Abstract
Indoleamine 2,3-dioxygenase (IDO) is a rate limiting enzyme in tryptophan-degrading pathways and IDO activity results in immune suppression. Targeting IDO is a strategy of cancer immunotherapies. Our previous studies demonstrate that delivery of short hairpin against IDO (IDO shRNA) suppresses tumor growth and increases neutrophils infiltration into tumor. Neutrophils reveal antitumorigenic “N1” or protumorigenic “N2” phenotype in tumor microenvironment. However, the function of IDO shRNA-induced neutrophils is not clear. The LLC1 lung cancer model was used to investigate the role of these neutrophils. Intramuscular injection of IDO shRNA or IDO inhibitor treatment delayed tumor growth and both treatments increased neutrophil infiltration in tumor. Enriched tumor-infiltrating neutrophils expressed both high level of tumor necrosis factor-α and tumor necrosis factor-β (N1 and N2 associated molecules, respectively). In addition, IDO shRNA treatment induced interferon-γ and tryptophan transfer RNA expression in splenocytes. Systematic depletion of neutrophils abolished the IDO shRNA-induced therapeutic effect but did not affect the effect of IDO inhibitor. The levels of interferon-γ and tumor necrosis factor-α were suppressed in IDO shRNA treatment splenocytes after neutrophils depletion. In conclusion, these tumor-infiltrating neutrophils show antitumorigenic phenotype in spleen after IDO shRNA treatment in a murine lung cancer model.
Introduction
Indoleamine 2,3-dioxygenase (IDO) is one of rate limiting enzymes in tryptophan catabolite pathway and converts tryptophan to kynurenine.1 The enzyme activity causes tryptophan depletion and kynurenine accumulation and inhibits effector T cells through activating the kinase general control nonderessible 2 pathway.2 Accumulation of tryptophan metabolites binding to acyl hydrogen receptor results in differentiating naive CD4+ T cell into regulatory T cells.3 In addition, IDO1-expressing plasmacytoid dendritic cells cause expansion of regulatory T cells in tumor draining lymph nodes.4 Targeting IDO1 has been a strategy of cancer immunotherapy.5 The IDO inhibitors such as D-1-methyl-tryptophan (D-1MT) and INCB024360 are in phase 1/2 clinical trials for evaluating the efficiency against multiple types of cancers.6 In addition, combination of IDO inhibitor and programmed death-ligand 1, programmed death-receptor 1, and cytotoxic T-lymphocyte associated protein 4 blockage further enhances T cell activation and therapeutic efficiency in animal tumor models.7,8
Delivery of short hairpin RNA against IDO (IDO shRNA) is another strategy to induced host antitumor immune responses. Systematic delivery of Salmonella typhimurium transformed IDO shRNA suppresses tumor growth in a murine melanoma model.9 Silencing IDO is associated with tumor infiltration of polymorphonuclear neutrophils which enhances production of reactive oxygen species (ROS) after IDO shRNA treatment.9 Our previous study, demonstrated that delivery of IDO shRNA through low pressure gene gun delays tumor growth in bladder, colon, and liver tumor models and enhances the efficiency of Her2/neu DNA vaccine.10,11 We also observed massive Gr-1+ cells (including Ly6G+ and Ly6C+ cells) infiltration into tumor after IDO shRNA treatment.10 It suggests that gene gun administration of IDO shRNA may increase neutrophils infiltration in tumor because Ly6G and Ly6C are respectively a marker of neutrophil and monocyte myeloid lineage.12 However, the role of these cells is not determined.
Tumor-associated neutrophils usually play a protumorigenic role (“N2” neutrophils. Transforming growth factor-β (TGF-β) phenotype). However, neutrophils reveal antitumorigenic role (“N1” neutrophils. It produces higher level of tumor necrosis factor-α (TNF-α), ROS, and nitrogen oxide) in certain situation.13,14 Tumor microenvironment is a critical factor to affect neutrophil polarization.13 The N2 cells promote angiogenesis and metastasis.15,16 In addition, N2 neutrophils suppress function of effector cells in tumor microenvironment.17 Increasing ratio of tumor-infiltrating polymorphonuclear cells to plasmacytic cells is associated with poor overall survival in several solid tumors and these polymorphonuclear cells are considered as granulocytic myeloid-derived suppressor cells (G-MDSC) which are characterized in CD11b+Gr-1+ phenotype.18,19 Although neutrophil phenotype (CD11b+Gr-1+) are the same, the function and some characteristics are different from G-MDSC. Compared with MDSC, neutrophils do not express CD244, produce lower level of ROS and higher level of TNF-α and express different transcription patterns from G-MDSC.20,21 Depletion N1 neutrophils increases tumor volume in murine mesothelioma model.13 Tumor entrained neutrophils inhibit premetastasic lung cancer.22 Another report indicates that tumor associated neutrophils shows N1 phenotype in early state of tumor progression.23 These evidences shows that neutrophil is a double edge sword in tumor.
Systematic delivery of bacteria-transformed IDO shRNA induces ROS-producing polymorphonuclear neutrophils in tumor.9 We wondered whether N1 phenotype of tumor-infiltrating neutrophils could be observed after IDO shRNA treatment through other delivery routes. Since lung cancer is one of the most serious diseases,24 the hypothesis was investigated in a murine lung cancer model. The number and the phenotype of tumor-infiltrating neutrophils were determined. The potential role in cancer immunotherapy was evaluated.
Results
IDO shRNA and D-1MT treatment inhibited LLC1 tumor growth and induced tumor-infiltrating CD11b+Ly6G+ neutrophils
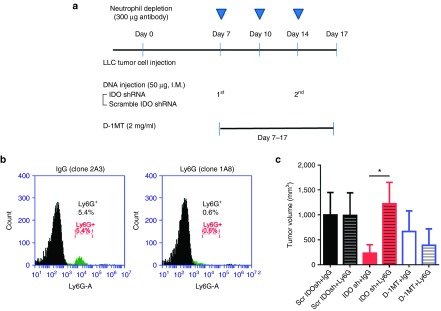
Our previous studies indicate skin delivery of IDO shRNA has an antitumor effect via low pressure gene gun bombardment on subcutaneous bladder and colon tumor and orthotopic liver tumor models.10,11 In addition, increasing number of tumor-infiltrating Gr-1+ (clone RB6-8C5) cells is observed after IDO shRNA treatment.10 However, the role of these neutrophils were not clear. In this study, IDO shRNA or scramble IDO shRNA was administrated through intramuscular injection. The other group was supplied with IDO inhibitor D-1MT (experimental design was shown in Figure 1a). The results showed that IDO shRNA and D-1MT treatment inhibited LLC1 tumor growth (Figure 1b). Although different delivery route (intramuscular injection in this study versus gene gun bombardment in our previous studies) were used, the number of tumor-infiltrated CD11b+Ly6G+ (clone 1A8) neutrophils significantly increased in IDO shRNA and D-1MT groups compared with scramble IDO shRNA group (Figure 1c). In contrast, the number of neutrophils in spleen were not significantly changed (Figure 1d).
Figure 1.
IDO shRNA and D-1MT treatment inhibits LLC1 tumor growth and causes increasing number of tumor-infiltrating neutrophils in a subcutaneous lung tumor model. (a) Experimental design. 2 × 105 LLC1 cells were subcutaneously injected into male inbred C57BL/6JNarl mice. Scramble IDO shRNA (Scr IDOsh), IDO shRNA (IDO sh), and 1-methyl-D-tryptophan (D-1MT) was treated on 7 days after LLC1 injection. (b) Tumor growth curve. *A statistically significant difference when compared with scramble IDO shRNA group on 16 days after LLC1 injection (P < 0.05). (c) Number of tumor-infiltrated CD11b+Ly6G+ neutrophils. *A statistically significant difference when compared with scramble IDO shRNA group (P < 0.05). (d) Number of CD11b+Ly6G+ neutrophils in spleen at 16 days after LLC1 injection. IDO, Indoleamine 2,3-dioxygenase; shRNA, short hairpin RNA.
Intramuscular injection of IDO shRNA induced Th1-bias immune responses
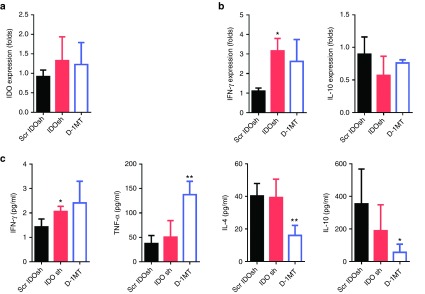
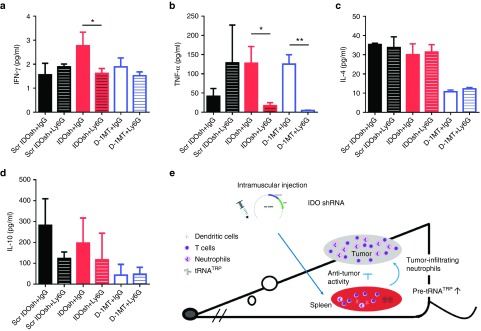
Our previous studies demonstrated that gene gun administration of IDO shRNA induced Th1-bias immune responses in splenocytes in an orthotopic liver and a subcutaneous bladder tumor models.11,25 After gene gun delivery of IDO shRNA, IDO expression in dendritic cells was suppressed. However, The IDO expression of total splenocytes was not altered.11 In this study, IDO expression of total splenocytes was not affected after intramuscular injection of IDO shRNA (Figure 2a). Because different routes of DNA plasmid delivery induced affects immune responses,26,27 the expression of Th1 and Th2 cytokines was analyzed. The elevated level of Th1 cytokine interferon-γ (IFN-γ) in IDO shRNA group and elevated level of tumor necrosis factor-α (TNF-α) in D-1MT group was observed (Figure 2b,c). In addition, D-1MT treatment suppressed the levels of Th2 cytokines interleukin (IL)-4 and IL-10. The patterns of cytokine expression were not identical between IDO shRNA and D-1MT treated groups.
Figure 2.
IDO shRNA and D-1MT treatment induced Th1-bias immune responses. Spleens were collected at 16 days after LLC1 injection. Splenocytes and culture medium were collected after 24 hours culture. (a) mRNA expression of IDO. (b) mRNA expression of IFN-γ and IL-10. (c) Cytokines concentration of IFN-γ, TNF-α, IL-4 and IL-10. * or **A statistically significant difference when compared with scramble IDO shRNA group (*P < 0.05; **P < 0.01). D-1MT, 1-methyl-D-tryptophan; IDO, Indoleamine 2,3-dioxygenase; IFN-γ, interferon- γ; IL-4, interlukein-4; shRNA, short hairpin RNA; TNF-α, tumor necrosis factor- α.
The frequency of regulatory T cells was not affected by intramuscular injection of IDO shRNA
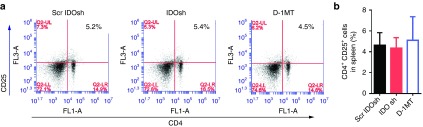
MDSC and regulatory T cells negatively regulate immune responses and attenuate function of effector T cells.28,29 In many previous studies, CD11b+Gr-1+ cells are considered as MDSC.19 IDO shRNA has been demonstrated to induce cytotoxic T activity and Th1 immune responses in spleen.10,11 Thus, the immune suppressive cells were determined in spleen. Our result revealed that the frequency of CD11b+Gr-1+ cells which were markers of granulocytic MDSC was not significantly changed in each group (Figure 1d). In addition, the frequency of CD4+CD25+ regulatory T cells was not affected after IDO shRNA and D-1MT treatment (Figure 3a,b). It suggested that these immune suppressive cells were not altered in spleen after each treatment.
Figure 3.
IDO shRNA and D-1MT did not significantly affect the CD4+CD25+ regulatory T cells. (a) Spleens were collected at 16 days after LLC1 injection and the population of CD4+CD25+ Treg cells were determined on a flow cytometry. Data are the representative of at least three independent experiments. (b) The quantitative results. D-1MT, 1-methyl-D-tryptophan; IDO, Indoleamine 2,3-dioxygenase; shRNA, short hairpin RNA.
IDO shRNA treatment enhanced expression of tryptophan transfer RNA in total splenocytes
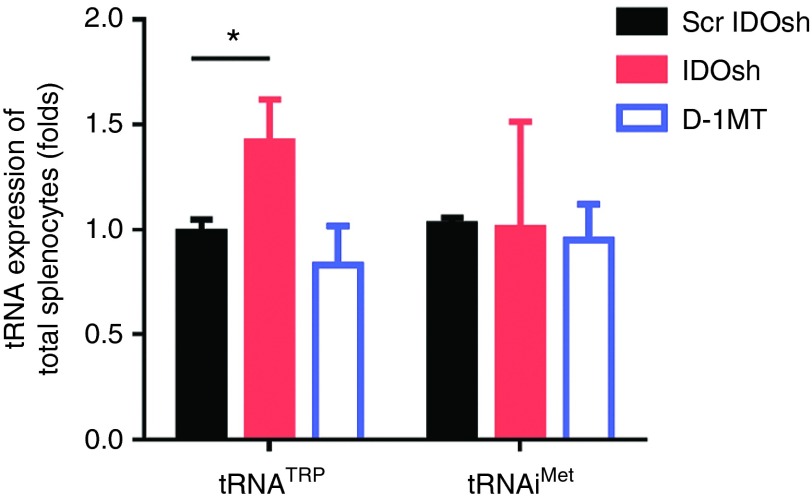
IDO over-activation leads to accumulating uncharged tryptophan transfer RNA (tRNATrp).2 It results in blocking translation and cell cycle arrest in T cells.2 There are generally 60–70% of CD8+ and CD4+ T cells in spleen.30 To determine whether tRNA was altered in T cells, tryptophan tRNA precursor (pre-tRNATrp) was determined in total splenocytes. It was interesting to note that only IDO shRNA treatment increased the expression level of pre-tRNATrp although IDO expression was not affected in total splenocytes after IDO shRNA and D-1MT treatment (Figure 4). The low level of initiator methionine transfer RNA (tRNAiMet) is associated with differentiating or arresting mammalian cells.31 However, similar expression levels of tRNAiMet was observed in all groups. Furthermore, the tRNATrp and tRNAiMet levels were not affected by IDO shRNA in tumor-infiltrating neutrophils (Supplementary Figure S1). It implied that tRNATrp involved in regulating IDO shRNA induced antitumor immune responses in spleen.
Figure 4.
The level of tryptophan transfer RNA was induced by IDO shRNA treatment in total splenocytes. Total splenocytes were collected at 16 days after LLC1 injection. The levels of tryptophan transfer RNA (tRNATrp) and initiator methionine transfer RNA (tRNAiMet) was measured. *A statistically significant difference when compared with scramble IDO shRNA group (*P < 0.05). IDO, Indoleamine 2,3-dioxygenase; shRNA, short hairpin RNA.
The phenotype of IDO shRNA-induced tumor-infiltrating CD11b+Ly6G+ neutrophils
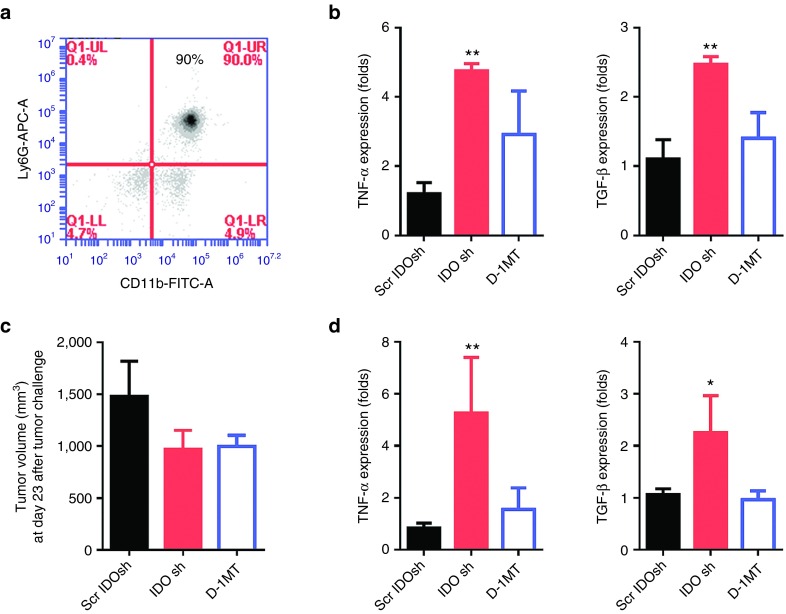
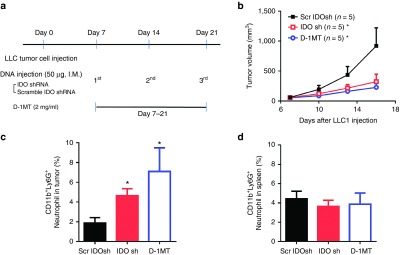
To determine the IDO shRNA induced-tumor-infiltrating neutrophils revealed antitumorigenic N1 phenotype or protumorigenic N2 phenotype, the cells were enriched by magnetic isolation (Figure 5a). The N1 cells are characterized by high TNF-α expression and N2 cells are associated with high TGF-β expression.14 In Figure 5b, significantly higher mRNA level of TNF-α and TGF-β was detected in IDO shRNA group at day 16 after LLC1 cells injection compared with scramble IDO shRNA group. Although the IDO shRNA and D-1MT treatment did not significantly suppress tumor growth at day 23 after LLC1 cells injection, similar mRNA expression pattern was detected (Figures 5c,d). The results could not indicate whether the neutrophils reveal antitumorigenic or protumorigenic phenotype.
Figure 5.
Tumor-infiltrating CD11b+Ly6G+ neutrophils expressed high level of TNF-α and TGF-β in IDO shRNA treated mice. (a) Enriched CD11b+Ly6G+ neutrophils from tumor-infiltrated cells. (b) The mRNA expression of tumor-infiltrating neutrophils which was collected on day 16 post LLC1 injection. (c) Tumor volume at day 23. (d) The mRNA expression of tumor-infiltrating neutrophils which was collected at day 23 post LLC1 injection. * or **A statistically significant difference when compared with scramble IDO shRNA group (*P < 0.05; **P < 0.01). IDO, Indoleamine 2,3-dioxygenase; shRNA, short hairpin RNA; TGF-β, Transforming growth factor-β. TNF-α, tumor necrosis factor-α.
Neutrophil depletion abolished IDO shRNA induced-Th1 immune responses and antitumor efficiency
In order to further confirm the role of neutrophil in our experimental model, depletion antibody (300 µg of anti-Ly6G, clone 1A8 or IgG control, clone 2A3) was administrated every 3 or 4 days (Figure 6a). Depletion efficiency was shown in Figure 6b. Neutrophil depletion abolished antitumor efficiency in IDO shRNA group but not in D-1MT group (Figure 6c). It indicated that neutrophils play an essential role in IDO shRNA-induced antitumor immune responses. In addition, the expression of IFN-γ and TNF-α was significantly suppressed in IDO shRNA group and the TNF-α level was suppressed in D-1MT group after neutrophil depletion (Figure 7a,b). In contrast, Th2 cytokines were not altered in all groups (Figure 7c,d).
Figure 6.
Neutrophils depletion abolished the antitumor effect of IDO shRNA. (a) Experimental design. 300 µg of anti-Ly6G antibody or IgG control was intraperitoneally injected at day 7, 10, and 14 after LLC1 cells injection. All mice were sacrificed at day 17. (b) Evaluation of depletion efficiency. The Ly6G+ cells were detected in total splenocytes after injection of depletion antibodies. (c) Tumor volume was measured at day 17. *A statistically significant difference between IgG and Ly6G groups (*P < 0.05). IDO, Indoleamine 2,3-dioxygenase; IgG, immunoglobulin G; shRNA, short hairpin RNA.
Figure 7.
Neutrophil depletion inhibit secretion of Th1 cytokines. Culture medium was collected after total splenocytes were cultured for 24 hours. The concentration of (a) IFN-γ, (b) TNF-α, (c) IL-4, and (d) IL-10 was measured. *A statistically significant difference between IgG and Ly6G groups (*P < 0.05). (e) A model is presented to illustrate the possible mechanism and role of neutrophils in IDO shRNA-induced antitumor immune responses. IDO, Indoleamine 2,3-dioxygenase; IgG, immunoglobulin G; IFN-γ, interferon- γ; IL-4, interlukein-4; shRNA, short hairpin RNA; TNF-α, tumor necrosis factor- α.
Discussion
We have demonstrated that low pressure gene gun delivered-IDO shRNA suppresses tumor growth in two subcutaneous tumor models and enhances therapeutic efficiency of Her2/neu DNA vaccine.10 In addition, it also delayed orthotopic liver tumor growth.11 Because low pressure gene gun efficiently deliver plasmid into skin dendritic which migrate to inguinal lymph nodes or spleen,32 skin delivery of IDO shRNA suppresses expression in CD11C+ dendritic cells in tumor draining lymph nodes and plasmatocytoid dendritic cells in spleen but did not affect IDO expression in total cells of lymph node, spleen, and tumor.10,11,25 Thus, we suppose that the levels of tryptophan metabolites are not changed in spleen and acyl hydrogen receptor pathway might not involve in IDO shRNA-mediated immune responses. In this study, IDO shRNA was delivered by intramuscular injection. IDO shRNA treatment still delayed tumor growth and induced Th1 cytokine IFN-γ in early stage after tumor cells injection. Similar immune responses were observed since different delivery routes were used. Intramuscular injection of DNA vaccine recruits mature dendritc cells in muscle and these dendritic cells are thought to present antigens of DNA vaccine.33 However, IDO shRNA plasmid did not express any specific tumor antigen in this study. The IDO shRNA uptake-dendritic cells trafficking to secondary lymphoid organs and then activating antitumor immune responses in secondary lymphoid organs might be a possible pathway. The detail mechanism needs to be further discussed.
Gene gun delivered-IDO shRNA increases tumor-infiltrating Gr-1+ cells. Gr-1 antibody recognizes Ly6G and Ly6C and only Ly6G is a good marker for detecting neutrophils.34 Our results confirmed that intramuscular injection of IDO shRNA results in neutrophils infiltration by detection of specific Ly6G+ antibody (clone 1A8). A transcriptomics analysis indicates tumor associated neutrophils (including N1 and N2) express higher levels of cytokines and chemokines (such as TNF-α, arginase 1, and chemokine (C–C motif) ligand 4) compared with naive bone marrow neutrophils.21 Both N1 and N2 express higher level of TNF-α compared with naive bone marrow neutrophils.35 The antitumorigenic N1 neutrophils expresses high level of TNF-α, intercellular adhesion molecule 1 (ICAM1) and low level of arginase 1.36 Our result showed the enriched tumor-infiltrating neutrophils had high TNF-α and TGF-β expression (Figure 5b,d). However, no significant difference of ICAM1 and arginase 1 expression was observed after IDO shRNA or IDO inhibitor treatment (data not shown). A report indicated that neutrophils exhibit different phenotypes in early and late stage of tumor progression in murine tumor models.20 We collected neutrophils from two different time points and observed similar expression pattern. The expression pattern of TNF-α and TGF-β was similar with that at day 16 although mean tumor volume did not reveal significant difference among three groups at day 23 (n = 3 in each group). Two time points might be too close to observe different neutrophil phenotypes. Therefore, our current results could not indicate the biological function of tumor-infiltrating neutrophils.
CD8+ T cells and natural killer (NK) cells are important effector immune cells which induce cytotoxic activity to inhibit tumor growth in cancer immunotherapy.37 CD8+ T cells but not NK cells were the most important effector cells in IDO shRNA-induced cytotoxicity in splenocytes.11 Systematic depletion CD8+ T cells abolished therapeutic effect of IDO shRNA.10 Our results revealed that systematic neutrophil depletion decreased the neutrophil population in spleen and compromised antitumor activity. In addition, Th1 cytokines IFN-γ and TNF-α were suppressed after depletion in IDO shRNA treated group. It suggested that neutrophils played an essential role in IDO shRNA-mediated Th1 immune responses in spleen and might play a minor role in IDO shRNA-activated cytotoxicity. Furthermore, spleen is a major origin of tumor-associated macrophage and neutrophils.38 IFN-γ is one of the known cytokines to lead N1 neutrophil polarization.36 Transcriptomics analysis indicates N1 neutrophils express higher mRNA levels of IFN-γ and TNF-α compared with N2 neutrophils.35 Because IDO shRNA treatment induced high level IFN-γ in total spleen, it might be a positive feedback loop among N1-polarized neutrophils, CD8+ T cells, and other immune cells in spleen. When splenic neutrophils trafficking to tumor, TGF-β expression of these neutrophils might be induced by tumor microenvironment. It might be a possible reason why tumor-infiltrating neutrophils expressed both high TNF-α and TGF-β.
IDO inhibitor D-1MT reveals antitumor efficiency against tryptophan catabolic enzymes (IDO, indoleamine 2,3-doxygenase 2 (IDO2), and tryptophan 2,3-dioxygenase (TDO)) overexpressing cancer.39,40 D-1MT treatment affects kinases mechanistic target of rapamycin (mTOR) and protein kinase C-θ (PKC-θ) and reverses the immunosuppressive signaling pathways.41 Increasing number of neutrophils was observed in tumor. Moreover, the mean tumor volume in neutrophil depletion group was smaller than that in IgG control group although no significant difference between two groups (P = 0.4881). Neutrophils depletion decreases TNF-α expression in D-1MT-treated group. Our results suggest D-1MT induced tumor-infiltrating neutrophils have different biological function from IDO shRNA-induced tumor-infiltrating neutrophils.
IDO overexpression results in accumulation of uncharged tRNATrp which activates general control nonderessible 2 kinase pathway to limit protein translation.2 D-1MT is a competitive inhibitor which serves as a mimic of tryptophan and affects mTOR signaling.5 Therefore, the protein translation is rescued after D-1MT treatment. In this study, pre-tRNATrp and pre- tRNAiMet was detected. Because pre-tRNA is not processed into mature tRNA, the level of pre-tRNA could not reflect the real level of uncharged-tRNA or mature tRNA. However, pre-tRNA could be represented the transcription efficiency of each tRNA. Our results indicated that IDO shRNA but not D-1MT induced the level of pre-tRNATrp in total splenocytes (Figure 4). A recent study demonstrates lipopolysaccharide stimulation activates NF-κB pathway, RNA polymerase III activity, and tRNA genes transcription.42 It suggests regulation of RNA polymerase III links to immune responses.42 Our results implies IDO shRNA treatment might affect regulatory elements of RNA polymerase III (such as transcription factor Myc, Jun, Fos, and repressor Maf1) in splenocytes.43 The hypothesis will be determined in the future.
In LLC1 lung cancer model, a single IDO shRNA treatment delayed tumor growth in early stage and was not beneficial to survival rate. Combination of a specific tumor antigen may enhance therapeutic potency of IDO shRNA. A report indicates that treatment of lipid A which is the hydrophobic anchor of lipopolysaccharide results in recruitment of N1 neutrophil in a rat colorectal tumor model.44 Combination of IDO shRNA and lipid A might be a strategy to induce synergistic antitumor immune responses. Further, our results suggest that IDO shRNA induce antitumorigenic neutrophils. IDO shRNA treatment might be a strategy to alter neutrophils polarization in immunotherapies.
In summary, our results indicated that IDO shRNA treatment through intramuscular injection inhibited tumor growth in early sate of tumor development and induced antitumorigenic neutrophils in an established lung cancer model. Depletion of neutrophil abolished therapeutic effect of IDO shRNA. In addition, tRNATrp might be associated with IDO shRNA-mediated antitumor immune responses (Figure 7e). Our results suggest that IDO shRNA treatment could be a strategy to induce N1 neutrophil polarization and combination IDO shDNA might be beneficial to improve the efficiency of other cancer immunotherapies.
Materials and methods
Cell culture. Mouse Lewis lung carcinoma cell line, LLC1 (LL/2), was obtained from Bioresource Collection and Research Center (Hsinchu, Taiwan) and was maintained in Dulbecco's modified Eagle's medium (DMEM) (Lonza, Walkersville, MD) which was supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (100 U/0.1 mg/ml) (Life Technologies, Grand Island, NY).
Plasmid preparation. U6 promoter-driven IDO shRNA plasmid (targeting sequence: 5′-GCACTGCACGACATAGCTA-3′) and Scramble IDO shRNA plasmid (sequence: 5′-GGCCATCTACCCATGAAGA-3′) which were described in our previous study.9 The endotoxin-free plasmids were prepared through NucleoBond Xtra Midi EF kit (Macherey-Nagel, Dliren, Germany) and was dissolved in phosphate-buffered saline (PBS) at a concentration of 0.5 µg/µl.
Evaluation of the therapeutic effect on established tumors. The use of all the animals in this study was approved by the Animal Care and Use Committee at the Kaohsiung Medical University. LLC1 cells (2 × 105 cells in 200 µl serum-free DMEM) were injected subcutaneously into six- to eight-week-old male inbred C57BL/6JNarl mice which were obtained from the National Laboratory Animal Center (Taiwan) and maintained in Experimental Animal Center of Kaohsiung Medical University. The mice were received with intramuscular injection (50 µg of IDO shRNA or scramble IDO shRNA in 100 µl PBS) or were provided with drinking water containing IDO inhibitor D-1MT, 2 mg/ml, pH = 7 (Sigma Aldrich) at 7 days after LLC1 cells injection. The IDO shRNA and scramble IDO shRNA-treated mice were supplied with drinking water at pH = 7 and the D-1MT treated mice were intramuscular injection of 100 µl PBS. These mice were sacrificed at days 16 or 23 after LLC1 cells injection. Tumor size was measured using calipers every 3–4 days. Tumor volume was calculated by the formula: volume A×A×B×0.5236, where A and B respectively represented the shortest and longest diameters.
Quantitative real-time polymerase chain reaction. Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) was performed using a PrimeScript RT reagent Kit (Clontech, Kusatsu, Shiga, Japan). The primer of mouse hypoxanthine phosphoribosyltransferase (HPRT) was 5′-GTTGGATACAGGCCAGACTTTGTTG-3′ and 5′-GATTCAACTTGCGCTCATCTTAGGC-3′; mouse IFN- γ was 5′-AACGCTACACACTGCATCTTGG-3′ and 5′-CAAGACTTCAAAGAGTCTGAGG-3; mouse TNF-α was 5′-CCCCAAAGGGATGAGAAGTT-3′ and 5′-CACTTGGTGGTTTGCTACGA-3′; mouse IL-10 was 5′-CCAGTTTTACCTGGTAGAAGTGATG-3′ and 5′-TGTCTAGGTCCTGGAGTCCAGCAGACTCAA-3′; mouse TGF-β was 5′-TGCGCTTGCAGAGATTAAAA-3′ and 5′-CGTCAAAAGACAGCCACTCA-3′; mouse ICAM-1 was 5′-GTGATGCTCAGGTATCCATCCA-3′ and 5′-CACAGTTCTC AAAGCACAGCG-3; mouse arginase 1 was 5′-CAGAAGAATGGAAGAGTCAG-3′ and 5′-CAGATATGCAGGGAGTCACC-3′. HPRT served as an internal control. The level of mRNA was determined on StepOne Plus Real-Time PCR System (Applied Biosystems) using a Fast SYBR Green Master Mix (Applied Biosystems). The relative gene expression was calculated according to comparative Ct method with normalization to HPRT. The results of ICAM-1 and arginase 1 were not shown.
Cytokines measurement. Spleens were obtained from the mice which were treated with scramble IDO shRNA, IDO shRNA, or D-1MT at 16 days after LLC1 cells injection. After removal of red blood cells, splenocytes (2 × 106 cells) were cultured in a 24-well plate with 1,000 µl Roswell Park Memorial Institute (RPMI) 1640 medium with 10% FBS and penicillin/streptomycin for 24 hours. The culture medium was collected and the mouse cytokines IFN-γ, TNF-α, IL-4, and IL-10 were detected by Mouse Cytokine/Chemokine Magnetic Bead Panel (EMD Millipore, Billerica, MA) according to the manufacturer's instructions. Data were acquired on Luminex xMAP Technology (Millipore, St Charles, MO) Concentrations of all cytokines were calculated by a five-parameter logistic curve fit curve method using the Milliplex Analyst 5.1 Software (Viagene Tech, Carlisle, MA).
Flow cytometry. Spleens were collected and red blood cells in slenocytes were removed. The splenocytes (1 × 106) were stained with FITC conjugated with anti-CD11b antibody (clone M1/70), APC conjugated anti-Ly6G antibody (clone 1A8), FITC conjugated with anti-CD4 antibody (clone RM4-5), or PerCP-Cy 5.5-conjugated with anti-CD25 antibody (clone PC61) for 30 minutes. All antibodies were obtained from BD Biosciences. The results were collected on a flow cytometry (Accuri C6, BD Biosciences, Ann Arbor, MI).
Transfer RNA detection. Total RNA extracted using Trizol reagent (Invitrogen, Carlsbad, CA). cDNA for detecting precursor tRNA was generated with random hexamers and PrimeScript RT reagent Kit (Clontech). Primers design was according to our previous study.45 Primers of pre-tRNATRP was 5′-GACCTCGTGGCGCAACGG-3′ and 5′-ACGCAACCTTCTGATCTGGAG-3′; pretRNAiMet was 5′-CTGGGCCCATAACCCAG AG-3′ and 5′-TGGTAGCAGAGGATGGTTTC-3′. The detection of tRNA was described in quantitative real-time polymerase chain reaction section.
Neutrophil enrichment. The LLC1 tumor was collected at day 16 or day 23 after LLC1 cells injection, and then was cut into small fragments and digested for 60 minutes at 37°C with 0.1% collagenase D (Roche Diagnostics, Mannheim, Germany). After removal of red blood cells, mouse neutrophils were enriched from tumor-infiltrated cells at days 16 postinjection of LLC1 cells by a mouse neutrophil isolation kit (catalog no. 1130-097-658, Miltenyi Biotec, Bergisch-Gladbach, Germany) according to the manufacturer's instruction. The purity of sorted cells was routinely more than 80%.
Neutrophil depletion. LLC1 tumor-bearing mice were intraperitoneally administrated 300 µg InVivoMAb antimouse Ly6G antibody (clone 1A8, BioXcell, West Lebanon, NH) or 300 µg InVivoMAb Rat IgG2a (clone 2A3, BioXcell) in 100 µl PBS. Antibodies were injected at the time of 7, 10, and 14 days after LLC1 cells injection. To evaluate depletion in the spleen, the spleens which were collected at 16 days after LLC1 cells injection and the depletion efficiency were evaluated by flow cytometry (BD Accuri C6 flow cytometer, BD Biosciences).
Statistical analysis. All of the numerical data and graphs were generated by GraphPad Prism 6.01 (GraphPad Software, San Diego, CA). Data represent mean ± SD. Student's t-test was used for analysis of difference between two groups, and a P-value < 0.05 was considered to indicate a statistically significant difference.
SUPPLEMENTARY MATERIAL Figure S1. The level of tryptophan tRNA and initiator methionine tRNA in tumor-infiltrating neutrophils.
Acknowledgments
This study was supported by grants from the Ministry of Science and Technology (MOST 104-2320-B-037-014-MY3), the Kaohsiung Medical University “Aim for the Top Journals Grant” (Grant No. KMU-DT106005), and the Kaohsiung Medical University Hospital (Grant No. KMUH102-2T04). We have no conflict of interests.
Supplementary Material
References
- Meirow, Y, Kanterman, J and Baniyash, M (2015). Paving the road to tumor development and spreading: myeloid-derived suppressor cells are ruling the fate. Front Immunol 6: 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn, DH, Sharma, MD, Baban, B, Harding, HP, Zhang, Y, Ron, D et al. (2005). GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22: 633–642. [DOI] [PubMed] [Google Scholar]
- Platten, M, von Knebel Doeberitz, N, Oezen, I, Wick, W and Ochs, K (2014). Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol 5: 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, MD, Baban, B, Chandler, P, Hou, DY, Singh, N, Yagita, H et al. (2007). Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest 117: 2570–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast, GC, Smith, C, Thomas, S, Mandik-Nayak, L, Laury-Kleintop, L, Metz, R et al. (2014). Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother 63: 721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, L, Spranger, S, Binder, DC, Gritsina, G, Lauing, KL, Giles, FJ et al. (2015). Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res 21: 5427–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgaard, RB, Zamarin, D, Munn, DH, Wolchok, JD and Allison, JP (2013). Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 210: 1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger, S, Koblish, HK, Horton, B, Scherle, PA, Newton, R and Gajewski, TF (2014). Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blache, CA, Manuel, ER, Kaltcheva, TI, Wong, AN, Ellenhorn, JD, Blazar, BR et al. (2012). Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res 72: 6447–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, MC, Lin, CC, Chen, YL, Huang, SS, Yang, HJ, Chang, CP et al. (2009). A novel cancer therapy by skin delivery of indoleamine 2,3-dioxygenase siRNA. Clin Cancer Res 15: 641–649. [DOI] [PubMed] [Google Scholar]
- Huang, TT, Yen, MC, Lin, CC, Weng, TY, Chen, YL, Lin, CM et al. (2011). Skin delivery of short hairpin RNA of indoleamine 2,3 dioxygenase induces antitumor immunity against orthotopic and metastatic liver cancer. Cancer Sci 102: 2214–2220. [DOI] [PubMed] [Google Scholar]
- Murray, PJ and Wynn, TA (2011). Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender, ZG, Sun, J, Kim, S, Kapoor, V, Cheng, G, Ling, L et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender, ZG and Albelda, SM (2012). Tumor-associated neutrophils: friend or foe? Carcinogenesis 33: 949–955. [DOI] [PubMed] [Google Scholar]
- De Larco, JE, Wuertz, BR and Furcht, LT (2004). The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 10: 4895–4900. [DOI] [PubMed] [Google Scholar]
- Shojaei, F, Singh, M, Thompson, JD and Ferrara, N (2008). Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci USA 105: 2640–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santo, C, Arscott, R, Booth, S, Karydis, I, Jones, M, Asher, R et al. (2010). Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol 11: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentles, AJ, Newman, AM, Liu, CL, Bratman, SV, Feng, W, Kim, D et al. (2015). The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 21: 938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel, D and Gabrilovich, DI (2015). Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 125: 3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn, JI, Collazo, M, Shalova, IN, Biswas, SK and Gabrilovich, DI (2012). Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol 91: 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender, ZG, Sun, J, Mishalian, I, Singhal, S, Cheng, G, Kapoor, V et al. (2012). Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One 7: e31524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot, Z, Henke, E, Comen, EA, King, TA, Norton, L and Benezra, R (2011). Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20: 300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishalian, I, Bayuh, R, Levy, L, Zolotarov, L, Michaeli, J and Fridlender, ZG (2013). Tumor-associated neutrophils (TAN) develop protumorigenic properties during tumor progression. Cancer Immunol Immunother 62: 1745–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, RL, Miller, KD and Jemal, A (2016). Cancer statistics, 2016. CA Cancer J Clin 66: 7–30. [DOI] [PubMed] [Google Scholar]
- Yen, MC, Weng, TY, Chen, YL, Lin, CC, Chen, CY, Wang, CY et al. (2013). An HDAC inhibitor enhances cancer therapeutic efficiency of RNA polymerase III promoter-driven IDO shRNA. Cancer Gene Ther 20: 351–357. [DOI] [PubMed] [Google Scholar]
- Wang, S, Zhang, C, Zhang, L, Li, J, Huang, Z and Lu, S (2008). The relative immunogenicity of DNA vaccines delivered by the intramuscular needle injection, electroporation and gene gun methods. Vaccine 26: 2100–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, K, Ito, K, Shinohara, N and Kato, S (2003). DNA immunization via intramuscular and intradermal routes using a gene gun provides different magnitudes and durations on immune response. Mol Immunol 39: 847–854. [DOI] [PubMed] [Google Scholar]
- Gabrilovich, DI and Nagaraj, S (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9: 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draghiciu, O, Lubbers, J, Nijman, HW and Daemen, T (2015). Myeloid derived suppressor cells: an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology 4: e954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noubade, R, Wong, K, Ota, N, Rutz, S, Eidenschenk, C, Valdez, PA et al. (2014). NRROS negatively regulates reactive oxygen species during host defence and autoimmunity. Nature 509: 235–239. [DOI] [PubMed] [Google Scholar]
- Gingold, H, Tehler, D, Christoffersen, NR, Nielsen, MM, Asmar, F, Kooistra, SM et al. (2014). A dual program for translation regulation in cellular proliferation and differentiation. Cell 158: 1281–1292. [DOI] [PubMed] [Google Scholar]
- Lin, CC, Yen, MC, Lin, CM, Huang, SS, Yang, HJ, Chow, NH et al. (2008). Delivery of noncarrier naked DNA vaccine into the skin by supersonic flow induces a polarized T helper type 1 immune response to cancer. J Gene Med 10: 679–689. [DOI] [PubMed] [Google Scholar]
- Sumida, SM, McKay, PF, Truitt, DM, Kishko, MG, Arthur, JC, Seaman, MS et al. (2004). Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines. J Clin Invest 114: 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey, MJ (2012). Has Ly6G finally found a job? Blood 120: 1352–1353. [DOI] [PubMed] [Google Scholar]
- Shaul, ME, Levy, L, Sun, J, Mishalian, I, Singhal, S, Kapoor, V, et al. (2016). Tumor-associated neutrophils display a distinct N1 profile following TGFβ modulation: a transcriptomics analysis of pro- vs antitumor TANs. Oncoimmunol (in press). [DOI] [PMC free article] [PubMed]
- Sionov, RV, Fridlender, ZG and Granot, Z (2015). The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron 8: 125–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka, K and Banchereau, J (2012). Cancer immunotherapy via dendritic cells. Nat Rev Cancer 12: 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez-Retamozo, V, Etzrodt, M, Newton, A, Rauch, PJ, Chudnovskiy, A, Berger, C et al. (2012). Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci USA 109: 2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz, CA, Litzenburger, UM, Sahm, F, Ott, M, Tritschler, I, Trump, S et al. (2011). An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478: 197–203. [DOI] [PubMed] [Google Scholar]
- Witkiewicz, AK, Costantino, CL, Metz, R, Muller, AJ, Prendergast, GC, Yeo, CJ et al. (2009). Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J Am Coll Surg 208: 781–7; discussion 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz, R, Rust, S, Duhadaway, JB, Mautino, MR, Munn, DH, Vahanian, NN et al. (2012). IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunol 1: 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk, D, White, RJ and Ryan, KM (2015). Involvement of RNA polymerase III in immune responses. Mol Cell Biol 35: 1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orioli, A, Pascali, C, Pagano, A, Teichmann, M and Dieci, G (2012). RNA polymerase III transcription control elements: themes and variations. Gene 493: 185–194. [DOI] [PubMed] [Google Scholar]
- Seignez, C, Martin, A, Rollet, CE, Racoeur, C, Scagliarini, A, Jeannin, JF et al. (2014). Senescence of tumor cells induced by oxaliplatin increases the efficiency of a lipid A immunotherapy via the recruitment of neutrophils. Oncotarget 5: 11442–11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, KC, Wang, CY, Liu, KT, Chen, YL, Chen, YC, Lai, MD et al. (2014). Optimization protein productivity of human interleukin-2 through codon usage, gene copy number and intracellular tRNA concentration in CHO cells. Biochem Biophys Res Commun 454: 347–352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.