Abstract
Low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6) are co-receptors for Wnt ligands. Upon ligand binding, LRP5/6 undergo glycogen synthase kinase 3 (GSK3)/casein kinase I (CKI)-mediated phosphorylation at multiple PPP(S/T)P motifs in the intracellular domain, which is essential for canonical Wnt signal transduction. On the other hand, in the Wnt-off state, the mitosis-specific CDK14-Cyclin Y kinase complex phosphorylates Ser-1490 of LRP5/6 at G2/M, thereby priming the receptor for Wnt-induced phosphorylation. However, it remains unclear how CDK14/Cyclin Y is recruited to LRP5/6 and whether there are other cofactors involved in this process. Previously, we identified Caprin-2 as a positive regulator of canonical Wnt signaling by promoting GSK3-depedent LRP5/6 phosphorylation upon Wnt stimulation. Here we uncovered that Caprin-2 positively regulates constitutive LRP5/6 Ser-1490 phosphorylation by complexing with CDK14/Cyclin Y. Caprin-2-mediated LRP5/6 phosphorylation is cell cycle-dependent in a pattern similar to that of CDK14/Cyclin Y-dependent LRP5/6 phosphorylation. Moreover, knockdown of Caprin-2 disrupts not only the interaction between CDK14 and Cyclin Y but also the interaction between CDK14/Cyclin Y and LRP6. Overall, our findings revealed an unrecognized role of Caprin-2 in facilitating LRP5/6 constitutive phosphorylation at G2/M through forming a quaternary complex with CDK14, Cyclin Y, and LRP5/6.
Keywords: cell cycle, cell signaling, protein phosphorylation, protein-protein interaction, Wnt pathway
Introduction
The evolutionarily conserved Wnt/β-catenin signaling plays a pivotal role in myriad physiological and pathological processes, including embryonic development, bone development, tissue homeostasis, neurogenesis, and tumorigenesis (1–4). At the cellular level, Wnt signals not only promote cell proliferation but also control fate determination or terminal differentiation of postmitotic cells (5). Dysregulation of Wnt signaling may lead to developmental defects and, thereby, underlies a variety of human pathologies such as colorectal cancer, osteoporosis, and neurodegenerative disorders (6).
Plenty of research has suggested a complex interplay between Wnt signaling and the cell cycle. Wnt engagement with the receptors Frizzled and LRP5/63 triggers a rapid assembly of a multiprotein complex known as the “LRP signalosome,” which is essential for full and sustained pathway activation. The formation of this signalosome is initiated by the recruitment of Axin to LRP5/6 phosphorylated sites (7). The intracellular LRP5/6 phosphorylation domain contains five PPPSPXS motifs. GSK3 and CK1 are responsible for these phosphorylations in the presence of Wnt ligands. Dvl-Frizzled interaction was also required for the recruitment of Axin, GSK3 and CK1, and PIP2-dependent translocation of adenomatous polyposis coli membrane recruitment 1 (Amer1) (also known as WTX) (8–11). Meanwhile, polymerization of Dvl and Axin facilitates clustering of LRP5/6 in the signalosome. The high local concentration of LRP5/6 further facilitates their phosphorylation mediated by GSK3 and CK1γ at Ser-1490 and Thr-1479, respectively (12, 13). Phosphorylated LRP5/6 inactivate the cytoplasmic β-catenin destruction machinery, leading to accumulation and nuclearization of β-catenin. This allows for β-catenin-dependent expression of various Wnt target genes associated with cell cycle progression, such as c-Myc and Cyclin D1, which directly regulates G1/S transition of the cell cycle (14, 15). In another scenario, without the presence of Wnt ligands, the activation of LRP5/6 is mediated by the mitosis-specific CDK14-Cyclin Y kinase complex, which phosphorylates LRP6 Ser-1490 during G2/M (known as constitutive phosphorylation) to increase the receptiveness of cells for the incoming Wnt signals (16). Therefore, β-catenin-dependent Wnt signaling is under cell cycle control and peaks in the G2/M phase of the cell cycles. In line with this, the protein levels of β-catenin and the Wnt target gene Axin2 also oscillate with the cell cycle, peaking at G2/M (17, 18). In addition, maximum GSK3 inhibition by constitutive LRP6 phosphorylation also occurs in mitosis, which activates Wnt-dependent stabilization of proteins (Wnt/STOP) signaling (19). Wnt/STOP signaling acts in a β-catenin-independent manner to slow down GSK3-dependent protein degradation and maintains a critical cell size required for cell division (19–21). Wnt/STOP signaling promotes cell division during early Xenopus embryogenesis and maintains protein homeostasis to promote sperm maturation in the mouse (22, 23).
Previously, we have identified cytoplasmic activation/proliferation-associated protein 2 (Caprin-2) as a positive regulator in canonical Wnt signaling. Caprin-2 directly binds to LRP5/6 and facilitates GSK3-mediated LRP5/6 phosphorylation (24). The C-terminal CRD of Caprin-2 forms a flexible homotrimer, and this homotrimerization of CRD is required for Caprin-2 to regulate LRP5/6 aggregation and canonical Wnt signaling (25). In this work, we found that Caprin-2 also participates in the regulation of constitutive LRP5/6 phosphorylation. Our findings suggested that Caprin-2 acts as a scaffold protein to potentiate the CDK14-Cyclin Y-LRP6 complex in the G2/M phase of the cell cycle, sensitizing cells for incoming Wnt signals when cells enter mitosis.
Results
Caprin-2 Promotes Constitutive LRP6 Ser-1490 Phosphorylation
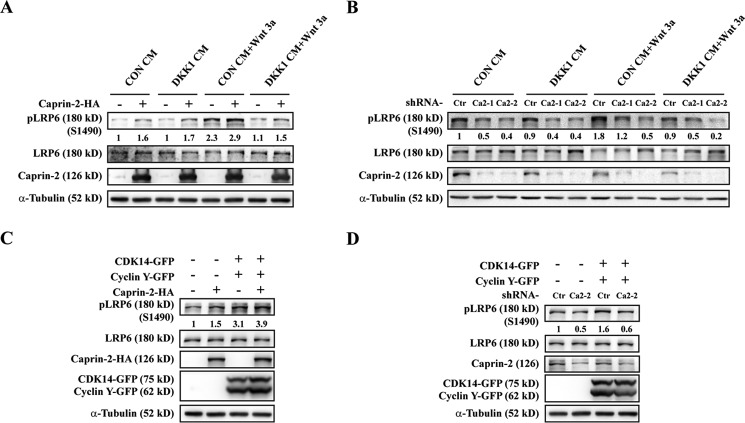
We previously showed that Caprin-2 was a positive regulator in canonical Wnt signaling through a mechanism of facilitating GSK3β-mediated LRP5/6 phosphorylation upon Wnt stimulation (24). Interestingly, several studies by Niehrs and co-workers revealed that phosphorylation of LRP5/6 is not exclusively Wnt-induced (12, 16). Their results showed that the CDK14-Cyclin Y complex phosphorylates Ser-1490 in the PPP(S/T)P motif of LRP5/6 in G2/M phase of the cell cycle, thereby priming LRP5/6 for an instant response to the incoming Wnt signals (16). This constitutive Wnt-independent LRP6 phosphorylation also triggers Wnt/STOP signaling, which plays essential roles in controlling daughter cell size and spermatogenes (19). Caprin-2, unlike Dvl, whose association with LRP5/6 requires Wnt stimulation, interacts with LRP5/6 in a Wnt ligand-independent manner (24). To investigate whether Caprin-2 is involved in regulating LRP5/6 constitutive phosphorylation, we first transfected HEK293 cells with HA-tagged Caprin-2 and examined its effects on the levels of phospho-LRP6 (Ser-1490) in the absence of Wnt stimulation. Consistent with our previous report, Caprin-2 overexpression and Wnt-3a treatment showed a synergistic effect on the increase of LRP6 phosphorylation (Fig. 1A); however, in the absence of Wnt stimulation, Caprin-2 also increased the phosphorylation levels of LRP6 (Fig. 1A). To exclude any potential effects because of the possible existence of Wnt proteins in the cell culture medium or secreted by cells per se, we supplemented the medium with DKK1 (Dickkopf 1), a secreted Wnt signaling antagonist that could prevent Frizzled-Wnt-LRP6 complex formation (26–28). As shown in Fig. 1A, the increase in phosphorylation in LRP6 Ser-1490 induced by Caprin-2 was not affected by the addition of the Dkk1-CM, whereas Wnt-3a-induced LRP6 Ser-1490 phosphorylation was completely blocked by Dkk1-CM treatment. Next, we performed a loss-of-function study by knocking down endogenous Caprin-2 using shRNA lentivirus infection. Two shRNAs were used to avoid siRNA off-target effects. According to our observation, endogenous Caprin-2 is quite stable, and we thus extended the virus incubation time up to 72 h for a maximal knockdown efficiency. As shown in Fig. 1B, knockdown of Caprin-2 significantly decreased both Wnt-3a-stimulated LRP6 phosphorylation and Dkk1-CM-treated Wnt-3a-independent LRP6 phosphorylation. These observations suggested that Caprin-2 plays a role not only in facilitating the Wnt-induced LRP5/6 Ser-1490 phosphorylation but also in regulating constitutive LRP6 Ser-1490 phosphorylation.
FIGURE 1.
Caprin-2 promotes constitutive LRP6 Ser-1490 phosphorylation. A, HEK293 cells were transfected with Caprin-2-HA. Cells were treated with control conditioned medium (CON CM), Wnt-3a protein, or Dkk1 CM for 30 min, and phosphorylation of endogenous LRP6 was then detected using an anti-pLRP6 (p1490) antibody. The anti-LRP6 antibody and anti-α-tubulin were used as the internal controls. The immunoblots were quantified by densitometry, and the intensity values were normalized with those of LRP6; values are given beneath each band. B, HEK293 cells were infected by a lentivirus encoding shRNAs of Caprin-2 for 72 h. Cells were treated with control CM, Wnt-3a protein, or Dkk1 CM for 30 min, and phosphorylation of LRP6 was then detected and quantified as indicated. C, HEK293 cells were transfected with CDK14-GFP, Cyclin Y-GFP, and Caprin-2-HA, and 24 h later, cells were collected and analyzed by Western blotting. D, HEK293 cells were infected by a lentivirus encoding shRNAs of Caprin-2 for 48 h, and then cells were transfected with CDK14-GFP and Cyclin Y-GFP. 24 h later, cells were collected and analyzed by Western blotting.
According to the findings of Davidson et al., coexpression of CDK14 and Cyclin Y resulted in strong enhancement of constitutive LRP6 phosphorylation (16). To investigate whether Caprin-2 affects CDK14/Cyclin Y-mediated constitutive LRP6 phosphorylation, we compared the effects of Caprin-2, CDK14/Cyclin Y, or their combination on LRP6 phosphorylation in HEK293 cells. Consistent with the previous report, transfection of CDK14/Cyclin Y alone induced a dramatic increase in LRP5/6 phosphorylation, which could be further elevated by their co-expression with Caprin-2 (Fig. 1C). Although Caprin-2, when overexpressed alone, exhibited only a mild effect on LRP5/6 phosphorylation compared with that of CDK14/Cyclin Y (Fig. 1C), Caprin-2 knockdown completely abolished CDK14/Cyclin Y-induced LRP6 Ser-1490 phosphorylation (Fig. 1D). This result indicates that CDK14/Cyclin Y-stimulated LRP6 Ser-1490 phosphorylation requires Caprin-2.
Caprin-2-mediated LRP6 Phosphorylation Peaks at G2/M
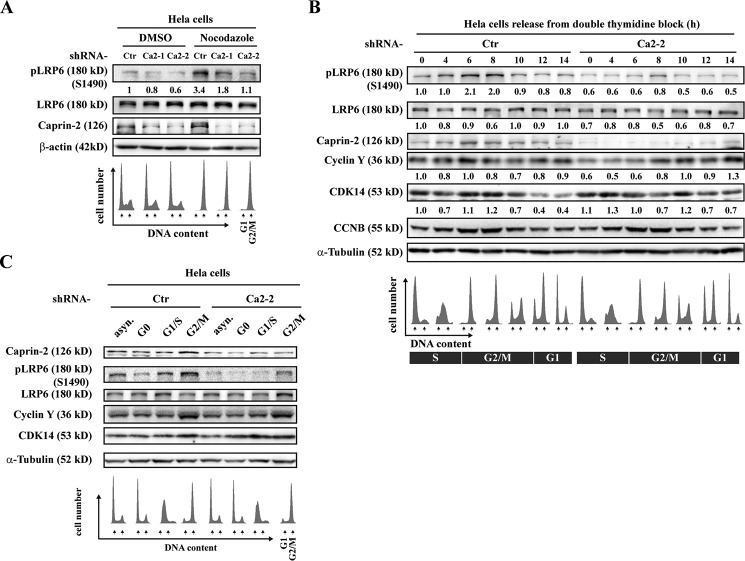
Because constitutive CDK14/Cyclin Y-mediated LRP6 phosphorylation is under the control of the cell cycle, peaking at G2/M (16), we next set out to examine whether Caprin-2-induced LRP6 constitutive phosphorylation is also related to the cell cycle. We first incubated HeLa cells with nocodazole to arrest the cell cycle at G2/M phase. Nocodazole-treated cells enter mitosis but cannot form metaphase spindles because microtubules cannot polymerize. Flow cytometry analysis confirmed the increase in mitotic cells in nocodazole-treated cells. Consistent with the previous report, endogenous Ser-1490 phosphorylation levels exhibited an apparent increase in G2/M-arrested cells; however, knockdown of Caprin-2 blocked this increase in LRP6 Ser-1490 phosphorylation at G2/M (Fig. 2A). To further dissect the role of Caprin-2 in cell cycle-dependent LRP6 phosphorylation, we performed a double thymidine block and release experiment in HeLa cells. Parallel FACS analysis confirmed cell cycle phase enrichment. As shown in Fig. 2B, LRP6 Ser-1490 phosphorylation peaked during G2/M, and so did the expression of the endogenous Cyclin Y. Of note, the expression of Caprin-2 also oscillated during the cell cycle and exhibited a peak in G2/M, implying a possible functional correlation between Caprin-2 and Cyclin Y. By contrast, knockdown of Caprin-2 not only decreased the overall phosphorylation levels of LRP6 but also compromised the oscillation pattern of LRP6 phosphorylation through the cell cycle (Fig. 2B). We also monitored the expression levels of Caprin-2 in drug-synchronized HeLa cells and observed a clear enrichment of both Caprin-2 and CDK14/Cyclin Y in nocodazole-treated cells (Fig. 2C). Moreover, consistent with the results from the double thymidine block and release assay, Caprin-2 knockdown led to decreased LRP6 phosphorylation through cell cycle phases in drug-synchronized HeLa cells (Fig. 2C). Taken together, these results suggested that Caprin-2-mediated LRP6 Ser-1490 phosphorylation is subject to cell cycle control and that Caprin-2 may synergize with CDK14/Cyclin Y to boost LRP6 Ser-1490 phosphorylation in G2/M.
FIGURE 2.
Caprin-2-mediated LRP6 Ser-1490 phosphorylation is cell cycle-dependent. A, HeLa cells were infected by a lentivirus encoding shRNAs of Caprin-2 for 56 h. Then cells were arrested in G2/M by nocodazole (100 ng/ml) treatment for 16 h. Cells were collected in SDS sample buffer and analyzed by Western blotting. Parallel FACS analysis was performed correspondingly. B, HeLa cells were infected by a lentivirus encoding shRNAs of Caprin-2 for 24 h. Then cells were synchronized at G1/S by double thymidine block. G1/S-arrested cells were then washed to progress through the cell cycle. Cells were collected at the indicated times and analyzed by Western blotting. FACS analysis of the cells is shown beneath the corresponding lanes. CCNB was used as a marker for cell cycle progression. C, HeLa cells with or without infection of Caprin-2 shRNAs were arrested at the indicated phases of the cell cycle by nocodazole treatment and then subjected to Western blotting analysis.
Caprin-2 Interacts with CDK14/Cyclin Y
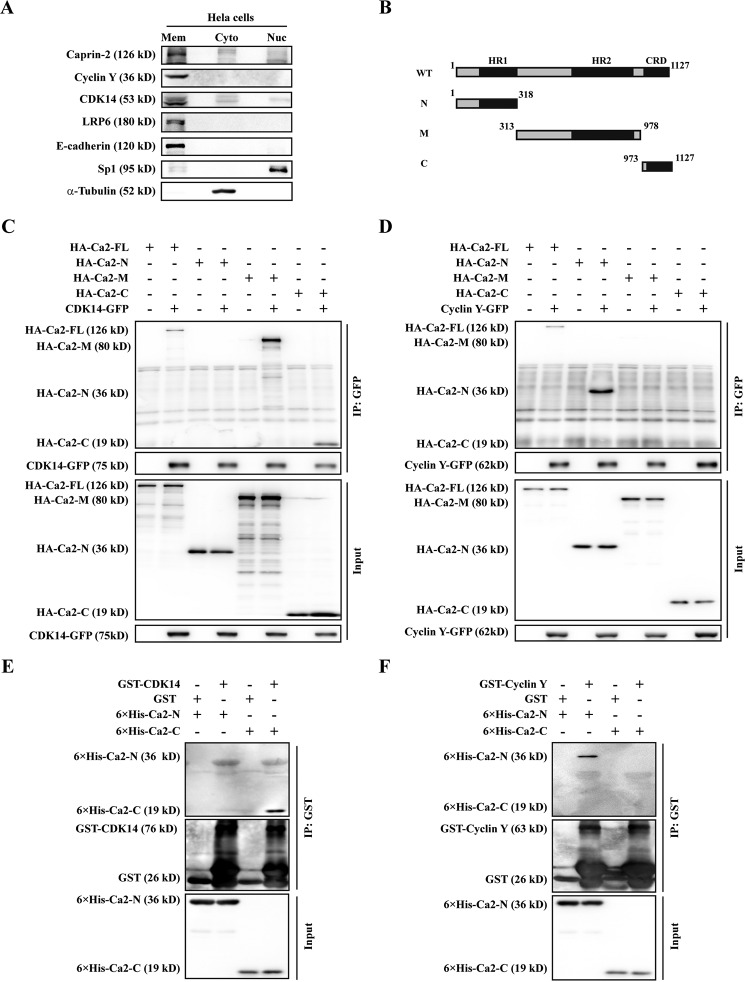
In a previous effort to identify the potential binding partners for Caprin-2, we carried out affinity purification coupled to mass spectrometry and found many CDKs in the list of candidate Caprin-2-interacting proteins (data not shown). We then asked whether Caprin-2 interacts with CDK14/Cyclin Y. Cyclin Y is localized to the plasma membrane via N-terminal myristoylation, and CDK14 is recruited to the plasma membrane by interacting with Cyclin Y. The subcellular distribution of Caprin-2 has so far not been clearly addressed. Thus, we first performed a subcellular fraction assay to examine the distribution of Caprin-2. We isolated the membrane, cytoplasmic, and nuclear proteins of HeLa cells and found that most of Caprin-2 proteins were in the membrane fraction, with very few distributed in the cytoplasmic and nuclear fractions (Fig. 3A). The co-localization of Caprin-2, CDK14, and Cyclin Y to the plasma membrane suggested that they may collaborate with each other in regulating constitutive LRP6 Ser-1490 phosphorylation. Next we set out to investigate whether Caprin-2 could form complexes with CDK14/Cyclin Y. Caprin-2 contains three domains, including the N-terminal homologous region 1 (HR-1) domain, the middle HR-2 domain, and a C1q-related domain (CRD) in the C terminus. To map the possible CDK14/Cyclin Y-interacting regions on Caprin-2, we generated three Caprin-2 fragments: Ca2-N (containing the HR1 domain), Ca2-M (containing the HR2 domain), and Ca2-C (containing the CRD domain) (Fig. 3B). We then co-transfected GFP-tagged CDK14 or Cyclin Y with HA-tagged full-length Caprin-2 or the three Caprin-2 fragments into HEK293T cells. At 24 h post-transfection, whole-cell lysates were used in a co-immunoprecipitation experiment using anti-GFP antibody. As shown in Fig. 3, C and D, CDK14 could be co-immunoprecipitated by the full-length Caprin-2, Ca2-M, and Ca2-C but not by Ca2-N, whereas Cyclin Y was co-immunoprecipitated with the full-length Caprin-2 and Ca2-N but not with Ca2-M or Ca2-C. These results indicate that Caprin-2 interacts with both CDK14 and Cyclin Y but through different regions. To determine whether Caprin-2 interacts with CDK14 or Cyclin Y directly, we performed an in vitro GST pulldown experiment using bacterially expressed recombinant proteins. We failed to obtain the full-length Caprin-2 and Ca2-M samples because of their extremely low solubility when expressed in Escherichia coli. We purified His6-tagged Ca2-N and Ca2-C and performed a GST pulldown assay using purified GST-tagged CDK14 or Cyclin Y. As shown in Fig. 3, E and F, GST-CDK14, but not the control GST, pulled down His6-Ca2-C, whereas GST-Cyclin Y pulled down His6-Ca2-N. These results are consistent with the observation from the co-immunoprecipitation assay, suggesting that Caprin-2 binds directly to both CDK14 and Cyclin Y through different regions.
FIGURE 3.
Caprin-2 interacts with CDK14/Cyclin Y. A, HeLa cells were fractionated and then subjected to Western blotting analysis. LRP6, α-tubulin, and Sp1 served as the loading controls for the membrane (Mem), cytosolic (Cyto), and nuclear (Nuc) fractions, respectively. B, schematic of Caprin-2 fragments. Numbers indicate amino acids. HR, homologous region. N, amino-terminal fragment of Caprin-2; M, middle fragment of Caprin-2; C, carboxyl-terminal fragment of Caprin-2. C and D, HEK293T cells expressing HA-Ca2-FL, HA-Ca2-N, HA-Ca2-M, or HA-Ca2-C were co-transfected with either CDK14-GFP or Cyclin Y-GFP. Then the cell lysates were immunoprecipitated with anti-GFP antibody, and the immunoprecipitates were probed with the indicated antibodies. E and F, GST-tagged CDK14 or Cyclin Y expressed in E. coli was bound to the glutathione-agarose beads. Then the His-tagged Caprin-2 fragments were added as indicated and incubated for 3 h. After three washes with lysis buffer, the proteins were eluted with the SDS loading buffer and analyzed by Western blotting using anti-GST and anti-His monoclonal antibodies.
Caprin-2 Scaffolds the CDK14-Cyclin Y-LRP6 Complex
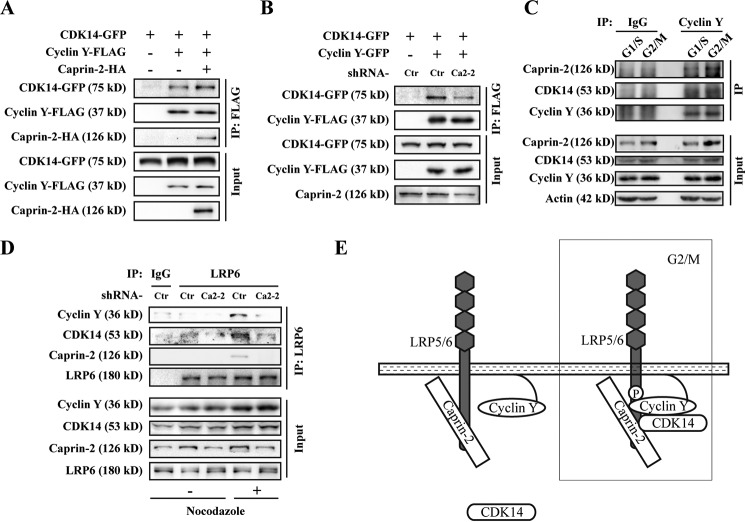
CDK14 and Cyclin Y are classic CDK/Cyclin partners. Cyclin Y is the regulatory subunit of CDK14, which is important for the kinase activity of CDK14. Our results above showed that Caprin-2 can interact directly with both CDK14 and Cyclin Y. We then asked whether Caprin-2 affects the CDK14-Cyclin Y interaction. We co-transfected CDK14 and Cyclin Y with or without Caprin-2 into HEK293T cells. As expected, Caprin-2 strengthened the interaction between CDK14 and Cyclin Y (Fig. 4A), which is also evidenced by the knockdown experiment using Caprin-2 shRNA (Fig. 4B). To examine whether Caprin-2-CDK14-Cyclin Y complex formation is subject to regulation by the cell cycle, we performed endogenous co-IP in different cell cycle phases. As shown in Fig. 4C, Caprin-2 and CDK14 co-immunoprecipitated by Cyclin Y were increased at G2/M. This result further confirmed that Caprin-2, CDK14, and Cyclin Y form a complex that is potentiated at G2/M. On the other hand, CDK14 and Cyclin Y both associate with LRP5/6, which is required for CDK14/Cyclin Y-mediated LRP5/6 constitutive phosphorylation (16). We then tested whether Caprin-2 plays a role in the interaction between CDK14/Cyclin Y and LRP6. We first examined the interactions between endogenous CDK14/Cyclin Y and LRP6. We immunoprecipitated endogenous LRP6 from 293 cell lysates using anti-LRP6 antibody and detected the presence of endogenous CDK14/Cyclin Y in the immunoprecipitates. Of note, these endogenous interactions of either LRP6-CDK14 or LRP6-Cyclin Y could only be observed in cells arrested at G2/M by nocodazole but not in unsynchronized cells. However, knockdown of endogenous Caprin-2 abolished both LRP6-CDK14 and LRP6-Cyclin Y interactions (Fig. 4D). These results, combined with those above, demonstrated that Caprin-2 serves as a scaffold to mediate the formation of the CDK14-Cyclin Y/LRP6 complex, in which LRP6 is phosphorylated by CDK14/Cyclin Y, thereby sensitizing cells for the incoming Wnt signals when cells enter mitosis (Fig. 4E).
FIGURE 4.
Caprin-2 scaffolds the CDK14-Cyclin Y-LRP6 complex. A, HEK293T cells were transfected with CDK14-GFP and Cyclin Y-FLAG with or without 5HA-Caprin-2 as indicated. Cell lysates were then immunoprecipitated with anti-FLAG antibody, and the immunoprecipitates were probed with the indicated antibodies. B, HEK-293T cells were infected by a lentivirus encoding shRNAs of Caprin-2 for 48 h, and then cells were transfected with the indicated plasmids. Co-IP was performed as described in A. C, endogenous interaction of Caprin-2/CDK14/Cyclin Y increased at G2/M. HeLa cells were synchronized by double thymidine block, and the cells lysates from G1/S- or G2/M-synchronized cells were immunoprecipitated by Cyclin Y-specific antibody, followed by Western blotting analysis with the indicated antibodies. D, endogenous Caprin-2/CDK14/Cyclin Y/LRP6 complexes were analyzed in HEK293 cells. Immunoprecipitation was performed with an anti-LRP6 polyclonal antibody. IgG was used as a control. Before co-immunoprecipitation, cells were treated with shCaprin-2 lentivirus for 56 h and nocodazole for another 16 h. E, model for Caprin-2 modulating constitutive LRP6 phosphorylation.
Discussion
We previously identified Caprin-2 as a new LRP5/6-binding protein that promotes Wnt-induced LRP5/6 phosphorylation. Here we revealed a new role of Caprin-2 in the regulation of the Wnt-independent LRP5/6 phosphorylation. Our findings showed that Caprin-2 acts as a scaffold protein to promote CDK14-Cyclin Y-LRP5/6 complex formation at G2/M, thereby positively regulating Wnt-independent phosphorylation of LRP5/6. Because Caprin-2 interacts with LRP6 independent of Wnt stimulation (24), Caprin-2 could be a constitutive binding partner of LRP5/6, which, in the Wnt-off state, promotes CDK14-Cyclin Y-mediated LRP6 phosphorylation, whereas, in the Wnt-on state, it acts synergistically with Wnt signals to promote GSK3β-mediated LRP5/6 phosphorylation. Therefore, Caprin-2 regulates both constitutive and Wnt-induced LRP5/6 phosphorylation, contributing to a full and quick activation of LRP5/6. It is possible that other signals may be required in the Wnt-off state to regulate the assembly of the Caprin-2-CDK14-Cyclin Y complex at G2/M. This complex may also include additional components, allowing for fine-tuning of the constitutive LRP5/6 phosphorylation.
Sequential phosphorylation of the five PPPSPXS motifs of LRP5/6 is the key event for the initiation of Wnt signaling. These phosphorylated sites provide docking sites for Axin. Axin recruitment to the phosphorylated LRP5/6 inactivated the β-catenin destruction complex, ultimately liberating β-catenin from the destiny of degradation. The phosphorylation of the five PPPSPXS motifs is hierarchically controlled. It is well known that Ser-1490 is phosphorylated by GSK3 upon Wnt stimulation, which primes the subsequent phosphorylation by CK1. But Ser-1490 is also constitutively phosphorylated by CDK14/Cyclin Y. The constitutive Ser-1490 phosphorylation of LRP5/6 may provide a weak binding site for the initial recruitment of Axin, thereby conferring LRP5/6 an easily activated status. We speculate that constitutive Ser-1490 phosphorylation may primesLRP5/6 to be phosphorylated by GSK3 and CK1 at more sites of the five PPPSP motifs upon Wnt engagement.
Caprin-2 contains a C1q-related domain at its C terminus (Cap2_CRD), and we previously determined its three-dimensional structure, which exhibits a trimeric assembly with a typical jelly roll topology shared by both C1q and TNF family proteins (25). Our previous study showed that trimerization of Cap2_CRD is not essential for Caprin-2 to interact with LRP5/6; however, it is required for Caprin-2 to regulate LRP5/6 phosphorylation upon Wnt stimulation (24). One possibility is that Caprin-2 constitutively interacts with LRP5/6 and recruits CDK14/Cyclin Y to LRP5/6 in G2/M for maintaining constitutive phosphorylation of LRP5/6, whereas Wnt stimulation may induce CRD domain-mediated aggregation of Caprin-2, which could facilitate LRP5/6 aggregation as well as the subsequent formation of the LRP5/6 signalosome.
In addition to priming Wnt-induced LRP5/6 phosphorylation, CDK14/Cyclin Y-mediated LRP5/6 phosphorylation also triggers Wnt/STOP signaling, which leads to GSK3 inactivation and thus protects proteins from GSK3-dependent degradation (19). Wnt/STOP signaling plays key roles in maintaining the size of daughter cells and the faithful execution of mitosis and has been found recently to be the dominant mode of Wnt signaling in several cancer cell lines (19, 29). Considering the essential role of Caprin-2 in CDK14/Cyclin Y-mediated LRP5/6 phosphorylation, it is very likely that Caprin-2 may also function in Wnt/STOP signaling. The role of Caprin-2 in Wnt/STOP signaling and whether this role may correlate with the generation and progression of some Wnt/STOP signaling-related cancers remains to be determined.
Based on current literature, both CDK14 and Cyclin Y were highly expressed in postmitotic tissues like the testis and brain (30, 31). Coincidentally, Caprin-2 is also highly expressed in the testis and brain (32, 33). These results imply a functional interaction between CDK14/Cyclin Y and Caprin-2 in these tissues. The majority of the cells in the brain and testis are not active in mitosis. The expression pattern of CDK14/Cyclin Y predominant in the brain and testis is consistent with the previous finding that the function of CDK14/Cyclin Y is different from the classic CDK-Cyclin complex (22, 34–36). Whether Caprin-2 exerts any physiological function in the testis or brain together with CDK14/Cyclin Y warrants further studies in a model organism, such as Caprin-2 knockout mice.
Experimental Procedures
Materials
Full-length human Caprin-2 was amplified from total RNA of HEK293T cells by RT-PCR, and the PCR product was cloned into the mammalian expression vector pCMV-HA. pEGPF-N3-CDK14 and pEGPF-N3-Cyclin Y were kindly provided by Dr. Jiangye Chen. HA-tagged and FLAG-tagged CDK14 and Cyclin Y were subcloned to pCMV-HA or pCMV-FLAG. Caprin-2, CDK14, and Cyclin Y constructs for recombinant expression in bacteria were created by PCR and then subcloned into pET28c or pGEX-4T2. Caprin-2 antibody was produced by Abgent. The following commercial antibodies were used for Western blotting and immunoprecipitation: HA (Covance); GST, FLAG (M2), α-tubulin, and His (Sigma-Aldrich); CDK14 (Abgent); Cyclin Y (Proteintech); LRP6 (C5C7) and phospho-LRP6 (Ser-1490) (Cell Signaling Technology); Cyclin B1 (CCNB) (Cell signaling); and GFP (Roche Applied Science).
Cell Culture, Transfection, and Reporter Gene Assay
HEK293T, HEK293, and HeLa cells were maintained in Dulbecco's modified Eagle's medium (Gibco) plus 10% fetal bovine serum (Gibco). Cells were seeded in plates 24 h before transfection, and plasmids were transfected using Lipo2000 reagent (Invitrogen) according to the instructions of the manufacturer. For the reporter gene assay, HEK293T cells in a 24-well plate were transfected with 250 ng of plasmids in total for each well, including 5 ng of TOPFlash reporter plasmid and 10 ng of GFP plasmid as the transfection control. After 18 h of transfection, cells were treated with Wnt3a-conditioned medium (Wnt3a-CM), Dkk1-conditioned medium (Dkk1-CM), or control medium (Ctrl-CM) for an additional 6 h. Cells were then lysed, and luciferase assays were performed. The luciferase activities presented were normalized against the levels of GFP expression as described previously (37).
Conditioned Medium
Wnt3a-CM and L cell control CM were prepared as described previously (7). For Dkk1 CM, HEK293T cells in 10-cm dishes were transfected with 10 μg of pcDNA3-Myc-Dkk1 using Lipo2000 according to the specifications of the manufacturer. The media were exchanged with fresh Dulbecco's minimum essential medium containing 10% fetal bovine serum 24 h after transfection. After further 48 h of incubation, the media were collected, centrifuged to remove cell debris, and stored at −80 °C.
Co-immunoprecipitation (Co-IP) and Western Blotting Assays
At 24 h post-transfection, cells were harvested and lysed in lysis buffer (50 mm Tris-HCl (pH 8.0), 1% Triton X-100, 150 mm NaCl, and 5 mm EDTA with protease and phosphatase inhibitors). The lysates were centrifuged for 15 min at 13,000 rpm at 4 °C. The supernatants were incubated with antibodies and protein A/G Plus-agarose (Santa Cruz Biotechnology, Inc.) for 3 h at 4 °C. Then, samples were washed three times with lysis buffer and denatured in SDS sample buffer for 10 min at 95 °C. Proteins were separated by SDS-PAGE and blotted onto PVDF membranes (Millipore). Membranes were blocked with 5% nonfat dry milk for 1 h and then incubated with primary antibodies for 1 h at room temperature or overnight at 4 °C. After being washed, membranes were incubated for 1 h at room temperature by using the appropriate horseradish peroxidase-conjugated secondary antibodies (Thermo Scientific) for 1 h at room temperature. The results were visualized by FujiFilm Las 4000 (FujiFilm).
shRNAs and Viral Packaging and Infection
The shRNAs were cloned into the pLKO.1-puro lentiviral vector (Addgene). The target sequences were as follows: 5′-GCAGAAAGAACAA GATCCAAA-3′ (shCaprin-2-1), 5′-AGCTCAAA CTGGAGGATTATA-3′ (shCaprin-2-2), and 5′-ACAG TTAACCACTTTTTGAAT-3′ (shCtr). Viral packaging and infection of cells were performed as described previously (38). Briefly, lentiviral particles were generated by co-transfecting the pLKO.1 transfer vector, pCMV-R8.74psPAX2, and pVSV-G into HEK293FT cells. 48 h later, the cell medium containing the packaged viruses was collected and added to the cells in the presence of 8 μg/ml Polybrene. After 12 h, the medium containing viruses was replaced with fresh growth medium, and 60 h later, the infected cells were used for the indicated assays. Experiments were performed in triplicate.
GST Pulldown Assay
Recombinant proteins (GST- or His-tagged) were expressed in E. coli host strain BL21(DE3). The cells were collected, resuspended in lysis buffer, and lysed using a high-pressure homogenizer. GST or GST-tagged fusion protein was incubated with glutathione-agarose beads (Sigma) for 3 h, and then His tagged proteins were added and mixed for another 3 h. The beads were washed three times with the lysis buffer. The samples were treated with SDS loading buffer and analyzed by Western blotting using anti-GST and anti-His monoclonal antibodies.
Cell Fractionations
Cells grown in 6-well plates were harvested with a cell scraper into 1.5 ml of PBS, followed by centrifugation at 700 × g for 10 min. The pelleted cells were resuspended in buffer A (10 mm HEPES (pH 7.9), 1.5 mm MgCl2, 10 mm KCl, 0.5 mm DTT, 10 mm NaF, 2 mm Na3VO4, 1 mm pyrophosphoric acid, and CompleteTM protease inhibitor) and incubated on ice for 10 min. Cells were then passed through a 0.4-mm needle point to break down the cell membrane and centrifuged at 700 × g at 4 °C for 10 min. The supernatant was collected and centrifuged at 100,000 × g at 4 °C for 1 h. The supernatant from the ultracentrifugation was collected as the cytosolic fraction. For nuclear protein extraction, the pellet from the 700 × g centrifugation was washed with buffer A, resuspended in buffer C (20 mm HEPES (pH 7.9), 1.5 mm MgCl2, 420 mm NaCl, 0.2 mm EDTA, 10 mm NaF, 2 mMNa3VO4, 1 mm pyrophosphoric acid, and CompleteTM protease inhibitor) and incubated on ice for 30 min. Nuclear extracts were recovered from the supernatants after centrifugation at 100,000 × g at 4 °C for 1 h.
Cell Cycle Assay
To arrest cells at the G2/M stage, cells were treated with nocodazole (100 ng/ml) for 16 h. G0 phase cells were obtained by serum starvation for 50 h in 0.2% serum. For the double thymidine block, cells were incubated in 2 mm thymidine twice for 17 h of exposure time separated by a 9-h recovery interval. G1/S-arrested cells were then allowed to progress through the cell cycle by removing thymidine. Cell cycle states were confirmed by flow cytometry after trypsinization of cells, fixation in 70% ethanol, and staining with 50 mg/ml propidium iodide for 30 min at 37 °C. Cells (at least 10,000) were analyzed on a FACSCalibur (BD Biosciences).
Author Contributions
L. L. and X. S. conceived and coordinated the study. X. W., Y. J., and C. F. designed, performed and analyzed the experiments. X. W., X. S., and L. L. wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
This work was supported by the National Natural Science Foundation of China (Grants 31530094, 31230044 and 31100532) and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grants XDB19000000 and XDA12010301). The authors declare that they have no conflicts of interest with the contents of this article.
- LRP
- lipoprotein receptor-related protein
- Dvl
- dishevelled
- Wnt/STOP
- Wnt-dependent stabilization of proteins
- CRD
- C1q-related domain
- CM
- conditioned medium
- co-IP
- co-immunoprecipitation.
References
- 1.Clevers H., and Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 [DOI] [PubMed] [Google Scholar]
- 2.MacDonald B. T., Tamai K., and He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland J. D., Klaus A., Garratt A. N., and Birchmeier W. (2013) Wnt signaling in stem and cancer stem cells. Curr. Opin Cell Biol. 25, 254–264 [DOI] [PubMed] [Google Scholar]
- 4.Kahn M. (2014) Can we safely target the WNT pathway? Nat. Rev. Drug. Discov. 13, 513–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reya T., and Clevers H. (2005) Wnt signalling in stem cells and cancer. Nature 434, 843–850 [DOI] [PubMed] [Google Scholar]
- 6.Verkaar F., and Zaman G. J. (2011) New avenues to target Wnt/β-catenin signaling. Drug Discov. Today 16, 35–41 [DOI] [PubMed] [Google Scholar]
- 7.Mao J., Wang J., Liu B., Pan W., Farr G. H. 3rd, Flynn C., Yuan H., Takada S., Kimelman D., Li L., and Wu D. (2001) Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7, 801–809 [DOI] [PubMed] [Google Scholar]
- 8.Bilic J., Huang Y. L., Davidson G., Zimmermann T., Cruciat C. M., Bienz M., and Niehrs C. (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316, 1619–1622 [DOI] [PubMed] [Google Scholar]
- 9.Pan W., Choi S. C., Wang H., Qin Y., Volpicelli-Daley L., Swan L., Lucast L., Khoo C., Zhang X., Li L., Abrams C. S., Sokol S. Y., and Wu D. (2008) Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science 321, 1350–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanneberger K., Pfister A. S., Brauburger K., Schneikert J., Hadjihannas M. V., Kriz V., Schulte G., Bryja V., and Behrens J. (2011) Amer1/WTX couples Wnt-induced formation of PtdIns(4,5)P2 to LRP6 phosphorylation. EMBO J. 30, 1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Q., and Gao N. (2015) Keeping Wnt signalosome in check by vesicular traffic. J. Cell. Physiol. 230, 1170–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson G., Wu W., Shen J., Bilic J., Fenger U., Stannek P., Glinka A., and Niehrs C. (2005) Casein kinase 1 γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438, 867–872 [DOI] [PubMed] [Google Scholar]
- 13.Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., and He X. (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438, 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He T. C., Sparks A. B., Rago C., Hermeking H., Zawel L., da Costa L. T., Morin P. J., Vogelstein B., and Kinzler K. W. (1998) Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 [DOI] [PubMed] [Google Scholar]
- 15.Tetsu O., and McCormick F. (1999) β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–426 [DOI] [PubMed] [Google Scholar]
- 16.Davidson G., Shen J., Huang Y. L., Su Y., Karaulanov E., Bartscherer K., Hassler C., Stannek P., Boutros M., and Niehrs C. (2009) Cell cycle control of wnt receptor activation. Dev. Cell 17, 788–799 [DOI] [PubMed] [Google Scholar]
- 17.Olmeda D., Castel S., Vilaró S., and Cano A. (2003) β-Catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol. Biol. Cell 14, 2844–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadjihannas M. V., Bernkopf D. B., Brückner M., and Behrens J. (2012) Cell cycle control of Wnt/β-catenin signalling by conductin/axin2 through CDC20. EMBO Rep. 13, 347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acebron S. P., Karaulanov E., Berger B. S., Huang Y. L., and Niehrs C. (2014) Mitotic wnt signaling promotes protein stabilization and regulates cell size. Mol. Cell 54, 663–674 [DOI] [PubMed] [Google Scholar]
- 20.Kim N. G., Xu C., and Gumbiner B. M. (2009) Identification of targets of the Wnt pathway destruction complex in addition to β-catenin. Proc. Natl. Acad. Sci. U.S.A. 106, 5165–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu D., and Pan W. (2010) GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 35, 161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch S., Acebron S. P., Herbst J., Hatiboglu G., and Niehrs C. (2015) Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell 163, 1225–1236 [DOI] [PubMed] [Google Scholar]
- 23.Huang Y. L., Anvarian Z., Döderlein G., Acebron S. P., and Niehrs C. (2015) Maternal Wnt/STOP signaling promotes cell division during early Xenopus embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 112, 5732–5737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Y., Xi Y., Chen T., Wang J. Y., Tao D. L., Wu Z. L., Li Y. P., Li C., Zeng R., and Li L. (2008) Caprin-2 enhances canonical Wnt signaling through regulating LRP5/6 phosphorylation. J. Cell Biol. 182, 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miao H., Jia Y., Xie S., Wang X., Zhao J., Chu Y., Zhou Z., Shi Z., Song X., and Li L. (2014) Structural insights into the C1q domain of Caprin-2 in canonical Wnt signaling. J. Biol. Chem. 289, 34104–34113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bafico A., Liu G., Yaniv A., Gazit A., and Aaronson S. A. (2001) Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3, 683–686 [DOI] [PubMed] [Google Scholar]
- 27.Mao B., Wu W., Li Y., Hoppe D., Stannek P., Glinka A., and Niehrs C. (2001) LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321–325 [DOI] [PubMed] [Google Scholar]
- 28.Semënov M. V., Zhang X., and He X. (2008) DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J. Biol. Chem. 283, 21427–21432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolz A., Neufeld K., Ertych N., and Bastians H. (2015) Wnt-mediated protein stabilization ensures proper mitotic microtubule assembly and chromosome segregation. EMBO Rep. 16, 490–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang M., Gao Y., Yang T., Zhu X., and Chen J. (2009) Cyclin Y, a novel membrane-associated cyclin, interacts with PFTK1. FEBS Lett. 583, 2171–2178 [DOI] [PubMed] [Google Scholar]
- 31.Yang T., and Chen J. Y. (2001) Identification and cellular localization of human PFTAIRE1. Gene 267, 165–172 [DOI] [PubMed] [Google Scholar]
- 32.Shiina N., and Tokunaga M. (2010) RNA granule protein 140 (RNG140), a paralog of RNG105 localized to distinct RNA granules in neuronal dendrites in the adult vertebrate brain. J. Biol. Chem. 285, 24260–24269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorén C. E., Schrader J. W., Ahlgren U., and Gunhaga L. (2009) FGF signals induce Caprin2 expression in the vertebrate lens. Differentiation 77, 386–394 [DOI] [PubMed] [Google Scholar]
- 34.Liu D., and Finley R. L. Jr. (2010) Cyclin Y is a novel conserved cyclin essential for development in Drosophila. Genetics 184, 1025–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stowers R. S., Garza D., Rascle A., and Hogness D. S. (2000) The L63 gene is necessary for the ecdysone-induced 63E late puff and encodes CDK proteins required for Drosophila development. Dev. Biol. 221, 23–40 [DOI] [PubMed] [Google Scholar]
- 36.Zi Z., Zhang Z., Li Q., An W., Zeng L., Gao D., Yang Y., Zhu X., Zeng R., Shum W. W., and Wu J. (2015) CCNYL1, but not CCNY, cooperates with CDK16 to regulate spermatogenesis in mouse. PLoS Genet. 11, e1005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M., Wang H., Huang T., Wang J., Ding Y., Li Z., Zhang J., and Li L. (2010) TAB2 scaffolds TAK1 and NLK in repressing canonical Wnt signaling. J. Biol. Chem. 285, 13397–13404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naldini L., Blömer U., Gage F. H., Trono D., and Verma I. M. (1996) Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. U.S.A. 93, 11382–11388 [DOI] [PMC free article] [PubMed] [Google Scholar]