9 | −8.1 | −6.5 | −4.2 |
| Scenario 2 (lower limit) | −6.6 | −6.0 | −6.2 | −5.0 | −3.2 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
The annual costs of funding the estimated increase in demand for LNG-IUS devices, for all scenarios, are presented in Table 47. This includes only the additional cost of publicly funding the devices and does not account for the potential cost-savings from decreased use of other types of treatment. Detailed calculations behind these costs are shown in Appendix 8, Tables A29 to A34. Results show a cost between $0.7 to $4.2 million per year.
Table 47:
New Annual Cost of Publicly Funding LNG-IUS Treatment
| $ million | |||||||
|---|---|---|---|---|---|---|---|
| Scenario | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | ||
| LNG-IUS vs. hysterectomy | |||||||
| Scenario 1 (upper limit) | 2.2 | 2.2 | 2.2 | 2.2 | 3.4 | ||
| Scenario 2 | 1.9 | 1.8 | 1.7 | 1.5 | 2.0 | ||
| Scenario 3 | 0.6 | 0.7 | 0.7 | 0.8 | 1.0 | ||
| Scenario 4 (lower limit) | 0.5 | 0.6 | 0.7 | 0.7 | 0.8 | ||
| LNG-IUS vs. endometrial ablation | |||||||
| Scenario 1 (upper limit) | 2.5 | 2.6 | 2.6 | 2.7 | 4.2 | ||
| Scenario 2 (lower limit) | 2.1 | 2.1 | 2.2 | 2.2 | 3.4 | ||
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Discussion
This budget impact analysis revealed that, in the first 5 years, publicly funding the LNG-IUS for heavy menstrual bleeding as an alternative to surgical interventions could result in savings of between $3 million and $30 million per year. This includes potential savings of $0.1 million to $23 million per year by replacing hysterectomies and $3.2 million to $8.5 million per year by replacing endometrial ablation procedures. Cost savings are expected to decrease over time as more women begin treatment with the LNG-IUS and the number of women currently on this treatment rises. This trend would likely stabilize at approximately year 10 as women who have used an LNG-IUS for close to a decade approach menopause.
In a supplementary analysis, we estimated the total new cost of publicly funding LNG-IUS treatment for heavy menstrual bleeding; this involves only the cost of providing the devices because physician fees related to the procedure are already covered. The total new cost for the first 5 years would be about $3 million to $8 million per year. This includes between $0.5 million and $3.4 million per year as LNG-IUS replaces some hysterectomies, and between $2.1 and $4.2 million per year as LNG-IUS replaces some endometrial ablations.
To our knowledge, no other budget impact analysis has been published on the use of the LNG-IUS to treat heavy menstrual bleeding. Our evaluation has several strengths. We included incremental cost data from our primary economic evaluation, which encompassed all downstream costs in the first 5 years. Calculating the 5-year budget impact allowed us to include costs for a complete lifetime of the LNG-IUS device. Further, our analysis considered three groups of potential users of LNG-IUS: women who would choose the LNG-IUS without public funding (they have private insurance or would pay out-of-pocket); women who would have chosen hysterectomy or endometrial ablation if LNG-IUS were not publicly funded; and women who need a repeat LNG-IUS procedure because their device has reached the end of its 5-year lifetime.
Our analysis also had two key limitations. First, our estimates of cohort size were based on volumes of procedures from administrative data. Due to limitations in reporting, the cohort sizes used in our calculations are likely an underestimation. The actual volumes are currently unknown. Second, and arguably the most important limitation, was the uncertainty regarding the number of women who would receive the LNG-IUS instead of hysterectomy or endometrial ablation if this treatment were publicly funded. We addressed this uncertainty by developing a variety of scenarios based on different assumptions. Comparing the LNG-IUS with hysterectomy, for the upper limit we included all women who would be expected to receive a hysterectomy for heavy menstrual bleeding; this is likely an overestimate since it does not consider that some patients may prefer hysterectomy. For the lower limit, we only included women who received an unnecessary hysterectomy; this is likely an underestimate since it does not consider women who may be appropriate for hysterectomy but would prefer the LNG-IUS. However, by exploring different volume estimates, we calculated the upper and lower boundaries between which the true budget impact is expected to fall.
Our study estimated the budget impact of publicly funding the LNG-IUS for heavy menstrual bleeding and does not include the cost of funding the LNG-IUS for contraception. The budget impact of the LNG-IUS for all indications would be much higher than the results in our analysis.
Conclusions
Overall, our analysis suggests that if the LNG-IUS were publicly funded as an alternative to surgical treatment for heavy menstrual bleeding, it would result in cost savings in the first 5 years. The amount of cost savings is uncertain but could be up to $23 million per year compared with hysterectomy and up to $9 million per year compared with endometrial ablation.
Acknowledgments
The medical editor was Amy Zierler. Others involved in the development and production of this report were Arshia Ali, Ana Laing, Claude Soulodre, Andree Mitchell, Anil Thota, Vivian Ng, Nancy Sikich, and Irfan Dhalla.
We are grateful to the following experts for their contributions to this report:
Jennifer Blake
CEO, Society of Obstetricians and Gynaecologists of Canada
Adjunct Professor, Department of Obstetrics and Gynaecology, University of Toronto
Adjunct Professor, Department of Obstetrics and Gynaecology, University of Ottawa
Dustin Costescu, MD, FRCSC
Assistant Professor (Family Planning Specialist), Department of Obstetrics and Gynaecology, McMaster University
Sheila Dunn, MD, MSc, CCFP(EM)
Associate Professor, Department of Family and Community Medicine, Women's College Hospital, University of Toronto
Nicholas Leyland, BASc, MD, MHCM, FRCSC
Professor and Chair, Department of Obstetrics and Gynaecology, Director of the Minimally Invasive Gynecologic Surgery (MIGS) Fellowship Program, McMaster University, Chief of Obstetrics and Gynaecology, Hamilton Health Sciences, Hamilton, Ontario
Kate Rheault
Chief of Obstetrics, Orillia Soldiers Memorial Hospital, Orillia, Ontario
Glossary
ABBREVIATIONS
- BMI
Body mass index
- EQ-5D
EuroQol five-dimension, a quality of life assessment tool
- EQ-VAS
EuroQol visual analogue scale, a quality assessment tool
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HRQOL-4
Four-item health-related quality of life assessment tool; also called the Healthy Days Measure
- LNG-IUS
Levonorgestrel-releasing intrauterine system
- MMAS
Menorrhagia multi-attribute scale
- PBAC
Pictorial blood loss assessment chart
- QALY
Quality-adjusted life-year
- RCT
Randomized controlled trial
- SF-36
Short Form 36, a 36-item quality of life assessment tool
Glossary
GLOSSARY
- Cost-effective
The technique or intervention has been determined to provide results sufficiently positive to justify the cost.
- Incremental cost
The extra cost associated with using one test or treatment instead of another.
- Parity
The number of pregnancies a person has had in which the fetus reached the point of viability, even if not carried to full term or successfully delivered.
- Quality-adjusted life-year (QALY)
A measurement that takes into account both the number of years gained by a patient from a procedure and the quality of those extra years (ability to function, freedom from pain, etc.). One QALY is expressed as a number between zero (no benefit) and one (perfect health). The QALY is commonly used as an outcome measure in cost–utility analyses.
- Randomized controlled trial
A type of study in which subjects are assigned randomly into different groups, with one group receiving the intervention or treatment under study and the other group(s) receiving a different intervention or treatment (or a placebo or no treatment) in order to determine the effectiveness of one approach over another.
- Sensitivity analysis
Every evaluation contains some degree of uncertainty. Study results can vary depending on the values taken by key parameters. Sensitivity analysis is a method that allows estimates for each parameter to be varied to show the impact on study results. There are various types of sensitivity analyses. Examples include deterministic, probabilistic, and scenario.
- Systematic review
A process to answer a research question by methodically identifying and assessing all available studies that evaluate the specified research question. The systematic review process is designed to be transparent and objective and is aimed at reducing bias in determining the answers to research questions.
- Utility
The perceived benefit (value) placed on a treatment by a person or society.
APPENDICES
Appendix 1: Clinical Literature Search Strategies
Databases searched: Ovid MEDLINE, Ovid MEDLINE In-Process, Ovid EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database and National Health Service (NHS) Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <July 2015>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to June 2015>, EBM Reviews - Database of Abstracts of Reviews of Effects <2nd Quarter 2015>, EBM Reviews - Health Technology Assessment <3rd Quarter 2015>, EBM Reviews - NHS Economic Evaluation Database <2nd Quarter 2015>, EMBASE <1980 to 2015 Week 33>, All Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
| 1 | Menorrhagia/ (11505) |
| 2 | (menorrhag* or hypermenorrh*).tw. (8019) |
| 3 | (((abnormal* or heavy or excessive* or irregular* or dysfunction* or prolonged or frequent) adj2 (menstrua* or period* or uter* bleeding or uter* blood or vaginal bleeding or vaginal blood)) or AUB or DUB or HMB).tw. (55064) |
| 4 | or/1–3 (66531) |
| 5 | exp Intrauterine Devices, Medicated/ (17410) |
| 6 | (((intrauterine or intrauterine) adj2 (device* or system*)) or IUD or IUDs or IUS or IUSs).tw. (24149) |
| 7 | Levonorgestrel/ (13820) |
| 8 | exp Progestins/ (205021) |
| 9 | exp Progesterone/ (140496) |
| 10 | (levonorgestrel* or LNG).tw. (9767) |
| 11 | mirena.tw. (1564) |
| 12 | or/5–11 (244698) |
| 13 | 4 and 12 (5953) |
| 14 | Meta Analysis.pt. (59760) |
| 15 | Meta-Analysis/ or Meta-Analysis as Topic/ or exp Technology Assessment, Biomedical/ (211030) |
| 16 | (((systematic* or methodologic*) adj3 (review* or overview*)) or pooled analysis or published studies or published literature or hand search* or handsearch* or medline or pubmed or embase or cochrane or cinahl or data synthes* or data extraction* or HTA or HTAs or (technolog* adj (assessment* or overview* or appraisal*))).ti,ab. (439892) |
| 17 | (meta analy* or metaanaly* or health technolog* assess*).mp. (310716) |
| 18 | or/14–17 (626281) |
| 19 | 13 and 18 (409) |
| 20 | limit 19 to english language [Limit not valid in CDSR,DARE; records were retained] (400) |
| 21 | 20 use pmoz (105) |
| 22 | 20 use cctr (7) |
| 23 | Menorrhagia/ (11505) |
| 24 | (menorrhag* or hypermenorrh*).tw. (8019) |
| 25 | (((abnormal* or heavy or excessive* or irregular* or dysfunction* or prolonged or frequent) adj2 (menstrua* or period* or uter* bleeding or uter* blood or vaginal bleeding or vaginal blood)) or AUB or DUB or HMB).tw. (55064) |
| 26 | or/1–3 (66531) |
| 27 | exp Intrauterine Contraceptive Device/ (24926) |
| 28 | (((intrauterine or intrauterine) adj2 (device* or system*)) or IUD or IUDs or IUS or IUSs).tw. (24149) |
| 29 | Levonorgestrel/ (13820) |
| 30 | exp Gestagen/ (140678) |
| 31 | Progesterone/ (127671) |
| 32 | (levonorgestrel* or LNG).tw. (9767) |
| 33 | mirena.mp. (1719) |
| 34 | or/27–33 (226375) |
| 35 | 26 and 34 (5583) |
| 36 | Meta Analysis.pt. (59760) |
| 37 | Meta Analysis/ or “Meta Analysis (Topic)”/ or Biomedical Technology Assessment/ (195987) |
| 38 | (((systematic* or methodologic*) adj3 (review* or overview*)) or pooled analysis or published studies or published literature or hand search* or handsearch* or medline or pubmed or embase or cochrane or cinahl or data synthes* or data extraction* or HTA or HTAs or (technolog* adj (assessment* or overview* or appraisal*))).ti,ab. (439892) |
| 39 | (meta analy* or metaanaly* or health technolog* assess*).mp. (310716) |
| 40 | or/36–39 (625180) |
| 41 | 35 and 40 (395) |
| 42 | limit 41 to english language [Limit not valid in CDSR,DARE; records were retained] (387) |
| 43 | 42 use emez (211) |
| 44 | 13 use coch (56) |
| 45 | 13 use dare (17) |
| 46 | 13 use clhta (7) |
| 47 | 13 use cleed (17) |
| 48 | or/21–22,43–47 (420) |
Appendix 2: Clinical Evidence Quality Assessment
Our first consideration was study design; we started with the assumption that randomized controlled trials are high quality, whereas observational studies are low quality. We then took into account five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias. Limitations in these areas resulted in downgrading the quality of evidence. Finally, we considered three main factors that may raise the quality of evidence: the large magnitude of effect, the dose-response gradient, and any residual confounding factors.56 For more detailed information, please refer to the latest series of GRADE articles.56
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | We are very confident that the true prognosis (probability of future events) lies close to that of the estimate |
| Moderate | We are moderately confident that the true prognosis (probability of future events) is likely to be close to the estimate, but there is a possibility that it is substantially different |
| Low | Our confidence in the estimate is limited: the true prognosis (probability of future events) may be substantially different from the estimate |
| Very Low | We have very little confidence in the estimate: the true prognosis (probability of future events) is likely to be substantially different from the estimate |
Table A1:
GRADE Evidence Profile for Conclusions Related to the Comparison of LNG-IUS and Hysterectomy
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Quality of life | |||||||
| 2 (RCTs) | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Menstrual bleeding | |||||||
| 1 (RCT) | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitationsb | Undetected | None | ⊕⊕⊕ Moderate |
| Satisfaction | |||||||
| 1 (RCT) | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; RCT, randomized controlled trial.
Lack of blinding. See Table A4 for full risk of bias assessment.
Single small study but achieved 100% completion for full 2-year study period.
Table A2:
GRADE Evidence Profile for Conclusions Related to the Comparison of LNG-IUS and Endometrial Ablation
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Quality of life | |||||||
| 5 (RCTs) | Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
| Menstrual bleeding | |||||||
| 8 (RCTs) | Serious limitations (−1)a | Serious limitations (−1)c | No serious limitations | Serious limitations (−1) | Undetected | None | ⊕ Very low |
| Satisfaction | |||||||
| 4 (RCTs) | Serious limitations (−1)a | Serious limitations (−1)c | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕ Very low |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; RCT, randomized controlled trial.
Inadequate allocation concealment, lack of blinding in all, intention-to-treat analysis was not conducted, and loss to follow-up. See Table A5 for full risk of bias assessment.
Very small sample sizes; loss to follow-up compromised power in most studies.
Mixed findings between studies and within them at various follow-up points with regards to superior benefit or lack of significant difference.
Table A3:
GRADE Evidence Profile for Conclusions Related to the Comparison of LNG-IUS and Usual Medical Therapy
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Quality of life | |||||||
| 2 (RCTs) | Serious limitations (−1)a | Serious limitations (−1)b | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Menstrual bleeding | |||||||
| 4 (RCTs) | Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)c | Undetected | None | ⊕⊕ Low |
| Satisfaction | |||||||
| 1 (RCT) | Serious limitations (−1)a | No Serious Limitationsd | No serious limitations | Serious limitations (−1)c | Undetected | None | ⊕⊕ Low |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; RCT, randomized controlled trial.
Lack of blinding. See Table A6 for full risk of bias assessment.
Results varied from favouring LNG-IUS to no difference depending on quality of life tool used and time point assessed.
Small samples and loss to follow-up, posing issues with adequate power.
Only 1 small study providing evidence.
Table A4:
Risk of Bias Among Randomized Controlled Trials for the Comparison of LNG-IUS and Hysterectomy
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Sesti et al, 201277 | No limitations | Limitationsa | No limitations | No limitations | No limitations |
| Hurskainen et al, 200179 | No limitations | Limitationsb | No limitations | No limitations | No limitations |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Only outcome assessors were blinded to treatment allocation.
No blinding was reported.
Table A5:
Risk of Bias Among Randomized Controlled Trials for the Comparison of LNG-IUS and Endometrial Ablation
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Ghazizadeh et al, 201181 | No limitations | Limitationsa | Limitationsb | No limitations | No limitations |
| de Souza et al, 201082 | Limitationsc | Limitationsa | Limitationsb | Limitationsd | No limitations |
| Shaw et al, 200784 | Limitationsc | Limitationsa | Limitationsb,e | No limitations | No limitations |
| Tam et al, 200685 | Limitationsc | Limitationsa | Limitationsb,f | No limitations | No limitations |
| Busfield et al, 200686 | No limitations | Limitationsa | Limitationsb | No limitations | No limitations |
| Malak and Shawki, 200687 | No limitations | Limitationsa | Limitationsb | No limitations | No limitations |
| Barrington et al, 200388 | Limitationsc | Limitationsa | Limitationsb | No limitations | No limitations |
| Kittelsen and Istre, 199890 | No limitations | Limitationsa | Limitationsb,g | No limitations | No limitations |
| Crosignani et al, 199792 | Limitationsa | Limitationsa | Limitationsb | No limitations | No limitations |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
RCTs were not blinded due to the nature of interventions.
Outcomes were analyzed only for those participants completing treatment, not as intention-to-treat.
Allocation concealment not reported so unclear risk of bias.
Satisfaction outcome not reported at 1 year but only at 5-year follow-up.
Loss to follow-up was 34.8% overall and those lost were excluded from the analysis.
Loss to follow-up was 25% overall.
Loss to follow-up was 30.5% overall.
Table A6:
Risk of Bias Among Randomized Controlled Trials for the Comparison of LNG-IUS and Medical Therapy
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Gupta et al, 201393 | No limitations | Limitationsa | No limitationsb | No limitations | No limitations |
| Shaaban et al, 201195 | No limitations | Limitationsa | No limitationsc | No limitations | No limitations |
| Kaunitz et al, 201097 | Limitationsd | Limitationsa | Limitationsc,e | No limitations | No limitations |
| Reid and Virtanen-Kari, 200598 | Limitationsf | Limitationsa | No limitationsc | No limitations | No limitations |
| Irvine et al, 199899 | No limitations | Limitationsa | Limitationsc,d | No limitations | No limitations |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
RCTs were not blinded due to the nature of interventions.
Loss to follow-up was 25.8% at 5-year follow-up, 16.3% at 2-year follow-up; however, planned power of the study was 90% and year 5 post hoc power was calculated to be 87%.
Loss to follow-up was less than 20% overall.
Allocation concealment not reported.
Primary analyses adhered to the intention-to-treat principle; however, secondary analyses were analyzed according to treatment received.
Principal investigator personally recruited, consented, randomized, and followed up all participants, and conducted 75% of analyses with known allocation.
Appendix 3: Economic Literature Search Strategies
Databases searched: Ovid MEDLINE, Ovid MEDLINE In-Process, Ovid EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database and National Health Service (NHS) Economic Evaluation Database
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <July 2015>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to June 2015>, EBM Reviews - Database of Abstracts of Reviews of Effects <2nd Quarter 2015>, EBM Reviews - Health Technology Assessment <3rd Quarter 2015>, EBM Reviews - NHS Economic Evaluation Database <2nd Quarter 2015>, EMBASE <1980 to 2015 Week 33>, All Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
| 1 | Menorrhagia/ (11506) |
| 2 | (menorrhag* or hypermenorrh*).tw. (8019) |
| 3 | (((abnormal* or heavy or excessive* or irregular* or dysfunction* or prolonged or frequent) adj2 (menstrua* or period* or uter* bleeding or uter* blood or vaginal bleeding or vaginal blood)) or AUB or DUB or HMB).tw. (55071) |
| 4 | or/1–3 (66539) |
| 5 | exp Intrauterine Devices, Medicated/ (17410) |
| 6 | (((intrauterine or intrauterine) adj2 (device* or system*)) or IUD or IUDs or IUS or IUSs).tw. (24150) |
| 7 | Levonorgestrel/ (13820) |
| 8 | exp Progestins/ (205023) |
| 9 | exp Progesterone/ (140498) |
| 10 | (levonorgestrel* or LNG).tw. (9767) |
| 11 | mirena.tw. (1564) |
| 12 | or/5–11 (244701) |
| 13 | 4 and 12 (5953) |
| 14 | economics/ (247515) |
| 15 | economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (699564) |
| 16 | economics.fs. (370014) |
| 17 | (econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmacoeconomic*).tw. (639364) |
| 18 | exp “costs and cost analysis”/ (487137) |
| 19 | cost*.ti. (219849) |
| 20 | cost effective*.tw. (229772) |
| 21 | (cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (143701) |
| 22 | models, economic/ (127581) |
| 23 | markov chains/ or monte carlo method/ (116576) |
| 24 | (decision adj1 (tree* or analy* or model*)).tw. (31175) |
| 25 | (markov or markow or monte carlo).tw. (92890) |
| 26 | quality-adjusted life years/ (26199) |
| 27 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (44922) |
| 28 | ((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (88425) |
| 29 | or/14–28 (2158916) |
| 30 | 13 and 29 (498) |
| 31 | limit 30 to english language [Limit not valid in CDSR,DARE; records were retained] (471) |
| 32 | 31 use pmoz,cctr,coch,dare,clhta (171) |
| 33 | Menorrhagia/ (11506) |
| 34 | (menorrhag* or hypermenorrh*).tw. (8019) |
| 35 | (((abnormal* or heavy or excessive* or irregular* or dysfunction* or prolonged or frequent) adj2 (menstrua* or period* or uter* bleeding or uter* blood or vaginal bleeding or vaginal blood)) or AUB or DUB or HMB).tw. (55071) |
| 36 | or/33–35 (66539) |
| 37 | exp Intrauterine Contraceptive Device/ (24926) |
| 38 | (((intrauterine or intrauterine) adj2 (device* or system*)) or IUD or IUDs or IUS or IUSs).tw. (24150) |
| 39 | Levonorgestrel/ (13820) |
| 40 | exp Gestagen/ (140678) |
| 41 | Progesterone/ (127673) |
| 42 | (levonorgestrel* or LNG).tw. (9767) |
| 43 | mirena.mp. (1719) |
| 44 | or/37–43 (226378) |
| 45 | 36 and 44 (5583) |
| 46 | Economics/ (247515) |
| 47 | Health Economics/ or exp Pharmacoeconomics/ (209294) |
| 48 | Economic Aspect/ or exp Economic Evaluation/ (376085) |
| 49 | (econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmacoeconomic*).tw. (639364) |
| 50 | exp “Cost”/ (487137) |
| 51 | cost*.ti. (219849) |
| 52 | cost effective*.tw. (229772) |
| 53 | (cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (143701) |
| 54 | Monte Carlo Method/ (47541) |
| 55 | (decision adj1 (tree* or analy* or model*)).tw. (31175) |
| 56 | (markov or markow or monte carlo).tw. (92890) |
| 57 | Quality-Adjusted Life Years/ (26199) |
| 58 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (44922) |
| 59 | ((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (88425) |
| 60 | or/46–59 (1766428) |
| 61 | 45 and 60 (457) |
| 62 | limit 61 to english language [Limit not valid in CDSR,DARE; records were retained] (434) |
| 63 | 62 use emez (270) |
| 64 | 13 use cleed (17) |
| 65 | or/32,63–64 (458) |
| 66 | remove duplicates from 65 (381) |
Appendix 4: Modified Methodological Checklist for Economic Evaluations
| Question topic: | ||
| Study reference: | ||
| Checklist completed by: | ||
| APPLICABILITY (relevance to question under review) | ||
| Item | Yes/Partly/ No/Unclear/NA | Comments |
| Is the study population appropriate to the question? | ||
| Are the interventions appropriate to the question? | ||
| Are all relevant interventions compared? | ||
| What country was this study conducted in? | ||
| Is the health care system in which the study was conducted sufficiently similar to Ontario with respect to this question/topic? Explain the ways in which they differ. | ||
| Are estimates of relative treatment effect the same as those included in the clinical report? | ||
| Are costs measured from a health care payer perspective? | ||
| Are non-direct health effects on individuals excluded? | ||
| Are both costs and health effects discounted at an annual rate of 5%? | ||
| Do the estimates of resource use differ from that which would be expected in an Ontario context? | ||
| Is the value of health expressed in terms of quality-adjusted life-years (QALYs)? | ||
| Are changes in health-related quality of life (HRQOL) obtained directly from patients and/or carers? | ||
| Was the valuation of changes in HRQOL (utilities) obtained from a representative sample of the general public? | ||
| Overall judgment (directly applicable/partially applicable/not applicable): | ||
| If a study is considered not applicable, there is no need to assess its quality. | ||
Appendix 5: Applicability of Studies in Economic Evidence Review
Table A7:
Applicability of Studies Given Full-Text Review
| Objective: To assess the cost-effectiveness of LNG-IUS | |||||
|---|---|---|---|---|---|
| Author, Year | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system in which the study was conducted sufficiently similar to the current Ontario context? | Was/were the perspective(s) clearly stated and what were they? | Are estimates of relative treatment effect from the best available source? |
| Bhattacharya et al, 201163 | Yes | Yes (LNG-IUS vs. surgical treatment) | No (United Kingdom) | Yes (public health care payer in secondary care) | Yes |
| Heliovaara-Peippo et al, 201378 | Yes | Partly (LNG-IUS vs. hysterectomy) | No (Finland) | No (likely societal) | Yes |
| Calaf et al, 2015120 | Yes | Yes (LNG-IUS vs. oral treatment) | No (Spain) | Yes (public health care payer) | Yes |
| You et al, 2006116 | Yes | Yes | No (Hong Kong) | Yes (public health care payer) | Yes |
| Sanghera et al, 2014121 | Yes | Yes (LNG-IUS vs. oral treatment) | No (United Kingdom) | Yes (public health care payer) | Yes |
| Lete et al, 2011122 | Yes | Yes (LNG-IUS vs. oral treatment) | No (Spain) | Yes (public health care payer) | Yes |
| Ganz et al, 2013115 | Yes | Yes | No (United States) | Yes (public health care payer) | Yes |
| Clegg et al, 2007118 | Yes | Yes (LNG-IUS vs. surgical treatment) | No (United Kingdom) | Yes (public health care payer) | Yes |
| Brown et al, 2006119 | Yes | Partly (LNG-IUS vs. thermal balloon endometrial ablation) | No (New Zealand) | No (likely societal) | Yes |
| Bhattacharya et al, 201163 | Yes (3.5%) | Yes | No | Partially applicable | |
| Heliovaara-Peippo et al, 201378 | Yes (3% base case, 5% sensitivity) | Yes | No | Partially applicable | |
| Calaf et al, 2015120 | Yes (3%) | Yes | No | Partially applicable | |
| You et al, 2006116 | Yes (3%) | Yes | No | Partially applicable | |
| Sanghera et al, 2014121 | Yes (3.5%) | Yes | No | Partially applicable | |
| Lete et al, 2011122 | Yes (3%) | Yes | No | Partially applicable | |
| Ganz et al, 2013115 | Yes (3%) | Yes | No | Partially applicable | |
| Clegg et al, 2007118 | Yes (3.5%) | Yes | No | Partially applicable | |
| Brown et al, 2006119 | Yes (5%) | No | No | Partially applicable | |
Appendix 6: Full Economic Model Inputs
Table A8:
Adverse Event Inputs Used in the Economic Model
| Model Parameters | Probability Reported in Study (Time Frame) | Converted Monthly Rate | Monthly Probability | Duration of Risk in the Model | Reference |
|---|---|---|---|---|---|
| LNG-IUS | |||||
| Device reinsertion | 0.04310 (1 year) | 0.0036 | 0.0037 | 1 year | Hurskainen et al, 200179 |
| Gastrointestinal obstruction | 0.1026 (10 years) | 0.0009 | 0.0009 | 9 years | Heliovaara-Peippo et al, 201378 |
| Lower abdominal pain | 0.1667 (5 years) | 0.0015 | 0.0015 | 9 years | Malak and Shawki, 200687 |
| Pelvic pain | 0.1 (5 years) | 0.0009 | 0.0009 | 9 years | Malak and Shawki, 200687 |
| Pelvic inflammatory disease | 0.0504 /0.0336 (10 years) | 0.0004 /0.0003 | 0.0004 /0.0003 | 9 years | Heliovaara-Peippo et al, 201378 |
| Endometrial ablation | |||||
| Hematometra | 0.033 (5 years) | 0.0003 | 0.0003 | 9 years | Malak and Shawki, 200687 |
| Pelvic pain | 0.1333 (5 years) | 0.0012 | 0.0012 | 9 years | Malak and Shawki, 200687 |
| Hysterectomy | |||||
| Gastrointestinal obstruction | 0.15179 (10 years) | 0.00137 | 0.0014 | 10 years | Heliovaara-Peippo et al, 201378 |
| Lower abdominal pain | 0.00893 (10 years) | 0.00007 | 0.00007 | 10 years | Heliovaara-Peippo et al, 201378 |
| Secondary hemorrhage | 0.01786 (10 years) | 0.00015 | 0.00015 | 10 years | Heliovaara-Peippo et al, 201378 |
| Urinary retention | 0.02678 (10 years) | 0.00023 | 0.00023 | 10 years | Heliovaara-Peippo et al, 201378 |
| Surgical infection | 0.3125 (1 year) | 0.0031 | 0.0031 | 1 year | Hurskainen et al, 200179 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A9:
Adverse Event Inputs for Secondary Analysis Using Administrative Data
| Model Parameters | Monthly Probability | Duration of Risk in the Model |
|---|---|---|
| Initial treatment LNG-IUS | ||
| Device reinsertion | 0.0020 | 1 year |
| Gastrointestinal obstruction | 0.00002 | 9 years |
| Lower abdominal pain | 0.0004 | 9 years |
| Pelvic pain | 0.00003 | 9 years |
| Pelvic inflammatory disease | 0.00007 | 9 years |
| Switch to endometrial ablation | ||
| Hematometra | 0.00003 | 9 years |
| Pelvic pain | 0.000003 | 9 years |
| Switch to hysterectomy | ||
| Gastrointestinal obstruction | 0 | 9 years |
| Lower abdominal pain | 0.00006 | 9 years |
| Secondary hemorrhage | 0 | 9 years |
| Urinary retention | 0 | 9 years |
| Surgical infection | 0.0042 | 1 year |
| Initial treatment endometrial ablation | ||
| Hematometra | 0.00003 | 9 years |
| Pelvic pain | 0.0005 | 9 years |
| Switch to another endometrial ablation | ||
| Hematometra | 0.000003 | 9 years |
| Pelvic pain | 0.00002 | 9 years |
| Switch to hysterectomy | ||
| Gastrointestinal obstruction | 0.000004 | 9 years |
| Lower abdominal pain | 0.00008 | 9 years |
| Secondary hemorrhage | 0.0000006 | 9 years |
| Urinary retention | 0.0000006 | 9 years |
| Surgical infection | 0.0042 | 1 year |
| Initial treatment hysterectomy | ||
| Gastrointestinal obstruction | 0.00004 | 9 years |
| Lower abdominal pain | 0.0008 | 9 years |
| Secondary hemorrhage | 0 | 9 years |
| Urinary retention | 0.00007 | 9 years |
| Surgical infection | 0.0042 | 1 year |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A10:
Treatment Switch Inputs Used in the Economic Model
| Model Parameters | Probability Reported in Study (Time Frame) | Converted Rate | Monthly Probability | Reference |
|---|---|---|---|---|
| LNG-IUS to endometrial ablation | ||||
| First 6 months | 0.0769 | 0.0133 | 0.0053 | Heliovaara-Peippo et al, 201378 |
| 7–12 months | 0.1563 | 0.0283 | 0.0112 | Heliovaara-Peippo et al, 201378 |
| 2–5 years | 0.3133 | 0.0078 | 0.0031 | Heliovaara-Peippo et al, 201378 |
| 6–10 years | 0.1020 | 0.0018 | 0.0007 | Heliovaara-Peippo et al, 201378 |
| LNG-IUS to hysterectomy | ||||
| First 6 months | 0.0769 | 0.0133 | 0.0080 | Heliovaara-Peippo et al,201378 |
| 7–12 months | 0.1563 | 0.0283 | 0.0168 | Heliovaara-Peippo et al, 201378 |
| 2–5 years | 0.3133 | 0.0078 | 0.0047 | Heliovaara-Peippo et al, 201378 |
| 6–10 years | 0.1020 | 0.0018 | 0.0011 | Heliovaara-Peippo et al, 201378 |
| Endometrial ablation to hysterectomy | ||||
| 1st year | 0.1 (1 year) | 0.0088 | 0.0088 | Silva-Filho et al, 201383 |
| 2nd year | 0.0370 (1 year) | 0.0031 | 0.0031 | Silva-Filho et al, 201383 |
| 3rd year | 0.0769 (1 year) | 0.0067 | 0.0066 | Silva-Filho et al, 201383 |
| 4th year | 0 | 0 | 0 | Silva-Filho et al, 201383 |
| 5th year | 0 | 0 | 0 | Silva-Filho et al, 201383 |
| 6th −13th years | 0 | 0 | 0 | Extrapolation |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A11:
Distribution of Pregnancy Outcomes Used in the Economic Model
| Model Parameters | Probability | Reference |
|---|---|---|
| Pregnancy post LNG-IUS | ||
| Spontaneous abortion – complete | 0.13 | Yin, 2010,129 assuming half of spontaneous abortions are complete |
| Spontaneous abortion – incomplete | 0.13 | Yin, 2010,129 assuming half of spontaneous abortions are incomplete |
| Ectopic pregnancy | 0.07 | Yin, 2010129 |
| Induced abortion | 0.67 | Assumption |
| Pregnancy post endometrial ablation | ||
| Spontaneous abortion – complete | 0.0025 | Blumenthal et al, 2006,112 assuming half of spontaneous abortions are complete |
| Spontaneous abortion – incomplete | 0.0025 | Blumenthal et al, 2006,112 assuming half of spontaneous abortions are complete |
| Ectopic pregnancy | 0.60 | Blumenthal et al, 2006112 |
| Induced abortion | 0.39 | Assumption |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A12:
Other Clinical Outcome Inputs Used in the Economic Model
| Model Parameters | Probability Reported in Study (Time Frame) | Converted Rate | Monthly Probability | Duration of Risk in the Model | Reference |
|---|---|---|---|---|---|
| Menorrhagia resolved; no further treatment | 0.1538 (10 years) | 0.0014 | 0.0014 | 10 years | Heliovaara-Peippo et al, 201378 |
| LNG-IUS risk for pregnancy | 0.006 (1 year) | 0.0005 | 0.0005 | 10 years | Mansour et al, 2010127 |
| Endometrial ablation risk for pregnancy | 0.007 (1 year) | 0.0006 | 0.0006 | 10 years | Lo and Pickersgill, 2006128 |
| Mortality (assuming base case initial age of 42) | |||||
| 1 year | 0.00094 | 0.00008 | 0.00008 | Life table 2006130 | |
| 2 years | 0.00102 | 0.00009 | 0.00009 | Life table 2006130 | |
| 3 years | 0.00111 | 0.00009 | 0.00009 | Life table 2006130 | |
| 4 years | 0.00122 | 0.00010 | 0.00010 | Life table 2006130 | |
| 5 years | 0.00133 | 0.00011 | 0.00011 | Life table 2006130 | |
| 6 years | 0.00145 | 0.00012 | 0.00012 | Life table 2006130 | |
| 7 years | 0.00158 | 0.00013 | 0.00013 | Life table 2006130 | |
| 8 years | 0.00173 | 0.00014 | 0.00014 | Life table 2006130 | |
| 9 years | 0.00188 | 0.00016 | 0.00016 | Life table 2006130 | |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A13:
Procedure Costs for Physician Services for LNG-IUS, Endometrial Ablation, and Hysterectomy
| Variable | Fee Code | Unit Cost, $ | Total Units/Procedure | Total Cost, $ | Reference |
|---|---|---|---|---|---|
| LNG-IUS | |||||
| Insertion of intrauterine contraceptive device | G378 | 25.50 | NA | 25.50 | Ontario Schedule of Benefits for Physician Services132 |
| When performed outside hospital | E542 | 11.15 | NA | 11.15 | Ontario Schedule of Benefits for Physician Services132 |
| Total | 36.65 | ||||
| Endometrial ablation | |||||
| Surgeon | S772 | 218.65 | NA | 218.65 | Ontario Schedule of Benefits for Physician Services132 |
| Anesthesiologist | S772 | 15.01 | 6 | 90.06 | Ontario Schedule of Benefits for Physician Services132 |
| Total | 308.71 | ||||
| Hysterectomy | |||||
| Surgeon | S757 | 463.00 | NA | 463.00 | Ontario Schedule of Benefits for Physician Services132 |
| Assistant surgeon | S757 | 12.04 | 6 | 72.24 | Ontario Schedule of Benefits for Physician Services132 |
| Anesthesiologist | S757 | 15.01 | 7 | 105.07 | Ontario Schedule of Benefits for Physician Services132 |
| Total | 640.31 | ||||
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system; NA not applicable.
Table A14:
Treatment-Related Adverse Event Costs for LNG-IUS, Endometrial Ablation, and Hysterectomy
| Variable | Unit Cost, $ (Standard Deviation) | Reference |
|---|---|---|
| LNG-IUS–related adverse events | ||
| Reinsertion | ||
| Procedure | 337.90 | Ontario Drug Benefit Formulary133 |
| Physician | 36.65 | Ontario Schedule of Benefits for Physician Services132 (code G378 and E542) |
| Gastrointestinal obstruction (assuming 2-day mean length of stay according to administrative data) | ||
| Hospital | 4,156.00 (2,615) | Administrative dataa |
| Admission assessment | 100.36 | Ontario Schedule of Benefits for Physician Services132 (code C003) |
| General surgery consultation | 90.30 | Ontario Schedule of Benefits for Physician Services132 (code A035) |
| Physician discharge assessment | 58.80 | Ontario Schedule of Benefits for Physician Services132 (code C124) |
| Lower abdominal pain (assuming most are outpatient visits according to administrative data) | ||
| Hospital | 492.00 (222) | Administrative dataa |
| Physician assessment | 77.20 | Ontario Schedule of Benefits for Physician Services132 (code C003) |
| Pelvic pain (assuming 2-day mean length of stay according to administrative data) | ||
| Hospital | 4,211.00 (1,063) | Administrative dataa |
| Admission assessment | 100.36 | Ontario Schedule of Benefits for Physician Services132 (code C003, E082) |
| Most responsible physician follow-up assessment – second day | 58.80 | Ontario Schedule of Benefits for Physician Services132 (code C122) |
| Physician discharge assessment | 58.80 | Ontario Schedule of Benefits for Physician Services132 (code C124) |
| Pelvic inflammatory disease (assuming most are outpatient visits according to administrative data) | ||
| Hospital | 503.00 (704) | Administrative dataa |
| Physician assessment | 77.20 | Ontario Schedule of Benefits for Physician Services132 (code C003) |
| Endometrial ablation–related adverse events | ||
| Hematometra (assuming 3-day mean length of stay according to administrative data) | ||
| Hospital | 3,854.00 (3,859) | Administrative dataa |
| Admission assessment | 100.36 | Ontario Schedule of Benefits for Physician Services132 (code C003) |
| General surgery consultation | 90.30 | Ontario Schedule of Benefits for Physician Services132 (code A035) |
| Most responsible physician follow-up assessment – second day | 58.80 | Ontario Schedule of Benefits for Physician Services132 (code C122) |
| Physician discharge assessment | 58.80 | Ontario Schedule of Benefits for Physician Services132 (code C124) |
| Pelvic pain (assuming most are outpatient visits according to administrative data) | ||
| Hospital | 1,372.00 (1,818) | Administrative dataa |
| Physician assessment | 77.20 | Ontario Schedule of Benefits for Physician Services132 (code C003) |
| Hysterectomy-related adverse events | ||
| Gastrointestinal obstruction (assuming 5-day mean length of stay according to administrative data) | ||
| Hospital | 3,823.00 (2,269) | Administrative dataa |
| Admission assessment | 100.36 | Ontario Schedule of Benefits for Physician Services132 (code C003) |
| Most responsible physician follow-up assessment – second day | 58.80 | Ontario Schedule of Benefits for Physician Services132 (code C122) |
| Most responsible physician follow-up assessment – third day | 58.80 | Ontario Schedule of Benefits for Physician Services132 (code C123) |
| Physician follow-up assessment – subsequent visit | 31.00 | Ontario Schedule of Benefits for Physician Services132 (code C002) |
| General surgery consultation | 90.30 | Ontario Schedule of Benefits for Physician Services132 (code A035) |
| Physician discharge assessment | 58.80 | Ontario Schedule of Benefits for Physician Services132 (code C124) |
| Lower abdominal pain (assuming most are outpatient visits according to administrative data) | ||
| Hospital | 390.00 (246) | Administrative dataa |
| Physician assessment | 77.20 | Ontario Schedule of Benefits for Physician Services132 (code C003) |
| Secondary hemorrhage (assuming 2-day mean length of stay according to administrative data) | ||
| Hospital | 1,878.00 (646) | Administrative dataa |
| Admission assessment | 100.36 | Ontario Schedule of Benefits for Physician Services132 (code C003) |
| Physician discharge assessment | $58.80 | Ontario Schedule of Benefits for Physician Services132 (code C124) |
| Urinary retention | ||
| Hospital | 549.00 (683.82) | Administrative dataa |
| Physician assessment | 77.20 | Ontario Schedule of Benefits for Physician Services132 (code C003) |
| Surgical infection | ||
| Hospital | 758.90 (1,093.67) | Administrative dataa |
| Physician assessment | 77.20 | Ontario Schedule of Benefits for Physician Services132 (code C003) |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Data provided by Ontario IntelliHEALTH.
Appendix 7: Administrative Data Sources for the Economic Model Population and Treatment-Related Adverse Events
The total number of women receiving LNG-IUS for heavy menstrual bleeding in Ontario was calculated from the OHIP physician billing database, using procedure fee code G378.
Women receiving endometrial ablation or hysterectomy were identified from the Discharge Abstract Database and the National Ambulatory Care Reporting System database (Table A15).
Table A15:
Administrative Data Codes Used to Identify Endometrial Ablation or Hysterectomy for Heavy Menstrual Bleeding
| Procedure Code | Diagnosis Code | |
|---|---|---|
| Endometrial ablation for heavy menstrual bleeding | 1.RM.59 | N920 |
| N921 | ||
| N924 | ||
| N925 | ||
| N926 | ||
| N938 | ||
| N939 | ||
| Hysterectomy for heavy menstrual bleeding | 1.RM.87 | N920 |
| 1.RM.89 | N921 | |
| N924 | ||
| N925 | ||
| N926 | ||
| N938 | ||
| N939 |
To ensure that only women with idiopathic heavy menstrual bleeding were included, we used the diagnosis codes shown in Table A16 to exclude from the Discharge Abstract Database women with a diagnosis of malignant neoplasms, carcinoma, fibroids, endometriosis, prolapse, or polyps of the uterus or cervix.
Table A16:
Administrative Data Codes Used to Exclude Non-idiopathic Heavy Menstrual Bleeding
| Diagnosis Code | |
|---|---|
| Malignant neoplasms | All C codes |
| Carcinoma | D05.9-D07.3 |
| Fibroids | D25 |
| Endometriosis | N80 |
| Prolapse | N81 |
| Polyps of the uterus or cervix | N84.0 and N84.1 |
Treatment-related adverse events were identified among the LNG-IUS, endometrial ablation, and hysterectomy cohorts by finding inpatient hospitalizations in the Discharge Abstract Database where the most responsible diagnosis was related to the adverse events identified in the clinical review. The specific diagnosis codes are presented in Table A17.
Table A17:
Diagnosis Codes Used to Identify Adverse Events Related to LNG-IUS, Endometrial Ablation, and Hysterectomy
| Diagnosis Code | |
|---|---|
| Gastrointestinal obstruction | K56.6 |
| Lower abdominal pain | R10.39 |
| Pelvic pain | R10.2 |
| Pelvic inflammatory disease | N73.8–N73.9 |
| Hematometra | N85.7 |
| Secondary hemorrhage | T81.0 |
| Urinary retention | R33 |
| Surgical infection | T81.4 |
Appendix 8: Budget Impact Analysis Inputs
The estimated number of women who could be expected to receive LNG-IUS regardless of public funding was calculated from administrative data. We used physician billing data for 2008 to 2012 to extract a cohort of individuals who received an IUD insertion procedure (fee code G378)132 with a diagnosis code for menstrual dysfunction. Since the proportion of women with menstrual dysfunction who specifically have menorrhagia is unknown, we assumed this to be 25% based on epidemiological data.136 Cohort projections were calculated using linear extrapolation in Microsoft Excel (Table A18).
Table A18:
Projected Incident Volumes of LNG-IUS Without Public Funding
| Volumes from Administrative Dataa | Projected Volumes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Women receiving LNG-IUS insertion procedure with diagnosis of menstrual dysfunction | 1,735 | 2,083 | 2,499 | 3,168 | 3,443 | 3,938 | 4,423 | 4,880 | 5,292 | 5,787 |
| Women receiving LNG-IUS for heavy menstrual bleeding (assuming 25% of menstrual dysfunction cohort) | 433 | 520 | 625 | 792 | 861 | 984 | 1,106 | 1,220 | 1,323 | 1,447 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Data provided by Ontario IntelliHEALTH.
The estimated number of women who could be expected to need a replacement procedure for the LNG-IUS because the device has reached the end of its life was calculated from the volumes of women receiving LNG-IUS for heavy menstrual bleeding 5 years earlier. We assumed that 70% women would remain on LNG-IUS after 5 years, as calculated in our primary economic model (Table A19).
Table A19:
Projected Incident Volumes of LNG-IUS Reinsertions
| Volumes per Year | |||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Women receiving LNG-IUS insertion procedure for heavy menstrual bleeding, 2008–2012 | 433 | 520 | 625 | 792 | 861 |
| Women remaining on LNG-IUS 5 years later and requiring a reinsertion | 304 | 365 | 439 | 556 | 605 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
The estimated number of women who could be expected to receive a hysterectomy or endometrial ablation for heavy menstrual bleeding was calculated from administrative data. Cohort projections were calculated using linear extrapolation in Microsoft Excel. The results are presented in Tables A20 and Table A21. Detailed calculations for the different scenarios are shown in Table A22, and Tables A23 to A34 provide detailed results for each scenario in the budget impact analysis.
Table A20:
Projected Incident Volumes of Hysterectomy for Heavy Menstrual Bleeding
| Volumes from Administrative Dataa | Projected Volumes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Women receiving hysterectomy for heavy menstrual bleeding | 6,161 | 5,989 | 5,835 | 5,596 | 5,391 | 5,215 | 5,021 | 4,828 | 4,635 | 4,441 |
Data provided by Ontario IntelliHEALTH.
Table A21:
Projected Incident Volumes of Endometrial Ablation for Heavy Menstrual Bleeding
| Volumes from Administrative Dataa | Projected Volumes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Women receiving endometrial ablation for heavy menstrual bleeding | 6,021 | 5,984 | 6,002 | 6,122 | 6,158 | 6,181 | 6,222 | 6,263 | 6,305 | 6,346 |
Data provided by Ontario IntelliHEALTH.
Table A22:
Detailed Calculations of Patient Volumes for Different Scenarios
| Strategy | Example Calculation | Incidence, N | ||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | ||
| Hysterectomy | ||||||
| Scenario 1 (upper limit): Women who would have received hysterectomy if LNG-IUS were not publicly funded (includes all women receiving hysterectomy for heavy menstrual bleeding) | Projected volumes based on administrative data | 5,215 | 5,021 | 4,828 | 4,635 | 4,441 |
| Scenario 2: Women who would have received hysterectomy if LNG-IUS were not publicly funded (assuming 12% of women offered LNG-IUS would prefer hysterectomy) | 4,763 × 0.88 = 4,191 (Scenario 1 volumes multiplied by 88%) |
4,191 | 4,045 | 3,900 | 3,754 | 3,608 |
| Scenario 3: Women who would have received hysterectomy if LNG-IUS were not publicly funded (assuming all with unsupportive pathology for hysterectomy [12%] did not have private health insurance coverage) | 4,763 × 0.12 = 572 (Scenario 1 volumes multiplied by 12%) |
572 | 552 | 532 | 512 | 492 |
| Scenario 4 (lower limit): Women who would have received hysterectomy if LNG-IUS were not publicly funded (assuming 50% with unsupportive pathology for hysterectomy did not have private health insurance coverage) | 4,763 × 0.12 × 0.5 = 286 (Scenario 1 volumes multiplied by 12% multiplied by 50%) |
286 | 276 | 266 | 256 | 246 |
| Endometrial Ablation | ||||||
| Scenario 1 (upper limit): Women who would have received endometrial ablation if LNG-IUS were not publicly funded (includes all women receiving endometrial ablation) | Projected volumes based on administrative data | 6,181 | 6,222 | 6,263 | 6,305 | 6,346 |
| Scenario 2 (lower limit): Women who would have received endometrial ablation if LNG-IUS were not publicly funded (assuming 22% of women offered LNG-IUS would prefer endometrial ablation) | 6,181 × 0.88 = 4,838 (Scenario 1 volumes multiplied by 88%) |
4,821 | 4,853 | 4,885 | 4,918 | 4,950 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A23:
Detailed Results of Budget Impact Analysis, LNG-IUS vs. Hysterectomy, Scenario 1
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | −23.1 | 2.0 | 1.1 | 1.8 | 1.7 |
| Year 2 cohort | −22.1 | 2.0 | 1.1 | 1.8 | |
| Year 3 cohort | −21.2 | 1.9 | 1.0 | ||
| Year 4 cohort | −20.2 | 1.8 | |||
| Year 5 cohort | −19.3 | ||||
| Total | −23.1 | −20.1 | −18.1 | −15.4 | −13.0 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A24:
Detailed Results of Budget Impact Analysis, LNG-IUS vs. Hysterectomy, Scenario 2
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | −18.4 | 1.6 | 0.9 | 1.5 | 1.3 |
| Year 2 cohort | −17.7 | 1.6 | 0.8 | 1.4 | |
| Year 3 cohort | −17.0 | 1.5 | 0.8 | ||
| Year 4 cohort | −16.3 | 1.5 | |||
| Year 5 cohort | −15.6 | ||||
| Total | −18.5 | −16.1 | −14.5 | −12.4 | −10.5 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A25:
Detailed Results of Budget Impact Analysis, LNG-IUS vs. Hysterectomy, Scenario 3
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | −2.1 | 0.2 | 0.1 | 0.2 | 0.2 |
| Year 2 cohort | −2.0 | 0.2 | 0.1 | 0.2 | |
| Year 3 cohort | −1.8 | 0.2 | 0.1 | ||
| Year 4 cohort | −1.7 | 0.2 | |||
| Year 5 cohort | −1.5 | ||||
| Total | −2.1 | −1.8 | −1.5 | −1.1 | −0.8 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A26:
Detailed Results of Budget Impact Analysis, LNG-IUS vs. Hysterectomy, Scenario 4
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | −0.8 | 0.1 | 0.06 | 0.1 | 0.09 |
| Year 2 cohort | −0.7 | 0.1 | 0.06 | 0.1 | |
| Year 3 cohort | −0.6 | 0.1 | 0.06 | ||
| Year 4 cohort | −0.5 | 0.1 | |||
| Year 5 cohort | −0.4 | ||||
| Total | −0.8 | −0.6 | −0.5 | −0.2 | −0.06 |
Abbreviation: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A27:
Detailed Results of Budget Impact Analysis, LNG-IUS vs. Endometrial Ablation, Scenario 1
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | −8.5 | 0.6 | −0.2 | 1.6 | 2.3 |
| Year 2 cohort | −8.5 | 0.6 | −0.2 | 1.6 | |
| Year 3 cohort | −8.6 | 0.6 | −0.2 | ||
| Year 4 cohort | −8.6 | 0.7 | |||
| Year 5 cohort | −8.6 | ||||
| Total | −8.5 | −7.9 | −8.1 | −6.5 | −4.2 |
Abbreviation: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A28:
Detailed Results of Budget Impact Analysis, LNG-IUS vs. Endometrial Ablation, Scenario 2
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | −6.6 | 0.5 | −0.2 | 1.2 | 1.8 |
| Year 2 cohort | −6.6 | 0.5 | −0.2 | 1.2 | |
| Year 3 cohort | −6.6 | 0.5 | −0.2 | ||
| Year 4 cohort | 6.6 | 0.5 | |||
| Year 5 cohort | −6.6 | ||||
| Total | −6.6 | −6.1 | −6.2 | −5.0 | −3.2 |
Abbreviation: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A29:
Detailed Results, New Annual Cost of Funding LNG-IUS vs. Hysterectomy, Scenario 1
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | 2.2 | 1.2 | |||
| Year 2 cohort | 2.2 | ||||
| Year 3 cohort | 2.2 | ||||
| Year 4 cohort | 2.2 | ||||
| Year 5 cohort | 2.2 | ||||
| Total | 2.2 | 2.2 | 2.2 | 2.2 | 3.4 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A30:
Detailed Results, New Annual Cost of Funding LNG-IUS vs. Hysterectomy, Scenario 2
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | 1.9 | 1.0 | |||
| Year 2 cohort | 1.8 | ||||
| Year 3 cohort | 1.7 | ||||
| Year 4 cohort | 1.5 | ||||
| Year 5 cohort | 1.0 | ||||
| Total | 1.9 | 1.8 | 1.7 | 1.5 | 2.0 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A31:
Detailed Results, New Annual Cost of Funding LNG-IUS vs. Hysterectomy, Scenario 3
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | 0.6 | 0.1 | |||
| Year 2 cohort | 0.7 | ||||
| Year 3 cohort | 0.7 | ||||
| Year 4 cohort | 0.8 | ||||
| Year 5 cohort | 0.8 | ||||
| Total | 0.6 | 0.7 | 0.7 | 0.8 | 0.9 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A32:
Detailed Results, New Annual Cost of Funding LNG-IUS vs. Hysterectomy, Scenario 4
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | 0.5 | 0.07 | |||
| Year 2 cohort | 0.6 | ||||
| Year 3 cohort | 0.7 | ||||
| Year 4 cohort | 0.7 | ||||
| Year 5 cohort | 0.8 | ||||
| Total | 0.5 | $0.6 | 0.7 | 0.7 | 0.8 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A33:
Detailed Results, New Annual Cost of Funding LNG-IUS vs. Endometrial Ablation, Scenario 1
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | 2.5 | 1.5 | |||
| Year 2 cohort | 2.1 | ||||
| Year 3 cohort | 2.2 | ||||
| Year 4 cohort | 2.2 | ||||
| Year 5 cohort | 2.3 | ||||
| Total | 2.5 | 2.6 | 2.6 | 2.7 | 4.2 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Table A34:
Detailed Results, New Annual Cost of Funding LNG-IUS vs. Endometrial Ablation, Scenario 2
| Year | $ million | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Year 1 cohort | 2.1 | 1.1 | |||
| Year 2 cohort | 2.1 | ||||
| Year 3 cohort | 2.2 | ||||
| Year 4 cohort | 2.2 | ||||
| Year 5 cohort | 2.2 | ||||
| Total | 2.1 | 2.1 | 2.2 | 2.2 | 3.4 |
Abbreviations: LNG-IUS, levonorgestrel-releasing intrauterine system.
Author contributions
This report was developed by a multi-disciplinary team from Health Quality Ontario. The lead clinical epidemiologist was Alexis Schaink, the lead health economist was Brian Chan, the medical librarian was Caroline Higgins.
KEY MESSAGES
Up to one-third of women experience heavy menstrual bleeding, which can be painful, distressing, and disruptive to daily life. Often there is no specific cause, but treatment can make a woman's periods more manageable or even eliminate symptoms. Options include medications, various types of surgery, and a device called the levonorgestrel-releasing intrauterine system (LNG-IUS) that is inserted into the uterus and releases medication. This health technology assessment looked at how effective and cost-effective the LNG-IUS is for treating heavy menstrual bleeding.
We found that the LNG-IUS works well and costs less compared with other options to treat heavy menstrual bleeding. Women using the LNG-IUS said their quality of life improved and their periods were lighter. Although some women experienced mild side effects, women seemed to be generally satisfied with the treatment.
We also found that funding the LNG-IUS to treat heavy menstrual bleeding would result in cost savings to the Ontario health care system. Currently, the insertion procedure is publicly funded but the cost of the device is not, while several other treatment options are publicly funded in their entirety.
Contributor Information
Health Quality Ontario:
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are.
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do.
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches.
Why It Matters.
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
REFERENCES
- (1).Abu Hashim H. Medical treatment of idiopathic heavy menstrual bleeding. What is new? An evidence based approach. Arch Gynecol Obstet. 2013;287(2): 251–60. [DOI] [PubMed] [Google Scholar]
- (2).National Collaborating Centre for Women's and Children's Health. Heavy menstrual bleeding [Internet]. London: National Institute for Health and Care Excellence; 2007. [cited 2015 Jul 1]. Available from: https://www.nice.org.uk/guidance/cg44/evidence/cg44-heavy-menstrual-bleeding-full-guideline2 [Google Scholar]
- (3).Singh S, Best C, Dunn S, Leyland N, Wolfman WL, Clinical Practice - Gynaecology Committee, et al. Abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can. 2013;35(5): 473–9. [DOI] [PubMed] [Google Scholar]
- (4).Copher R, Le Nestour E, Law A, Pocoski J, Zampaglione E. Retrospective analysis of variation in heavy menstrual bleeding treatments by age and underlying cause. Curr Med Res Opin. 2013;29(2): 127–39. [DOI] [PubMed] [Google Scholar]
- (5).Cole SK, Billewicz WZ, Thomson AM. Sources of variation in menstrual blood loss. J Obstet Gynaecol Br Commonw. 1971;78(10): 933–9. [DOI] [PubMed] [Google Scholar]
- (6).Hallberg L, Hogdahl AM, Nilsson L, Rybo G. Menstrual blood loss–a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand. 1966;45(3): 320–51. [DOI] [PubMed] [Google Scholar]
- (7).Beatty MN, Blumenthal PD. The levonorgestrel-releasing intrauterine system: safety, efficacy, and patient acceptability. Ther Clin Risk Manag. 2009;5(3): 561–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Matteson KA, Clark MA. Questioning our questions: do frequently asked questions adequately cover the aspects of women's lives most affected by abnormal uterine bleeding? Opinions of women with abnormal uterine bleeding participating in focus group discussions. Women Health. 2010;50(2): 195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Duckitt K, Collins S. Menorrhagia. BMJ Clin Evid. 2012;2012(01): 1–65. [PMC free article] [PubMed] [Google Scholar]
- (10).Greenberg M. The meaning of menorrhagia: an investigation into the association between the complaint of menorrhagia and depression. J Psychosom Res. 1983;27(3): 209–14. [DOI] [PubMed] [Google Scholar]
- (11).Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99(2): 229–34. [DOI] [PubMed] [Google Scholar]
- (12).Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3): 183–94. [DOI] [PubMed] [Google Scholar]
- (13).Peuranpaa P, Heliovaara-Peippo S, Fraser I, Paavonen J, Hurskainen R. Effects of anemia and iron deficiency on quality of life in women with heavy menstrual bleeding. Acta Obstet Gynecol Scand. 2014;93(7): 654–60. [DOI] [PubMed] [Google Scholar]
- (14).Higham JM, O'Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97(8): 734–9. [DOI] [PubMed] [Google Scholar]
- (15).Zakherah MS, Sayed GH, El-Nashar SA, Shaaban MM. Pictorial blood loss assessment chart in the evaluation of heavy menstrual bleeding: diagnostic accuracy compared to alkaline hematin. Gynecol Obstet Invest. 2011;71(4): 281–4. [DOI] [PubMed] [Google Scholar]
- (16).Reid PC, Coker A, Coltart R. Assessment of menstrual blood loss using a pictorial chart: a validation study. BJOG. 2000;107(3): 320–2. [DOI] [PubMed] [Google Scholar]
- (17).Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010; 2: CD003855. [DOI] [PubMed] [Google Scholar]
- (18).McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14(4): 399–406. [DOI] [PubMed] [Google Scholar]
- (19).Medical Advisory Secretariat. Thermal balloon endometrial ablation for dysfunctional uterine bleeding: an evidence-based analysis. Ont Health Technol Assess Ser. 2004;4(11): 1–89. [PMC free article] [PubMed] [Google Scholar]
- (20).Wishall KM, Price J, Pereira N, Butts SM, Della Badia CR. Postablation risk factors for pain and subsequent hysterectomy. Obstet Gynecol. 2014;124(5): 904–10. [DOI] [PubMed] [Google Scholar]
- (21).Stankiewicz A, Pogany L, Popadiuk C. Prevalence of self-reported hysterectomy among Canadian women, 2000/2001–2008. Chronic Dis Inj Can. 2014;34(1): 30–5. [PubMed] [Google Scholar]
- (22).Kives S, Lefebvre G, Wolfman W, Leyland N, Allaire C, Awadalla A, et al. Supracervical hysterectomy. J Obstet Gynaecol Can. 2010;32(1): 62–8. [DOI] [PubMed] [Google Scholar]
- (23).Ghomi A, Hantes J, Lotze EC. Incidence of cyclical bleeding after laparoscopic supracervical hysterectomy. J Minim Invasive Gynecol. 2005;12(3): 201–5. [DOI] [PubMed] [Google Scholar]
- (24).Lieng M, Qvigstad E, Istre O, Langebrekke A, Ballard K. Long-term outcomes following laparoscopic supracervical hysterectomy. BJOG. 2008;115(13): 1605–10. [DOI] [PubMed] [Google Scholar]
- (25).Lethaby A, Hussain M, Rishworth JR, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015; 4: CD002126. [DOI] [PubMed] [Google Scholar]
- (26).Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet Gynecol. 2013;121(3): 654–73. [DOI] [PubMed] [Google Scholar]
- (27).Kennedy AD, Sculpher MJ, Coulter A, Dwyer N, Rees M, Abrams KR, et al. Effects of decision aids for menorrhagia on treatment choices, health outcomes, and costs: a randomized controlled trial. JAMA. 2002;288(21): 2701–8. [DOI] [PubMed] [Google Scholar]
- (28).Vuorma S, Rissanen P, Aalto AM, Kujansuu E, Hurskainen R, Teperi J. Factors predicting choice of treatment for menorrhagia in gynaecology outpatient clinics. Soc Sci Med. 2003;56(8): 1653–60. [DOI] [PubMed] [Google Scholar]
- (29).Bourdrez P, Bongers MY, Mol BW. Treatment of dysfunctional uterine bleeding: patient preferences for endometrial ablation, a levonorgestrel-releasing intrauterine device, or hysterectomy. Fertil Steril. 2004;82(1): 160–6. [DOI] [PubMed] [Google Scholar]
- (30).Hauck B, Costescu D. Barriers and misperceptions limiting widespread use of intrauterine contraception among Canadian women. J Obstet Gynaecol Can. 2015;37(7): 606–16. [DOI] [PubMed] [Google Scholar]
- (31).Garside R, Britten N, Stein K. The experience of heavy menstrual bleeding: a systematic review and meta-ethnography of qualitative studies. J Adv Nurs. 2008;63(6): 550–62. [DOI] [PubMed] [Google Scholar]
- (32).Nelson AL. Levonorgestrel intrauterine system: a first-line medical treatment for heavy menstrual bleeding. Womens Health (Lond Engl). 2010;6(3): 347–56. [DOI] [PubMed] [Google Scholar]
- (33).Bayer Inc. Product monograph: Mirena. Submission Control No.: 166699 ed. Mississauga (ON): Bayer; 2014. [Google Scholar]
- (34).Qiu J, Cheng J, Wang Q, Hua J. Levonorgestrel-releasing intrauterine system versus medical therapy for menorrhagia: a systematic review and meta-analysis. Med Sci Monit. 2014; 20: 1700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kaunitz AM, Meredith S, Inki P, Kubba A, Sanchez-Ramos L. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113(5): 1104–16. [DOI] [PubMed] [Google Scholar]
- (36).Xiao B, Wu SC, Chong J, Zeng T, Han LH, Luukkainen T. Therapeutic effects of the levonorgestrel-releasing intrauterine system in the treatment of idiopathic menorrhagia. Fertil Steril. 2003;79(4): 963–9. [DOI] [PubMed] [Google Scholar]
- (37).Milsom I. The levonorgestrel-releasing intrauterine system as an alternative to hysterectomy in peri-menopausal women. Contraception. 2007; 75(6 Suppl): S152–4. [DOI] [PubMed] [Google Scholar]
- (38).Goni AZ, Lacruz RL, Paricio JJ, Hernandez Rivas FJ. The levonorgestrel intrauterine system as an alternative to hysterectomy for the treatment of idiopathic menorrhagia. Gynecol Endocrinol. 2009;25(9): 581–6. [DOI] [PubMed] [Google Scholar]
- (39).Soysal M, Soysal S, Ozer S. A randomized controlled trial of levonorgestrel releasing IUD and thermal balloon ablation in the treatment of menorrhagia. Zentralbl Gynakol. 2002;124(4): 213–9. [DOI] [PubMed] [Google Scholar]
- (40).Sayed GH, Zakherah MS, El-Nashar SA, Shaaban MM. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. Int J Gynaecol Obstet. 2011;112(2): 126–30. [DOI] [PubMed] [Google Scholar]
- (41).Ozdegirmenci O, Kayikcioglu F, Akgul MA, Kaplan M, Karcaaltincaba M, Haberal A, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95(2): 497–502. [DOI] [PubMed] [Google Scholar]
- (42).Kilic S, Yuksel B, Doganay M, Bardakci H, Akinsu F, Uzunlar O, et al. The effect of levonorgestrel-releasing intrauterine device on menorrhagia in women taking anticoagulant medication after cardiac valve replacement. Contraception. 2009;80(2): 152–7. [DOI] [PubMed] [Google Scholar]
- (43).Van Houdenhoven K, van Kaam KJ, van Grootheest AC, Salemans TH, Dunselman GA. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73(3): 257–60. [DOI] [PubMed] [Google Scholar]
- (44).Heinemann K, Reed S, Moehner S, Minh TD. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91(4): 274–9. [DOI] [PubMed] [Google Scholar]
- (45).Andersson K, Ryde-Blomqvist E, Lindell K, Odlind V, Milsom I. Perforations with intrauterine devices. Report from a Swedish survey. Contraception. 1998;57(4): 251–5. [DOI] [PubMed] [Google Scholar]
- (46).Caliskan E, Ozturk N, Dilbaz BO, Dilbaz S. Analysis of risk factors associated with uterine perforation by intrauterine devices. Eur J Contracept Reprod Health Care. 2003;8(3): 150–5. [PubMed] [Google Scholar]
- (47).Veldhuis HM, Vos AG, Lagro-Janssen AL. Complications of the intrauterine device in nulliparous and parous women. Eur J Gen Pract. 2004;10(3): 82–7. [DOI] [PubMed] [Google Scholar]
- (48).Prager S, Darney PD. The levonorgestrel intrauterine system in nulliparous women. Contraception. 2007; 75(6 Suppl): S12–5. [DOI] [PubMed] [Google Scholar]
- (49).Soini T, Hurskainen R, Grenman S, Maenpaa J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 Pt 1): 292–9. [DOI] [PubMed] [Google Scholar]
- (50).Inki P, Hurskainen R, Palo P, Ekholm E, Grenman S, Kivela A, et al. Comparison of ovarian cyst formation in women using the levonorgestrel-releasing intrauterine system vs. hysterectomy. Ultrasound Obstet Gynecol. 2002;20(4): 381–5. [DOI] [PubMed] [Google Scholar]
- (51).Laberge P, Leyland N, Murji A, Fortin C, Martyn P, Vilos G, et al. Endometrial ablation in the management of abnormal uterine bleeding. J Obstet Gynaecol Can. 2015;37(4): 362–76. [DOI] [PubMed] [Google Scholar]
- (52).Kirkham YA, Ornstein MP, Aggarwal A, McQuillan S, Canpago C, Allen L, et al. Menstrual suppression in special circumstances. J Obstet Gynaecol Can. 2014;36(10): 915–26. [DOI] [PubMed] [Google Scholar]
- (53).University of Texas at Austin, School of Nursing, Family Nurse Practitioner Program. Evaluation and management of ovulatory heavy menstrual bleeding (HMB) in primary care. Austin (TX): University of Texas at Austin, School of Nursing; 2012. Mar. 18 p. [Google Scholar]
- (54).Society of Obstetricians and Gynaecologists of Canada. Best practices to minimize risk of infection with intrauterine device insertion. No. 305 [Internet]. Ottawa (ON): The Society; 2014. [cited 2016 Jan 20]. Available from: http://sogc.org/guidelines/best-practices-minimize-risk-infection-intrauterine-device-insertion/ [Google Scholar]
- (55).Health Canada. Non-insured health benefits: First Nations and Inuit Health Branch: drug benefit list [Internet]. Ottawa (ON): Health Canada; 2014. [cited 2016 Jan 20]. Available from: http://www.hc-sc.gc.ca/fniah-spnia/nihb-ssna/provide-fournir/pharmaprod/med-list/index-eng.php [Google Scholar]
- (56).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4): 380–2. [DOI] [PubMed] [Google Scholar]
- (57).Bitzer J, Heikinheimo O, Nelson AL, Calaf-Alsina J, Fraser IS. Medical management of heavy menstrual bleeding: a comprehensive review of the literature. Obstet Gynecol Surv. 2015;70(2): 115–30. [DOI] [PubMed] [Google Scholar]
- (58).Uhm S, Perriera L. Hormonal contraception as treatment for heavy menstrual bleeding: a systematic review. Clin Obstet Gynecol. 2014;57(4): 694–717. [DOI] [PubMed] [Google Scholar]
- (59).Hartmann KE, Jerome RN, Lindegren ML, Potter SA, Shields TC, Surawicz TS, et al. Primary care management of abnormal uterine bleeding [Internet]. Rockville (MD): Agency for Healthcare Research and Quality; 2013. [cited 2015 Jul 1]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23617013 [PubMed] [Google Scholar]
- (60).Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013; 1: CD000400. [DOI] [PubMed] [Google Scholar]
- (61).Matteson KA, Abed H, Wheeler TL, 2nd, Sung VW, Rahn DD, Schaffer JI, et al. A systematic review comparing hysterectomy with less-invasive treatments for abnormal uterine bleeding. J Minim Invasive Gynecol. 2012;19(1): 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72(2): 193–215. [DOI] [PubMed] [Google Scholar]
- (63).Bhattacharya S, Middleton LJ, Tsourapas A, Lee AJ, Champaneria R, Daniels JP, et al. Hysterectomy, endometrial ablation and Mirena(R) for heavy menstrual bleeding: a systematic review of clinical effectiveness and cost-effectiveness analysis. Health Technol Assess. 2011; 15(19):iii–xvi, 1–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Middleton LJ, Champaneria R, Daniels JP, Bhattacharya S, Cooper KG, Hilken NH, et al. Hysterectomy, endometrial destruction, and levonorgestrel releasing intrauterine system (Mirena) for heavy menstrual bleeding: systematic review and meta-analysis of data from individual patients. BMJ. 2010; 341: c3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Blumenthal PD, Dawson L, Hurskainen R. Cost-effectiveness and quality of life associated with heavy menstrual bleeding among women using the levonorgestrel-releasing intrauterine system. Int J Gynaecol Obstet. 2011;112(3): 171–8. [DOI] [PubMed] [Google Scholar]
- (66).Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2008(1): CD001016. [DOI] [PubMed]
- (67).Iavazzo C, Salakos N, Bakalianou K, Vitoratos N, Vorgias G, Liapis A. Thermal balloon endometrial ablation: a systematic review. Arch Gynecol Obstet. 2008;277(2): 99–108. [DOI] [PubMed] [Google Scholar]
- (68).Varma R, Sinha D, Gupta JK. Non-contraceptive uses of levonorgestrel-releasing hormone system (LNG-IUS)–a systematic enquiry and overview. Eur J Obstet Gynecol Reprod Biol. 2006;125(1): 9–28. [DOI] [PubMed] [Google Scholar]
- (69).Stewart A, Cummins C, Gold L, Jordan R, Phillips W. The effectiveness of the levonorgestrel-releasing intrauterine system in menorrhagia: a systematic review. BJOG. 2001;108(1): 74–86. [DOI] [PubMed] [Google Scholar]
- (70).Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Cameron IT, Leask R, Kelly RW, Baird DT. The effects of danazol, mefenamic acid, norethisterone and a progesterone-impregnated coil on endometrial prostaglandin concentrations in women with menorrhagia. Prostaglandins. 1987;34(1): 99–110. [DOI] [PubMed] [Google Scholar]
- (72).Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164(3): 879–83. [DOI] [PubMed] [Google Scholar]
- (73).Ergun B, Kuru O, Sen S, Kilic Y, Bastu E. Roller-ball endometrial ablation versus levonorgestrel releasing intrauterine system in the management of abnormal uterine bleeding. Ginecoro. 2012;8(4): 199–201. [Google Scholar]
- (74).Lahteenmaki P, Haukkamaa M, Puolakka J, Riikonen U, Sainio S, Suvisaari J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ. 1998;316(7138): 1122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Endrikat J, Shapiro H, Lukkari-Lax E, Kunz M, Schmidt W, Fortier M. A Canadian, multicentre study comparing the efficacy of a levonorgestrel-releasing intrauterine system to an oral contraceptive in women with idiopathic menorrhagia. J Obstet Gynaecol Can. 2009;31(4): 340–7. [DOI] [PubMed] [Google Scholar]
- (76).Kucuk T, Ertan K. Continuous oral or intramuscular medroxyprogesterone acetate versus the levonorgestrel releasing intrauterine system in the treatment of perimenopausal menorrhagia: a randomized, prospective, controlled clinical trial in female smokers. Clin Exp Obstet Gynecol. 2008;35(1): 57–60. [PubMed] [Google Scholar]
- (77).Sesti F, Piancatelli R, Pietropolli A, Ruggeri V, Piccione E. Levonorgestrel-releasing intrauterine system versus laparoscopic supracervical hysterectomy for the treatment of heavy menstrual bleeding: a randomized study. J Womens Health (Larchmt). 2012;21(8): 851–7. [DOI] [PubMed] [Google Scholar]
- (78).Heliovaara-Peippo S, Hurskainen R, Teperi J, Aalto AM, Grenman S, Halmesmaki K, et al. Quality of life and costs of levonorgestrel-releasing intrauterine system or hysterectomy in the treatment of menorrhagia: a 10-year randomized controlled trial. Am J Obstet Gynecol. 2013; 209(6): 535.e1–e14. [DOI] [PubMed] [Google Scholar]
- (79).Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivela A, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357(9252): 273–7. [DOI] [PubMed] [Google Scholar]
- (80).Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivela A, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291(12): 1456–63. [DOI] [PubMed] [Google Scholar]
- (81).Ghazizadeh S, Bakhtiari F, Rahmanpour H, Davari-Tanha F, Ramezanzadeh F. A randomized clinical trial to compare levonorgestrel-releasing intrauterine system (Mirena) vs transcervical endometrial resection for treatment of menorrhagia. Int J Womens Health. 2011; 3: 207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).de Souza SS, Camargos AF, de Rezende CP, Pereira FA, Araujo CA, Silva Filho AL. A randomized prospective trial comparing the levonorgestrel-releasing intrauterine system with thermal balloon ablation for the treatment of heavy menstrual bleeding. Contraception. 2010;81(3): 226–31. [DOI] [PubMed] [Google Scholar]
- (83).Silva-Filho AL, Pereira Fde A, de Souza SS, Loures LF, Rocha AP, Valadares CN, et al. Five-year follow-up of levonorgestrel-releasing intrauterine system versus thermal balloon ablation for the treatment of heavy menstrual bleeding: a randomized controlled trial. Contraception. 2013;87(4): 409–15. [DOI] [PubMed] [Google Scholar]
- (84).Shaw RW, Symonds IM, Tamizian O, Chaplain J, Mukhopadhyay S. Randomised comparative trial of thermal balloon ablation and levonorgestrel intrauterine system in patients with idiopathic menorrhagia. Aust N Z J Obstet Gynaecol. 2007;47(4): 335–40. [DOI] [PubMed] [Google Scholar]
- (85).Tam WH, Yuen PM, Shan Ng DP, Leung PL, Lok IH, Rogers MS. Health status function after treatment with thermal balloon endometrial ablation and levonorgestrel intrauterine system for idiopathic menorrhagia: a randomized study. Gynecol Obstet Invest. 2006;62(2): 84–8. [DOI] [PubMed] [Google Scholar]
- (86).Busfield RA, Farquhar CM, Sowter MC, Lethaby A, Sprecher M, Yu Y, et al. A randomised trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113(3): 257–63. [DOI] [PubMed] [Google Scholar]
- (87).Abdel Malak K, Shawki O. Management of menorrhagia with the levonorgestrel intrauterine system versus endometrial resection. Gynecol Surg. 2006;3(4): 275–80. [Google Scholar]
- (88).Barrington JW, Arunkalaivanan AS, Abdel-Fattah M. Comparison between the levonorgestrel intrauterine system (LNG-IUS) and thermal balloon ablation in the treatment of menorrhagia. Eur J Obstet Gynecol Reprod Biol. 2003;108(1): 72–4. [DOI] [PubMed] [Google Scholar]
- (89).Istre O, Trolle B. Treatment of menorrhagia with the levonorgestrel intrauterine system versus endometrial resection. Fertil Steril. 2001;76(2): 304–9. [DOI] [PubMed] [Google Scholar]
- (90).Kittelsen N, Istre O. A randomized study comparing the levonorgestrel intrauterine system (LNG IUS) and transcervical resection of the endometrium (TCRE) in the treatment of menorrhagia: preliminary results. Gynaecol Endosc. 1998;7(2): 61–5. [Google Scholar]
- (91).Rauramo I, Elo I, Istre O. Long-term treatment of menorrhagia with levonorgestrel intrauterine system versus endometrial resection. Obstet Gynecol. 2004;104(6): 1314–21. [DOI] [PubMed] [Google Scholar]
- (92).Crosignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, De Giorgi O. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstet Gynecol. 1997;90(2): 257–63. [DOI] [PubMed] [Google Scholar]
- (93).Gupta J, Kai J, Middleton L, Pattison H, Gray R, Daniels J, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med. 2013;368(2): 128–37. [DOI] [PubMed] [Google Scholar]
- (94).Gupta JK, Daniels JP, Middleton LJ, Pattison HM, Prileszky G, Roberts TE, et al. A randomised controlled trial of the clinical effectiveness and cost-effectiveness of the levonorgestrel-releasing intrauterine system in primary care against standard treatment for menorrhagia: the ECLIPSE trial. Health Technol Assess. 2015;19(88): 1–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Shaaban MM, Zakherah MS, El-Nashar SA, Sayed GH. Levonorgestrel-releasing intrauterine system compared to low dose combined oral contraceptive pills for idiopathic menorrhagia: a randomized clinical trial. Contraception. 2011;83(1): 48–54. [DOI] [PubMed] [Google Scholar]
- (96).Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, DeSanctis Y, Jensen J. Levonorgestrel-releasing intrauterine system for heavy menstrual bleeding improves hemoglobin and ferritin levels. Contraception. 2012;86(5): 452–7. [DOI] [PubMed] [Google Scholar]
- (97).Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116(3): 625–32. [DOI] [PubMed] [Google Scholar]
- (98).Reid PC, Virtanen-Kari S. Randomised comparative trial of the levonorgestrel intrauterine system and mefenamic acid for the treatment of idiopathic menorrhagia: a multiple analysis using total menstrual fluid loss, menstrual blood loss and pictorial blood loss assessment charts. BJOG. 2005;112(8): 1121–5. [DOI] [PubMed] [Google Scholar]
- (99).Irvine GA, Campbell-Brown MB, Lumsden MA, Heikkila A, Walker JJ, Cameron IT. Randomised comparative trial of the levonorgestrel intrauterine system and norethisterone for treatment of idiopathic menorrhagia. Br J Obstet Gynaecol. 1998;105(6): 592–8. [DOI] [PubMed] [Google Scholar]
- (100).Herman MC, van den Brink MJ, Geomini PM, van Meurs HS, Huirne JA, Eising HP, et al. Levonorgestrel releasing intrauterine system (Mirena) versus endometrial ablation (Novasure) in women with heavy menstrual bleeding: a multicentre randomised controlled trial. BMC Womens Health. 2013; 13: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6): 473–83. [PubMed] [Google Scholar]
- (102).How to use EQ-5D [Internet]. Rotterdam, NL: EuroQol Research Foundation; c2016. [updated n.r.; cited 2015 Oct 28]. Available from: http://www.euroqol.org/about-eq-5d/how-to-use-eq-5d.html [Google Scholar]
- (103).Ohinmaa A, Sintonen H. Inconsistencies and modelling of the Finnish EuroQol (EQ-5D) preference values. In: Gainer W, Graf v d. Schulenburg J, Piercy J, eds. Discussion Papers, EuroQol, Plenary Meeting; 1998. Oct 1–2; Hannover, Germany. Hannover, Germany: Center of Health Economics and Health System Research, University of Hannover, 1998: 57–74. [Google Scholar]
- (104).EuroQol Group. EuroQol—a new facility for the treatment of health-related quality of life. Health Policy. 1990:199–208. [DOI] [PubMed]
- (105).CDC HRQOL–14 “Healthy Days Measure” [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; c2016. [updated 2011 Mar 15; cited 2015 Oct 28]. Available from: http://www.cdc.gov/hrqol/hrqol14_measure.htm#1 [Google Scholar]
- (106).Centers for Diease Control and Prevention. Measuring healthy days: population assessment of health-related quality of life. Atlanta (GA): Centers for Disease Control and Prevention; 2000. Nov. [Google Scholar]
- (107).Chassany O, Dimenäs E, Dubois D, Wu A, Dupuy H. The Psychological General Well-Being Index (PGWBI) user manual. Lyon, France: MAPI Research Institute; 2004. [Google Scholar]
- (108).Shaw RW, Brickley MR, Evans L, Edwards MJ. Perceptions of women on the impact of menorrhagia on their health using multi-attribute utility assessment. Br J Obstet Gynaecol. 1998;105(11): 1155–9. [DOI] [PubMed] [Google Scholar]
- (109).Pattison H, Daniels JP, Kai J, Gupta JK. The measurement properties of the menorrhagia multi-attribute quality-of-life scale: a psychometric analysis. BJOG. 2011;118(12): 1528–31. [DOI] [PubMed] [Google Scholar]
- (110).Shields M, Tjepkema M. Regional differences in obesity. Health Rep. 2006;17(3): 61–7. [PubMed] [Google Scholar]
- (111).National Institute for Health and Care Excellence. Process and methods guides. The guidelines manual: appendix G: methodology checklist [Internet]. London (UK): National Institute for Health and Care Excellence; 2012. [cited 2015 May 27]. Available from: http://publications.nice.org.uk/the-guidelines-manual-appendices-bi-pmg6b/appendix-g-methodology-checklist-economic-evaluations [Google Scholar]
- (112).Blumenthal PD, Trussell J, Singh RH, Guo A, Borenstein J, Dubois RW, et al. Cost-effectiveness of treatments for dysfunctional uterine bleeding in women who need contraception. Contraception. 2006;74(3): 249–58. [DOI] [PubMed] [Google Scholar]
- (113).Hurskaienen R, Teperi J, Rissanen P. A levonorgestrel releasing intrauterine system was more cost effective than was hysterectomy for menorrhagia. Evid Based Med. 2001; 6(4): 127. [Google Scholar]
- (114).Roberts TE, Tsourapas A, Middleton LJ, Champaneria R, Daniels JP, Cooper KG, et al. Hysterectomy, endometrial ablation, and levonorgestrel releasing intrauterine system (Mirena) for treatment of heavy menstrual bleeding: cost effectiveness analysis. BMJ. 2011; 342: d2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Ganz ML, Shah D, Gidwani R, Filonenko A, Su W, Pocoski J, et al. The cost-effectiveness of the levonorgestrel-releasing intrauterine system for the treatment of idiopathic heavy menstrual bleeding in the United States. Value Health. 2013;16(2): 325–33. [DOI] [PubMed] [Google Scholar]
- (116).You JHS, Sahota DS, MoYuen P. A cost-utility analysis of hysterectomy, endometrial resection and ablation and medical therapy for menorrhagia. Hum Reprod. 2006;21(7): 1878–83. [DOI] [PubMed] [Google Scholar]
- (117).Bhattacharya S, Middleton LJ, Tsourapas A, Lee AJ, Champaneria R, Daniels JP, et al. Hysterectomy, endometrial ablation and Mirena for heavy menstrual bleeding: a systematic review of clinical effectiveness and cost-effectiveness analysis. Health Technol Assess. 2011;15(19): 1–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Clegg JP, Guest JF, Hurskainen R. Cost-utility of levonorgestrel intrauterine system compared with hysterectomy and second generation endometrial ablative techniques in managing patients with menorrhagia in the UK. Curr Med Res Opin. 2007;23(7): 1637–48. [DOI] [PubMed] [Google Scholar]
- (119).Brown PM, Farquhar CM, Lethaby A, Sadler LC, Johnson NP. Cost-effectiveness analysis of levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113(7): 797–803. [DOI] [PubMed] [Google Scholar]
- (120).Calaf J, Lete I, Canals I, Crespo C, Espinos B, Cristobal I. Cost-effectiveness analysis in the treatment of heavy menstrual bleeding in Spain. Eur J Obstet Gynecol Reprod Biol. 2015; 184: 24–31. [DOI] [PubMed] [Google Scholar]
- (121).Sanghera S, Roberts TE, Barton P, Frew E, Daniels J, Middleton L, et al. Levonorgestrel-releasing intrauterine system vs. usual medical treatment for menorrhagia: an economic evaluation alongside a randomised controlled trial. PLoS One; 2014; 9(3): e91891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Lete I, Cristobal I, Febrer L, Crespo C, Arbat A, Hernandez FJ, et al. Economic evaluation of the levonorgestrel-releasing intrauterine system for the treatment of dysfunctional uterine bleeding in Spain. Eur J Obstet Gynecol Reprod Biol. 2011;154(1): 71–80. [DOI] [PubMed] [Google Scholar]
- (123).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013; 16(2): e1–5. [DOI] [PubMed] [Google Scholar]
- (124).Ontario Ministry of Health and Long-Term Care. Ontario wait times: surgical and diagnostic imaging wait times [Internet]. 2015. [cited 2016 Jan 21]. Available from: http://www.health.gov.on.ca/en/pro/programs/waittimes/surgery/default.aspx
- (125).National Health Service. Hysterectomy - recovery [Internet]. 2014. [cited 2016 May 02]. Available from: http://www.nhs.uk/Conditions/Hysterectomy/Pages/Recovery.aspx
- (126).WebMD. Hysterectomy recovery: what can you expect? [Internet]. 2016. [cited 2016 May 02]. Available from: http://www.webmd.com/women/guide/hysterectomy-recovery
- (127).Mansour D, Inki P, Gemzell-Danielsson K. Efficacy of contraceptive methods: a review of the literature. Eur J Contracept Reprod Health Care. 2010; 15 Suppl 2:S19–31. [DOI] [PubMed] [Google Scholar]
- (128).Lo JS, Pickersgill A. Pregnancy after endometrial ablation: English literature review and case report. J Minim Invasive Gynecol. 2006;13(2): 88–91. [DOI] [PubMed] [Google Scholar]
- (129).Yin CS. Pregnancy after hysteroscopic endometrial ablation without endometrial preparation: a report of five cases and a literature review. Taiwan J Obstet Gynecol. 2010;49(3): 311–9. [DOI] [PubMed] [Google Scholar]
- (130).Statistics Canada. Life Tables, Canada, Provinces and Territories, 2009 to 2011. Ottawa (ON): Statistics Canada; 2015. [Google Scholar]
- (131).Sculpher M. A cost-utility analysis of abdominal hysterectomy versus transcervical endometrial resection for the surgical treatment of menorrhagia. Int J Technol Assess Health Care. 1998;14(2): 302–19. [DOI] [PubMed] [Google Scholar]
- (132).Ontario Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto (ON): Ontario Ministry of Health and Long-Term Care; 2015. [cited 2015 Jul 28]. Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/sob_master11062015.pdf [Google Scholar]
- (133).Ontario Ministry of Health and Long-Term Care. Ontario drug benefit formulary: formulary search [Internet]. Toronto, ON: Queen's Printer for Ontario; 2015. [cited 2015 Jul 28]. Available from: https://www.healthinfo.moh.gov.on.ca/formulary/ [Google Scholar]
- (134).Bayer HealthCare Pharmaceuticals Inc. Mirena(R) IUD [Internet]. 2015. [cited 2015 May 02]. Available from: http://www.mirena-us.com/index.php
- (135).Corona LE, Swenson CW, Sheetz KH, Shelby G, Berger MB, Pearlman MD, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015; 212(3): 304 e1–7. [DOI] [PubMed] [Google Scholar]
- (136).Shapley M, Jordan K, Croft PR. An epidemiological survey of symptoms of menstrual loss in the community. Br J Gen Pract. 2004;54(502): 359–63. [PMC free article] [PubMed] [Google Scholar]