Abstract
Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine that has been implicated in a broad range of inflammatory and oncologic diseases. MIF is unique among cytokines in terms of its release profile and inflammatory role, notably as an endogenous counter-regulator of the anti-inflammatory effects of glucocorticoids. In addition, it exhibits a catalytic tautomerase activity amenable to the design of high affinity small molecule inhibitors. Although several classes of these compounds have been identified, biologic characterization of these molecules remains a topic of active investigation. In this study, we used in vitro LPS-driven assays to characterize representative molecules from several classes of MIF inhibitors. We determined that MIF inhibitors exhibit distinct profiles of anti-inflammatory activity, especially with regard to TNFα. We further investigated a molecule with relatively low anti-inflammatory activity, compound T-614 (also known as the anti-rheumatic drug iguratimod), and found that, in addition to exhibiting selective MIF inhibition in vitro and in vivo, iguratimod also has additive effects with glucocorticoids. Furthermore, we found that iguratimod synergizes with glucocorticoids in attenuating experimental autoimmune encephalitis, a model of multiple sclerosis. Our work identifies iguratimod as a valuable new candidate for drug repurposing to MIF-relevant diseases, including multiple sclerosis.
Keywords: drug discovery, drug screening, glucocorticoid, inflammation, substrate specificity, MIF, iguratimod
Introduction
Macrophage migration inhibitory factor (MIF)3 is one of the first cytokine-like proteins to be discovered (1, 2). Although MIF was originally characterized as a T-cell factor that inhibits random migration of macrophages, subsequent investigations have shown that it is ubiquitously expressed and has pleiotropic effects on inflammation, chemotaxis, and cell survival and proliferation (3–5). As a cytokine, MIF binds a CD74-CD44 receptor complex to initiate downstream signaling via the p44/p42 mitogen-activated protein kinase pathway, which leads to up-regulation of prostaglandins and phospholipase A2 with subsequent pro-inflammatory effects (5–7). These effects are relevant to multiple inflammatory states, and MIF has been shown to have an important pathologic role in inflammatory conditions such as sepsis, rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis (8–11).
Glucocorticoids are the cornerstone of treatment for several diseases due to their potent anti-inflammatory and immunosuppressive effects. Unfortunately, chronic systemic glucocorticoid use is also associated with a potentially severe adverse effect profile that is a significant source of iatrogenic illness among patients with chronic disease (12, 13). Glucocorticoids initiate their effects via a range of genomic and nongenomic mechanisms, many of which rely on interactions with membrane-bound or cytosolic glucocorticoid receptors (14). A common theory holds that most therapeutic effects are mediated by transrepression downstream of the glucocorticoid receptor that results in decreased production of pro-inflammatory proteins, whereas adverse (along with some therapeutic) effects appear to be caused by transactivation. Recent work has indicated that the actual mechanistic properties of glucocorticoids are likely to be more complex (14, 15). Nonetheless, dissociation of these events remains a key goal in steroid therapeutics, and a true “steroid-sparing therapy” would allow for lower doses of glucocorticoids to be used to achieve the same therapeutic result with fewer adverse effects (16). The plant-derived compound A is an important candidate in this growing field of therapeutics (17, 18).
Unlike other cytokines, MIF is capable of overriding the anti-inflammatory effects of glucocorticoids, likely based on interference downstream of the glucocorticoid receptor at the level of MKP-1 (mitogen-activated protein kinase phosphatase-1) and glucocorticoid-induced leucine zipper, with subsequent effects on transrepression (19–21). In addition, MIF release is triggered rather than suppressed by glucocorticoids in multiple cell types, and plasma MIF levels fluctuate in a circadian fashion that mirrors plasma levels of glucocorticoids (19, 22). These properties have led some to suggest that MIF's primary function is as an endogenous counter-regulator of glucocorticoids (23). Human genetic studies have validated the importance of this interaction, showing a relationship between MIF promoter polymorphisms and glucocorticoid sensitivity in diseases such as juvenile arthritis and Crohn's disease (24, 25). Because MIF's interference with glucocorticoids exists downstream of the glucocorticoid receptor at the level of transrepression, it is reasonable to assume that an anti-MIF therapy would strengthen the therapeutic anti-inflammatory and immunosuppressive effects of glucocorticoids without increasing their adverse effects. Although multiple research groups have explored this concept by using anti-MIF small molecules to attenuate MIF override of glucocorticoids (26–29), these studies did not show whether the molecules had any effect in the absence of exogenously added MIF. This type of synergistic effect has been observed in the context of anti-MIF antibodies and MIF silencing by siRNA, and it has been suggested in studies of the small molecule ISO-1. However, a dedicated study of anti-MIF small molecules in this context is still lacking (20, 30–32).
In addition to its unique interaction with glucocorticoids, MIF has multiple properties that make it an attractive drug target. MIF is ubiquitously expressed and detectable in plasma at nanogram/ml concentrations (22, 33). Although MIF deletion is beneficial in multiple disease conditions, the MIF KO animal is healthy and long-lived, and in the absence of intervention it has no associated health problems (8, 10, 34). MIF also possesses multiple enzymatic activities, including a well characterized dopachrome tautomerase activity whose active site is relevant to MIF interactions with the CD74 receptor. This site has been exploited for the design and high throughput screening of small molecule inhibitors with disease-modifying potential in multiple inflammatory states (31, 35–38). Using the dopachrome tautomerase assay as a foundation, research groups have discovered multiple classes of reversible MIF inhibitors, including isoxazolines (27, 36, 39, 40), pyrimidazoles (41), coumarin and chromene analogs (42, 43), and aromatic amino acid Schiff bases (44). In addition, multiple individual compounds of interest have been identified; notable among these are the l- and d-isomers of the metabolic hormone T4, the former of which may function as an endogenous ligand of MIF (45). Although these compounds hold great potential for clinical application, preclinical studies of MIF inhibitors have proven problematic, in large part due to a lack of a unified approach to developing bioassays for MIF activity. Although many candidate assays are employed, including cytokine production (46), signal transduction through the ERK pathway (6), proliferation and apoptosis studies (5, 47), and chemotaxis experiments (4), these assays do not always produce robust results and are easily confounded by factors such as contaminating endotoxin in recombinant protein preparations.
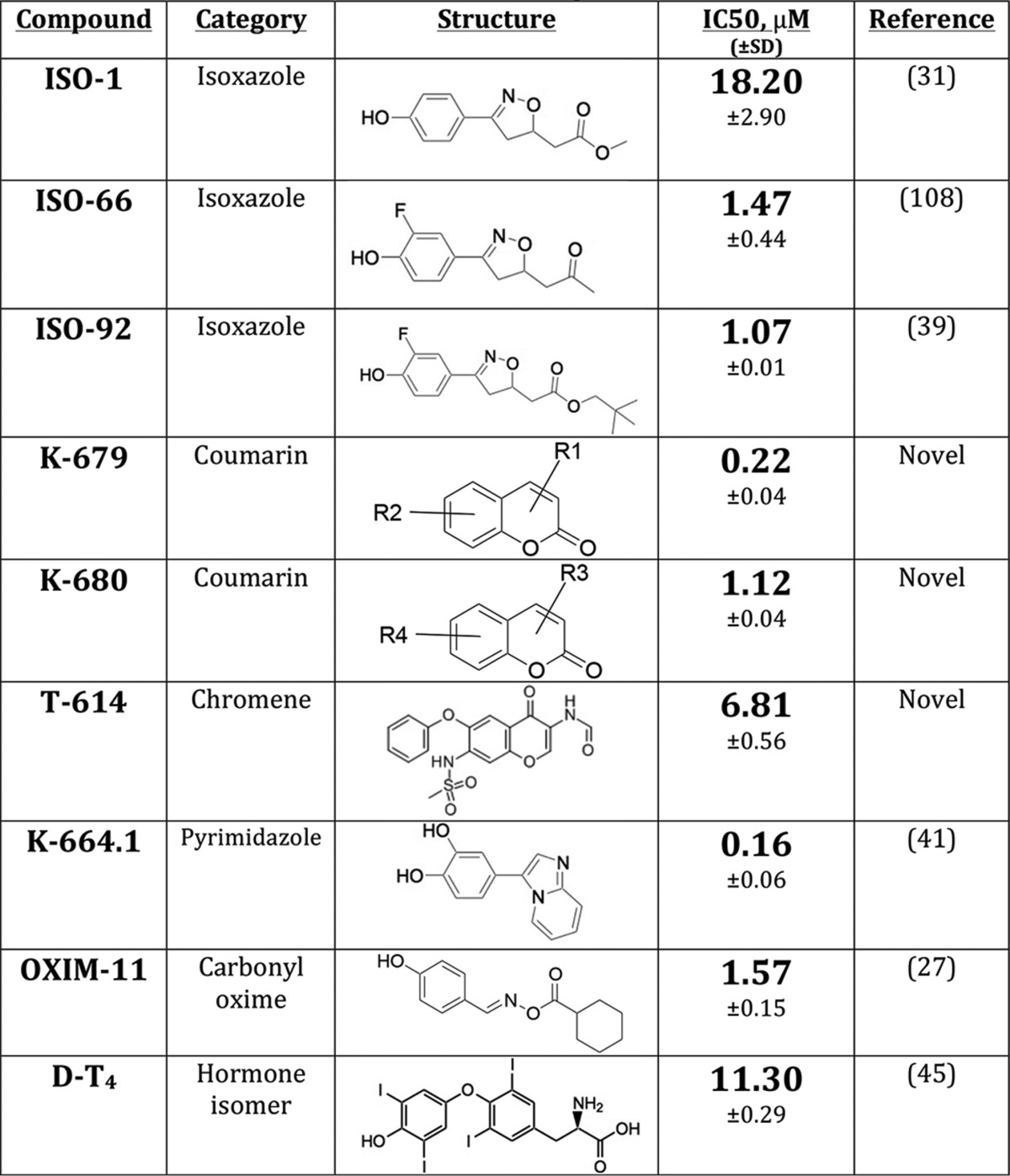
In our study, we selected compounds from multiple classes of MIF small molecule inhibitors (detailed in Table 1) and screened them for anti-inflammatory activity in an LPS-treated monocyte system. We discovered that, even within our small cohort, compounds segregated into at least three groups with distinct anti-inflammatory profiles. We selected the clinically utilized chromene derivative T-614 (iguratimod), which had low anti-inflammatory activity in our screen, for further study. Using MIF-dependent in vitro and in vivo studies, we determined that T-614 inhibits MIF and attenuates inflammatory disease in an MIF-dependent fashion. When tested alongside glucocorticoids in an in vitro context, we found that T-614 has significant additive effects with glucocorticoids in suppressing inflammation. We verified this in vivo in experimental autoimmune encephalomyelitis (EAE), showing that combination therapy with dexamethasone and T-614 is more efficacious in treating the disease phenotype than either drug alone. Our data suggest that iguratimod may exert its clinically observed anti-inflammatory activities via MIF inhibition and that this drug should be explored further as a potential steroid-sparing therapeutic in diseases such as multiple sclerosis.
TABLE 1.
Representative MIF inhibitory compounds selected for characterization
IC50 values are based on MIF dopachrome tautomerase activity as detailed under “Experimental Procedures”. Compounds previously characterized as MIF inhibitors are noted with a reference; other compounds are designated as “Novel”.
Results
Cytokine Release by Monocytes, Different Profiles of MIF Inhibitors
Small molecules of various classes with micromolar or lower IC50 in the MIF tautomerase assay were selected for biologic characterization in the context of LPS-treated human monocytes (Table 1). None of these compounds exhibited significant toxicity up to 50 μm in this context (data not shown). We found that although these molecules all have a similar profile of inhibition of MIF enzymatic activity, they exhibit diverse profiles of anti-inflammatory activity in this bioassay. The clearest distinctions were observable in TNFα release. The coumarin derivatives K-679 and K-680 almost completely suppressed TNFα release in monocytes; two isoxazole compounds (ISO-1 and ISO-66) as well as the pyrimidazole compound K-664.1 exhibited moderate suppression of TNFα release; whereas the chromene-derived T-614, isoxazole ISO-92, carbonyl oxime OXIM-11, and hormone isomer d-T4 almost completely spared TNFα at concentrations up to 50 μm (Fig. 1A). The release of the chemokines MCP-1 (macrophage chemoattractant protein 1) and IL-8 segregated similarly, with some exceptions: ISO-92 and K664.1 were both stronger suppressors of MCP-1 than TNFα; d-T4 exhibited moderate suppression of IL-8 compared with sparing of TNFα; and ISO-66 spared IL-8 despite moderately suppressing TNFα (Fig. 1B).
FIGURE 1.
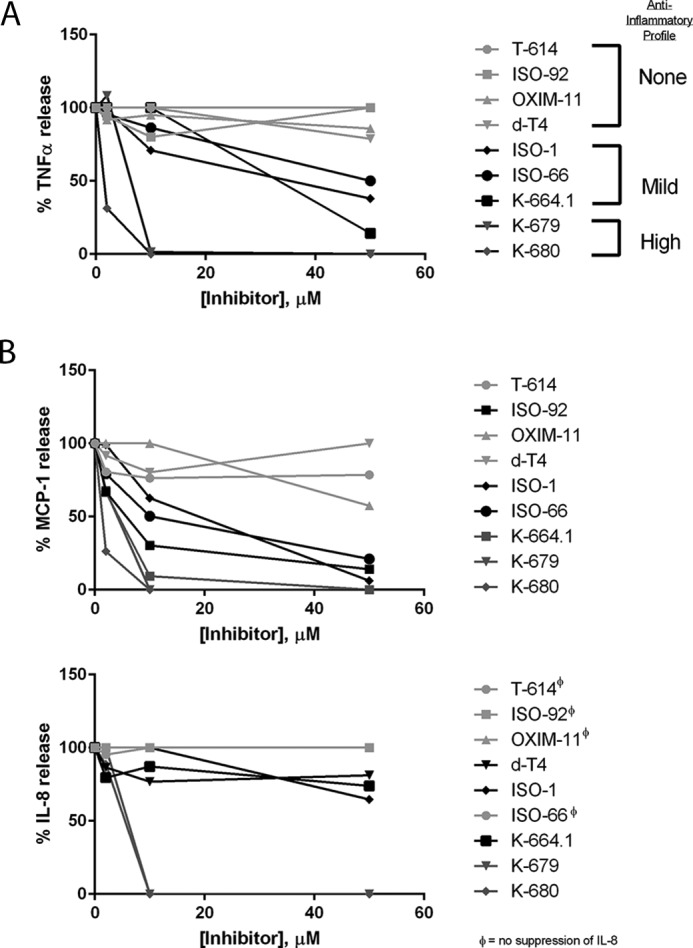
MIF inhibitors have distinct anti-inflammatory profiles, which are MIF-independent. A and B, human peripheral blood monocytes purified by negative selection were pretreated with the indicated dose of MIF inhibitor for 1 h prior to a 24-h stimulation with 1 ng/ml E. coli R515LPS, with results converted to percentage of maximum cytokine release and shown as an average of two independent experiments. MIF inhibitors showed at least three patterns of anti-TNFα activity (A) that are mostly consistent upon examination of chemokines MCP-1 and IL-8 (B). Compounds with no suppressive effect on IL-8 are indicated by φ. Results are shown as means of two independent experiments using duplicate samples.
The results of our phenotypic screen show that some MIF inhibitors are more anti-inflammatory than others, which might suggest that only strong anti-inflammatory compounds should advance to further testing in MIF-specific assays. However, we hypothesized that an MIF inhibitor with less profound effects on TNFα and other cytokines could still have usefulness in disease conditions where strong cytokine suppression may be undesirable, such as multiple sclerosis (48). Both T-614 and OXIM-11 exhibited minimal suppression for all the cytokines analyzed in this study. T-614 is clinically utilized in Asia, and its inventors have shown it to be strongly anti-inflammatory and efficacious in treating rheumatoid arthritis (49–51), which contrasts with the results of our phenotypic screen. Although putative mechanisms have been ascribed to the molecule, including COX-2 inhibition (52) or modulation of NFκB translocation (51), the data from these studies suggest that the molecular target of T-614 lies upstream of these factors. Identification of MIF as a molecular target for this drug would help clarify its experimental and clinical activities, as well as offering an anti-MIF clinical candidate with immediate applications as a repurposed drug. For these reasons, we determined that T-614 merited further investigation, and we sought to characterize it in MIF-specific assays.
T-614 Inhibits MIF in Vitro
Multiple approaches have been used to examine MIF activity in vitro, including measurement of glucocorticoid override and ERK phosphorylation. Our experience has been that these assays can produce inconsistent results and may involve non-MIF effects. To examine T-614 as a selective MIF inhibitor, we chose two bioassays where the readout is directly elicited by exogenously added rMIF protein. In the first assay, rMIF was found to significantly increase BrdU incorporation in synchronized Raji B cells at a concentration of 1 ng/ml, which mirrors the findings of Leng et al. (5) using thymidine incorporation in the same cells. T-614 did not affect BrdU incorporation on its own at concentrations as high as 200 μm, which is similar to previous observations (53); however, it did attenuate the rMIF effect at a concentration of 100 μm (Fig. 2A). For the second bioassay, IL-8 production was analyzed in adherence-purified peripheral blood monocytes, where we observed 100 ng/ml rMIF triggered a significant increase in IL-8 production over 24 h. Although T-614 did not affect IL-8 on its own and did not attenuate IL-8 release from LPS stimulation in enriched monocytes (Fig. 1B), it did dose-dependently suppress exogenous rMIF-induced IL-8 release from adherence-purified monocytes (Fig. 2B).
FIGURE 2.
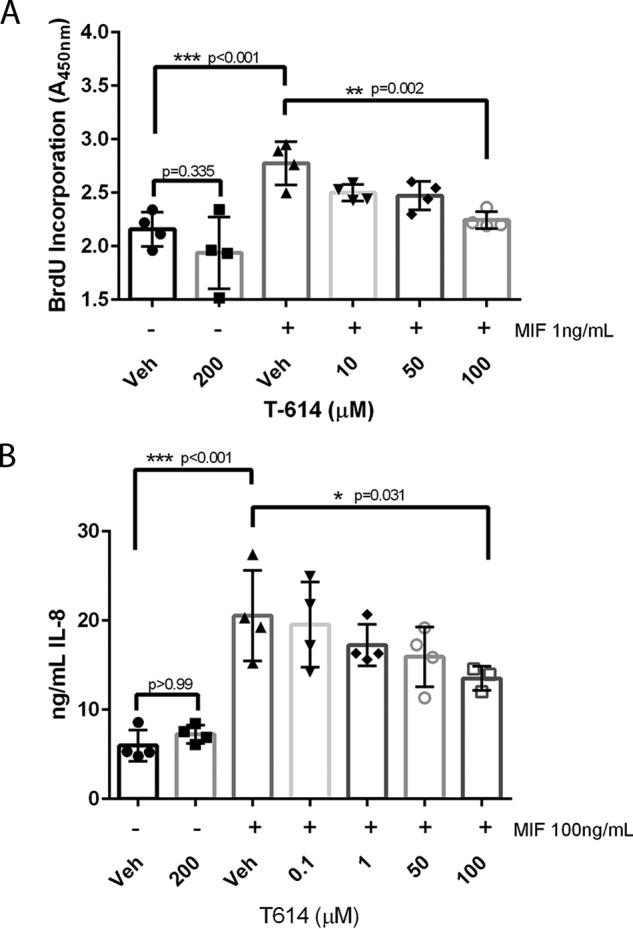
T-614 exhibits MIF-specific inhibition in vitro. A, human Raji B cells were synchronized for 24 h in 0.1–0.5% FBS, pretreated with the indicated doses of T-614 for 20 min, and then stimulated with 1 ng/ml rMIF or control for 24 h with BrdU added at 20 h. Results are expressed as absorbance values from a BrdU incorporation kit (Cell Signaling Technologies) and are representative of two independent experiments using quadruplicate samples. Results are expressed as mean ± S.D., and a one-way ANOVA was performed with indicated comparisons selected for a Bonferroni correction. B, adherence-purified human peripheral blood monocytes were pretreated with indicated doses of T-614 for 20 min and then stimulated with 100 ng/ml rMIF or control for 24 h before analysis with IL-8 ELISA. Results shown are representative of three independent experiments using quadruplicate samples. Results are expressed as mean ± S.D., and a one-way ANOVA was performed with indicated comparisons selected for a Bonferroni correction.
T-614 Inhibits MIF in Vivo
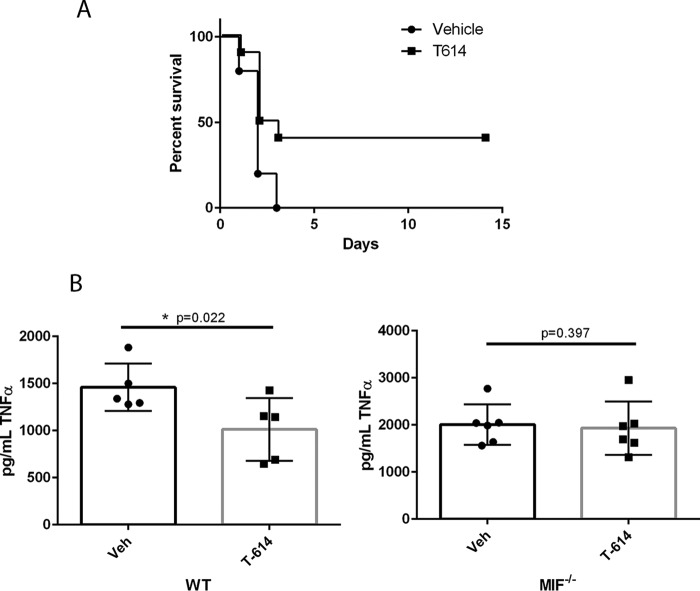
To test in vivo efficacy and selectivity of T-614 as an MIF inhibitor, we employed a murine endotoxemia model that has been well characterized in the context of MIF using both knock-out and inhibitory approaches (8, 33, 36). Using BALB/c mice that are vulnerable to endotoxemia, T-614-treated animals showed significantly increased survival after a lethal dose of LPS compared with vehicle-treated controls (Fig. 3A). T-614 treatment also attenuated TNFα release measured in serum isolated 90 min post-LPS administration in wild-type C57BL/6 mice. In MIF−/− miceon the same background, however, there was no significant effect of T-614 on serum TNFα 90 min post-LPS administration (Fig. 3B). These data suggest that T-614 can attenuate a systemic inflammatory response in vivo and suggest that its effects are MIF-specific in this context.
FIGURE 3.
T-614 exhibits MIF-specific inhibition in vivo. A, male BALB/c mice (n = 10/group) were treated with 5 mg/kg E. coli O111:B4 LPS to induce lethal endotoxemia and monitored for survival over 2 weeks. Survival data were analyzed using a log-rank test. B and bottom panel, male C57BL/6 (B, n = 5/group) and matched MIF−/− mice (bottom panel, n = 6/group) were administered a non-lethal dose of LPS and euthanized at 90 min for tissue collection. Data are expressed as mean ± S.D. with data from individual animals indicated and were analyzed using unpaired one-tailed t tests with p values indicated.
T-614 Is Additive with Glucocorticoids in Vitro
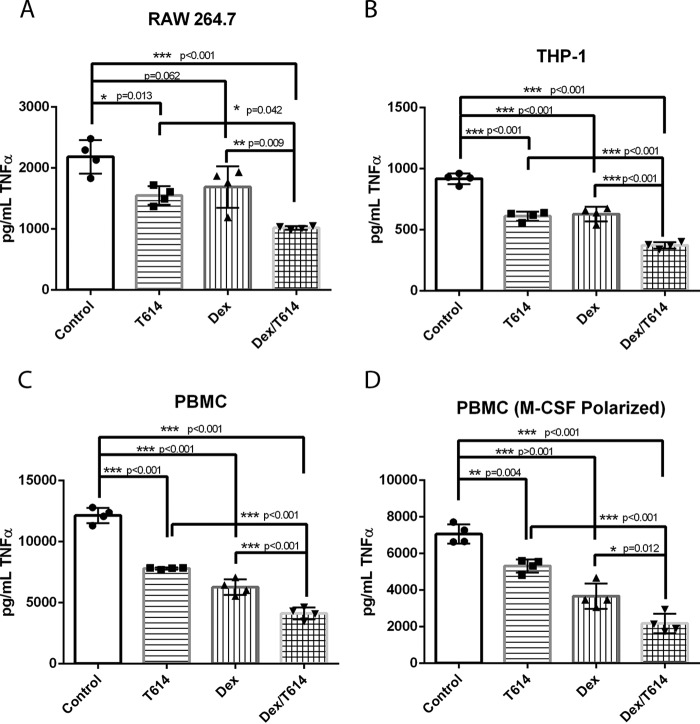
Glucocorticoid synergy has been demonstrated in the context of MIF in previous studies using RNA silencing and anti-MIF antibodies (20, 30). Additionally, the small molecule ISO-1 has shown some synergy with glucocorticoids in suppressing cytokines in LPS-treated peripheral blood monocytes (31) as well as dexamethasone-treated cultured nasal polyps (32). Because these studies used murine RAW 264.7 macrophages, human THP-1 monocytes, and primary human peripheral blood monocytes, we adapted similar systems for our study. For RAW 264.7 cells, individual pretreatment with inhibitor and dexamethasone attenuated TNFα release induced by 4-h stimulation with LPS, and the combination of the two drugs had a significant additive effect (Fig. 4A). A similar additive effect was observed in the setting of THP-1 cells stimulated with LPS for 16 h (Fig. 4B). To confirm the effect in primary cells, we tested adherence-purified human peripheral blood monocytes as well as M-CSF-polarized macrophages and again found that both T-614 and dexamethasone can individually attenuate LPS-induced TNFα release and that the combination suppressed it further (Fig. 4, C and D). We noted that although T-614 was generally cytokine-sparing up to 50 μm in primary monocytes enriched by magnetic selection (Fig. 1A), in the context of primary adherence-purified monocytes the drug did suppress TNFα at concentrations as low as 10 μm (data not shown). These variations might be attributable to differences between these monocyte preparations, and, indeed, it has been reported that the standard adherence protocol yields a relatively lower purity monocyte population that may have distinct inflammatory responses (54, 55).
FIGURE 4.
T-614 has additive anti-inflammatory effects when used with glucocorticoids in vitro. All cells were pretreated 20 min with T-614 or vehicle (PBS, pH 7.8) and 20 min with dexamethasone or vehicle (0.01% DMSO) and then stimulated with the indicated dose of E. coli O111:B4 LPS. Cell-free supernatants were collected at the indicated time points and analyzed for TNFα by ELISA. A, RAW 264.7 cells were treated using 100 μm T-614, 50 nm dexamethasone, 0.1 ng/ml LPS, and a 4-h stimulation with LPS. B, THP-1 cells were treated using 100 μm T-614, 50 nm dexamethasone, 5 ng/ml LPS, and a 16-h stimulation with LPS. C, adherence-purified human peripheral blood monocytes (PBMC, peripheral blood mononuclear cells) were treated using 100 μm T-614, 50 nm dexamethasone, 1 ng/ml LPS, and a 16-h stimulation with LPS. D, M-CSF polarized human macrophages were treated using 200 μm T-614, 50 nm dexamethasone, 0.5 ng/ml LPS, and a 16-h stimulation with LPS. All results are expressed as mean ± S.D. with replicate samples shown and are representative of three independent experiments using samples in quadruplicate. Data were analyzed using a one-way ANOVA with a Bonferroni correction.
T-614 Augments Low-dose Glucocorticoids in Vivo
Although MIF emerged early as a potential target in diseases where steroid therapy is a mainstay of treatment, to our knowledge no study has been undertaken actually pursuing anti-MIF treatment as a steroid-sparing therapy in disease or a disease model (56). Here, we chose a model of EAE for a pilot study exploring this question. EAE is a model of multiple sclerosis, a disease characterized by immune-mediated demyelination of the central nervous system, frequently resulting in disability (57). Intravenous or oral glucocorticoid treatment is commonly used in combination with other drugs for treatment of acute episodes and progressive disease (58, 59). The EAE model is characterized by T-cell infiltration into the central nervous system causing inflammation and myelin damage (60). Notably, previous work has established that MIF plays a significant role in this model, likely related to its ability to override the immunosuppressive effects of glucocorticoids; deletion of the MIF gene significantly increases the efficacy of glucocorticoid treatment after disease induction (61). T-614 has also been tested in this model and was found to significantly attenuate disease progression in vivo, likely in relation to inhibition of T-cell proliferation and cytokine production (62).
To examine potential synergy between glucocorticoids and T-614, we induced EAE in C57BL/6 mice by immunization with the MOG(35–55) peptide, and EAE disease peaked around day 20 post-immunization. Animals treated with either 0.1 mg/kg dexamethasone (3 days post-onset) or ∼12.5 mg/kg T-614 (daily post-onset) showed minor attenuation in disease scores (Fig. 5A). Animals co-treated with the two drugs showed a further decrease in disease scores compared with either drug alone, which was significantly different from control animals during peak disease (days 18–23). Additionally, animals receiving the combination therapy had a significantly lower area under the curve measure (AUC) for clinical scores, an indicator of less severe disease overall (Fig. 5B) (63). Together with our studies in vitro, these in vivo data suggest that T-614 may have promise as a steroid-sparing therapy.
FIGURE 5.
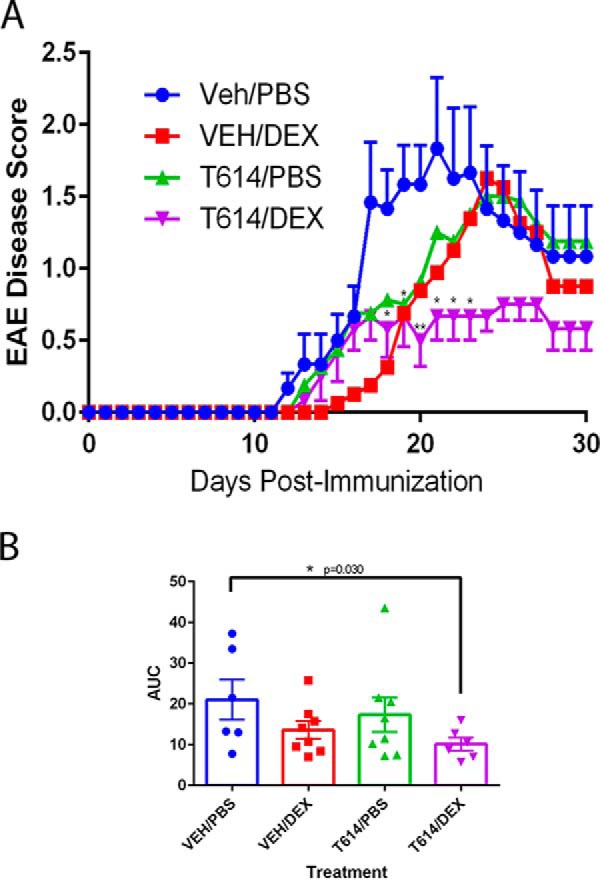
T-614 in combination with glucocorticoids attenuates disease in EAE. A, wild-type C57BL/6 mice (n = 6–8/group) were immunized to induce EAE using MOG(35–55) peptide and monitored daily for clinical disease. Results are expressed as mean ± S.E. for VEH/PBS and T-614/DEX groups, and data were analyzed using unpaired Student's t tests compare VEH/PBS and T614/DEX groups with * indicating significant differences at p < 0.05 and ** indicating significant differences at p < 0.01 (one-tailed p values). B, area under the curve (AUC) values for treatment groups over the entire EAE disease course. Results are expressed as mean ± S.E. with data from individual animals indicated, and data were analyzed using an unpaired one-tailed Student's t test comparing VEH/PBS and T614/DEX groups.
Discussion
Biologic systems are complex, and this complicates target-based drug design. Although a target such as MIF may be implicated in multiple disease processes via well elucidated mechanisms, probing the full scope of interactions between that target and the vast array of proteins and small molecules in the cellular milieu can be a daunting task. MIF is classically viewed as a pro-inflammatory cytokine, with candidate anti-MIF therapies promoted as potentially valuable anti-inflammatory drugs (3, 40). Suppression of an acute-phase cytokine such as TNFα would seem to be desirable in a general anti-inflammatory drug. However, several studies have shown that even this classical pro-inflammatory cytokine can have protective roles in tissue repair and regeneration. In the central nervous system, TNFα has been shown to induce proliferation of neural stem cells, likely via interactions with TNFR2 (48, 64, 65). Treatment with anti-TNFα has been linked to the development of demyelinating disease, which may relate to TNFα's roles in repair and neurogenesis (66–68). It stands to reason that when developing a drug for use in multiple sclerosis, such as an MIF inhibitor (61), it would be useful to design the drug to spare TNFα to avoid worsening of the demyelinating disease.
Our study has determined that diverse compounds with MIF-inhibitory activity segregate into at least three populations when tested in a broad in vitro inflammatory assay, LPS stimulation of monocytes (Fig. 1, A and B). There are multiple possibilities that could explain this observation. The differential profiles may originate from non-MIF off-target effects, such that highly anti-inflammatory compounds such as K-680 are interacting with an unknown substrate. Our endotoxemia data would support this possibility, because the poorly anti-inflammatory T-614 had no effect in the absence of MIF (Fig. 3B). Unfortunately, the toxicity profile of K-680 barred further study in murine cells or in vivo models, preventing us from demonstrating a true difference in MIF selectivity between these molecules. Another possibility could involve differing modes of action on MIF itself, where some molecules deactivate MIF pro-inflammatory activity in a manner independent of tautomerase inhibition. The utility of the tautomerase site in predicting MIF inflammatory activities has been a topic of some debate, with several groups showing inflammatory activity even in tautomerase-compromised MIF proteins or peptide fragments (69, 70), and a recent study indicated that the extent of solvent exposure in MIF tautomerase inhibitors may be an important element in their interference with MIF-CD74 binding and subsequent inflammatory effects (71). Future study is clearly needed to clarify the source of these anti-inflammatory profiles, which could have important implications to MIF drug design. However, because we hypothesized that cytokine-sparing MIF inhibitors might have unique clinical utility, we selected the clinically utilized chromene derivative T-614 for further investigation.
Compound T-614, better known as iguratimod, was created in the late 1980s and characterized as an anti-inflammatory drug by phenotypic screening in the early 1990s. Early communications indicated that the drug is orally bioavailable and capable of attenuating edema and joint destruction in arthritis models as well as exhibiting analgesic properties (72). These effects were viewed as potentially related to cyclooxygenase inhibition, because T-614 inhibits both the activity and transcription of COX-2. However, the mechanism of action of T-614 seems distinct from standard non-steroidal anti-inflammatory drugs (NSAIDs) (52, 73). Notably, one study found that T-614 inhibits both release and intracellular accumulation of the cytokine IL-1β in LPS-stimulated human peripheral blood monocytes, whereas the NSAID indomethacin inhibits release but increases intracellular accumulation of this cytokine (74). T-614 was also found to inhibit production of cytokines such as TNFα, IL-1β, IL-6, IL-8, and MCP-1 in a variety of cell types (50, 51, 74, 75). Over time, several more activities were attributed to T-614, including inhibition of immunoglobulin production by B cells (76), suppression of the IL-17 axis (77, 78), and promotion of bone anabolism by modulation of both osteoblastic and osteoclastic differentiation (79, 80). Multiple studies have been conducted searching for a mechanism of action of this drug, which has variably been reported as COX-2 inhibition (52) or NFκB modulation (50, 51). Both of these studies provided evidence that T-614 was affecting a target upstream of these inflammatory regulators. To our knowledge, no subsequent study has identified a molecular target for this drug, a daunting task given the diverse activities involved (81).
In our study, we have for the first time identified a molecular target that may explain some of the observed activities for T-614. T-614 interacts with the MIF trimer, inhibiting MIF's tautomerase enzymatic activity with an IC50 value comparable with ISO-1, the best-characterized MIF inhibitor (Table 1) (40). This interaction is relevant to MIF biology because T-614 was able to inhibit MIF-induced proinflammatory effects, including proliferation of B cells and cytokine release from monocytes (Fig. 2, A and B). Moreover, these effects are selective for MIF, because T-614 did not suppress systemic inflammation in the absence of MIF (Fig. 3B). MIF is known to exist in human plasma in nanogram/ml concentrations, which based on our IC50 data would be inhibited by T-614 at steady state concentrations (23, 82). All these data suggest that anti-inflammatory effects of T-614 may be mediated at least partially through MIF inhibition.
MIF is a pleiotropic molecule, and MIF inhibition could potentially underlie other observed activities of T-614. For example, MIF may be involved in T-614-mediated inhibition of immunoglobulin production from B cells; MIF has been previously linked to immunoglobulin production (83) and has a well known role in promoting survival of B cells (84). T-614 is known to suppress the IL-17 signaling axis, and MIF is known to stimulate this axis (85). T-614 has been shown to inhibit osteoclastic differentiation, which is induced by MIF (86, 87). It is not difficult to imagine that MIF may be targeted by T-614 in all of these activities, and it could be responsible for some or all of the efficacy of T-614 as a disease-modifying anti-rheumatic drug. Of further note, several studies have found that MIF has a significant role in the pathogenesis of rheumatoid arthritis and may be a relevant target in the disease (9, 88–91). Future studies of this drug may consider investigating the potential MIF dependence of these activities.
In addition to establishing T-614 as a MIF-targeting molecule, we also provide evidence for novel clinical applications of this drug. Also known as iguratimod, T-614 is currently clinically available in Japan and China as a daily oral formulation administered at 25–50 mg daily, which has shown safety and efficacy in improving symptoms and disease progression in rheumatoid arthritis both as a monotherapy (92–94) and in combination with methotrexate (95). Several preclinical studies have suggested that the drug may also have utility in other settings, such as multiple sclerosis (62) and cachexia in the context of adenocarcinoma (96). MIF is an influential player in a large variety of disease processes, including autoimmune (10, 11, 97, 98), neurologic (99, 100), metabolic (101), and oncologic conditions (102–105). Several of these disease processes benefit from treatment with glucocorticoids, of which MIF is an endogenous counter-regulator (19). Here, we show that T-614, acting as an MIF inhibitor, can synergize with glucocorticoids in an in vitro inflammatory model (Fig. 4, A–D) as well as in vivo in the EAE model (Fig. 5, A and B), suggesting potential clinical applications as a steroid adjunct. We have also determined that T-614 exhibits fewer effects on TNFα compared with other MIF inhibitors in the context of enriched primary human monocytes (Fig. 1, A and B). This latter finding stands in contrast to previous findings that found that T-614 has significant cytokine-suppressing effects (51, 74, 75) as well as our own findings in adherence purified monocytes (Fig. 4C), which we would attribute to differences in the preparation of these cells (54, 55). Because T-614 is steroid-sparing and lacks profound TNFα suppression in certain contexts, we believe that it would be an excellent candidate for preclinical study as a steroid adjunct in multiple sclerosis, where TNFα inhibition may exacerbate demyelination (66). However, further study is needed to determine whether T-614 impacts the transactivation effects of glucocorticoids, which would be undesirable in a steroid-sparing therapeutic (17, 18).
In sum, our findings highlight the complexities of target-based drug design, because we observed that even high affinity MIF inhibitors have distinct anti-inflammatory effects in vitro. Our screen highlighted the clinically available chromene derivative compound T-614 (iguratimod) as a MIF inhibitor with a low anti-inflammatory profile, which further investigation revealed has potential as a steroid-sparing therapeutic. We hope that our work encourages the repurposing of iguratimod to other MIF-relevant diseases, including multiple sclerosis.
Experimental Procedures
Reagents
All reagents were purchased from Sigma or Fisher unless otherwise indicated. Compound T-614 (iguratimod) was purchased from Ontario Chemical (Guelph, Ontario, Canada); it was solubilized in alkaline solution, pH 7.8 (106), for in vitro and endotoxemia studies and in 5% dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS) for EAE studies. Recombinant human MIF protein for catalytic characterization and in vitro use was expressed in Escherichia coli BLD1(DE3) cells and purified as described previously (31). In vitro readouts of MIF bioactivity were confirmed (where applicable) with bioactive recombinant human MIF purchased from Shenandoah Biotechnologies (Warwick, PA). Prior to in vitro use, endotoxin content was confirmed to be less than 0.05 EU/μg protein by a colorimetric end point Limulus amebocyte lysate (LAL) assay (Lonza, Allendale NJ). Unless otherwise indicated, cytokine ELISAs were purchased as DuoSet kits from R&D Systems (Minneapolis, MN) and used according to the manufacturer's instructions.
MIF Dopachrome Tautomerase Activity
The enzymatic activity of MIF on freshly prepared l-dopachrome methyl ester was assayed as described previously (44). Sterile recombinant human MIF was maintained in TBS, pH 7.4, at concentrations ranging from 0.5 to 1 mg/ml for up to 6 months without significant loss of activity. Inhibitory compounds were solubilized in DMSO, added to a cuvette containing 1 μg/ml rMIF in PBS, and mixed thoroughly; dopachrome substrate was then added, and absorbance at 475 nm was monitored for 20 s to measure activity.
Cell Culture
Human Raji B and THP-1 cells were maintained in suspension culture in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin/streptomycin, 2 mm l-glutamine, and 55 μm 2-mercaptoethanol (THP-1 cells only) (Life Technologies, Inc.). Cells were passaged by dilution three times weekly with total media replacement every 3 weeks. Raji B cells were used for 2 months post-thaw, and THP-1 cells were used only during weeks 2 and 3 post-thaw. RAW 264.7 macrophages were maintained in adherent culture in DMEM, 4.5 g/dl glucose supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin/streptomycin, and 2 mm l-glutamine; cells were passaged by scraping and used only until passage 20. All cells were cultured in a humidified incubator at 37 °C, 5% CO2. Unless otherwise indicated, all cells were purchased from the American Tissue Culture Collection (ATCC, Manassas, VA) and stored as passage 5 aliquots in liquid nitrogen.
Preparation of Peripheral Blood Cells
Human peripheral blood was collected in sodium heparin (IRB 12-200A) or obtained as Leuko PAKs from New York Blood Center (New York). Mononuclear cells were isolated by density gradient centrifugation in Ficoll-Paque Plus (GE Healthcare). Monocytes were either purified by 2-h adherence to Primaria culture plates (Corning Life Sciences) or enriched by negative magnetic selection using monocyte isolation kit II (Miltenyi Biotech, Auburn, CA). All monocyte preparations were cultured in RPMI 1640 medium supplemented with 10% human AB serum, 100 units/ml penicillin/streptomycin, and 2 mm l-glutamine and used within 24 h. Macrophages were differentiated from adherence-purified monocytes by incubation with 10 ng/ml human M-CSF (Sigma) for 7 days, with media replenishment performed on days 3 and 5.
Cytokine Production Assays
For LPS-induced cytokine release, negative selection-enriched human monocytes were plated at a density of 2 × 105 cells/ml in 96-well plates. Cells were pretreated with MIF inhibitors in 0.1% DMSO (final) for 1 h before stimulation with LPS from E. coli R515 (Axxora, Farmingdale, NY) for 24 h. Cell-free supernatants were collected and stored at −80 °C for cytokine determinations by ELISA; 1:2 dilutions were used for TNFα and 1:3 dilutions for IL-8 and MCP-1. Cytotoxicity was assessed using a Cytotox96 non-radioactive cytotoxicity kit (Promega, Madison, WI); no significant cytotoxicity was observed for the compounds used unless otherwise indicated.
Although the production of cytokines in response to MIF stimulation alone is controversial, the production of IL-8 by MIF stimulation of peripheral blood monocytes has been confirmed in multiple contexts (46, 69). Human peripheral blood monocytes purified by adherence were plated at a density of ∼2 × 105 cells/well in a 96-well plate. Cells were pretreated with T-614 or vehicle for 30 min prior to stimulation with MIF for 24 h. Cell-free supernatants were recovered and subjected to IL-8 MAX standard ELISA using a 1:100 dilution (Biolegend, San Diego). Cytotoxicity was assessed by a neutral red assay as described previously, using a 1-h incorporation period (107); no significant cytotoxicity effects were observed unless otherwise indicated.
MIF-induced Proliferation
For this bioassay, we adapted the method of Leng et al. (5) with some modifications. Briefly, human Raji B cells were plated at a density of 0.5 × 104 cells/well in a 96-well plate and synchronized by incubation for 24 h in RPMI 1640 medium supplemented with 0.1–0.5% FBS. Synchronized cells were pretreated with T-614 or vehicle for 30 min prior to stimulation with MIF for 24 h. At 20 h BrdU was added to cells and quantified using a BrdU Cell proliferation assay kit (Cell Signaling Technology, Danvers, MA).
Glucocorticoid Synergy in Vitro
The methods of Roger et al. (20) and Kerschbaumer et al. were adapted for these experiments (30). RAW 264.7 cells were plated at a density of 1 × 105 cells/well in a 96-well plate and incubated overnight (16 h) before pretreatment with T-614 or vehicle for 20 min, treatment with dexamethasone (Alfa Aesar, Ward Hill, MA) solubilized in 0.01% DMSO for 20 min, and finally stimulation with LPS from E. coli O111:B4 (Sigma) for 4 h. Cell-free supernatants were collected and analyzed immediately with a murine TNFα ELISA at a 1:10 dilution. THP-1 cells were plated at a density of 2 × 105 cells/well in a 96-well plate and used the same day with the same treatment conditions as RAW 264.7 cells, except that THP-1 cells were incubated with LPS for 16 h prior to isolation of cell-free supernatants. Supernatants were analyzed using a human TNFα ELISA at a 1:10 dilution. Human peripheral blood mononuclear cells isolated by adherence (described above) were plated at a density of 2 × 105 cells/well in a 96-well plate and used the same day with the same treatment conditions as THP-1 cells. Supernatants were analyzed using a human TNFα ELISA at a 1:20 dilution. Human macrophages were differentiated from adherence-purified monocytes by a 1-week culture with 10 ng/ml M-CSF on cells plated at a density of 2 × 105 cells/well and used when an astrocytic morphology was clearly observed in >50% of the cells. Cells were treated under the same conditions as THP-1 cells, and supernatants were analyzed using a human TNFα ELISA at a 1:10 dilution.
For all above experiments, cytotoxicity was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (1–2-h incorporation); no significant cytotoxicity effects were observed under any treatment conditions unless otherwise noted.
Animal Experiments
The Institutional Animal Care and Use Committees of the Feinstein Institute for Medical Research and/or the University of Texas at San Antonio reviewed and approved all animal protocols prior to initiation of experiments. Male BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used for endotoxemia experiments at ages 8–10 weeks. MIF KO animals were maintained on a C57BL/6 NCr background from Charles River Laboratories (Stone Ridge, NY); these animals were used alongside matched wild-type animals for in vivo experiments at ages 8–12 weeks.
Endotoxemia
Endotoxemia was induced by intraperitoneal injection of LPS from E. coli O111:B4 (Sigma). In BALB/c animals, 5 mg/kg LPS was used as a lethal dose for survival experiments; animals were treated with T-614 (20 mg/kg i.p.) 0.5 h prior to LPS, 6 h after LPS, and then once daily for 3 days and monitored for survival over 2 weeks. In C57BL/6 animals, 20 mg/kg LPS was used as non-lethal dose for plasma cytokine experiments; animals were pretreated with T-614 (20 mg/kg i.p.) twice, one dose each at 2 and 0.5 h prior to LPS administration, and euthanized at 90 min post-LPS by CO2 asphyxiation with cervical dislocation. Blood was collected by cardiac puncture and allowed to clot 20 min at room temperature and 20 min at 4 °C; sera were isolated by centrifugation at 300 × g for 10 min and stored at −20 °C for further analysis by TNFα ELISA (1:3 dilution).
EAE
EAE was induced by subcutaneous injection of C57BL/6 mice with 200 μg of MOG(35–55) peptide (United Biochemical Research, Seattle) in 50 μl of complete Freund's adjuvant, in addition to 400 ng of pertussis toxin administered intraperitoneally on days 0 and 2. Animals were monitored and scored daily for clinical signs of EAE as follows (61): 1, limp tail; 2, moderate hind limb weakness; 3, complete hind limb paralysis; 4, quadriplegia or premoribund state; 5, death. Upon reaching a disease score of 1.0, mice were treated intraperitoneally with 0.1 mg/kg dexamethasone (DEX) or phosphate-buffered saline (PBS) daily for 3 days, as well as 250 μg of T-614 or vehicle (VEH) daily until the end of the study.
Author Contributions
C. M., T. C., and B. S. collaborated to produce the data in Fig. 1. K. F. C. and M. H. synthesized/provided MIF inhibitory compounds in Table 1, characterized their IC50 values, and helped prepare T-614 for in vitro and in vivo experiments. J. B. conducted experiments encompassing Figs. 2–4 and wrote most of the paper. S. N., J. C., and T. F. collaborated to produce the data in Fig. 5. Y. A. conceived the idea of the project, oversaw the preparation of the paper, and gave final approval of the version to be published.
This work was supported by National Institutes of Health Grant R21AI110771 from NIAID (to T. F.) and Small Business Innovation Research Grant 1R43AR062401 (to T. C.). Yousef Al-Abed is the inventor or co-inventor of several small molecule inhibitors of MIF.
- MIF
- macrophage migration inhibitory factor
- ANOVA
- analysis of variance
- AUC
- area under the curve
- EAE
- experimental autoimmune encephalomyelitis
- LAL
- Limulus amebocyte lysate
- M-CSF
- macrophage colony-stimulating factor
- MOG
- myelin oligodendrocyte glycoprotein
- NSAID
- non-steroidal anti-inflammatory drug
- PBS
- phosphate-buffered saline
- rMIF
- recombinant MIF
- RPMI
- Roswell Park Memorial Institute
- DEX
- dexamethasone
- VEH
- vehicle
- T4
- thyroxine.
References
- 1.David J. R. (1966) Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. U.S.A. 56, 72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom B. R., and Bennett B. (1966) Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science 153, 80–82 [DOI] [PubMed] [Google Scholar]
- 3.Calandra T., and Roger T. (2003) Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3, 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernhagen J., Krohn R., Lue H., Gregory J. L., Zernecke A., Koenen R. R., Dewor M., Georgiev I., Schober A., Leng L., Kooistra T., Fingerle-Rowson G., Ghezzi P., Kleemann R., McColl S. R., Bucala R., et al. (2007) MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13, 587–596 [DOI] [PubMed] [Google Scholar]
- 5.Leng L., Metz C. N., Fang Y., Xu J., Donnelly S., Baugh J., Delohery T., Chen Y., Mitchell R. A., and Bucala R. (2003) MIF signal transduction initiated by binding to CD74. J. Exp. Med. 197, 1467–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell R. A., Metz C. N., Peng T., and Bucala R. (1999) Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J. Biol. Chem. 274, 18100–18106 [DOI] [PubMed] [Google Scholar]
- 7.Shi X., Leng L., Wang T., Wang W., Du X., Li J., McDonald C., Chen Z., Murphy J. W., Lolis E., Noble P., Knudson W., and Bucala R. (2006) CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 25, 595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozza M., Satoskar A. R., Lin G., Lu B., Humbles A. A., Gerard C., and David J. R. (1999) Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189, 341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onodera S., Kaneda K., Mizue Y., Koyama Y., Fujinaga M., and Nishihira J. (2000) Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J. Biol. Chem. 275, 444–450 [DOI] [PubMed] [Google Scholar]
- 10.de Jong Y. P., Abadia-Molina A. C., Satoskar A. R., Clarke K., Rietdijk S. T., Faubion W. A., Mizoguchi E., Metz C. N., Alsahli M., ten Hove T., Keates A. C., Lubetsky J. B., Farrell R. J., Michetti P., van Deventer S. J., et al. (2001) Development of chronic colitis is dependent on the cytokine MIF. Nat. Immunol. 2, 1061–1066 [DOI] [PubMed] [Google Scholar]
- 11.Powell N. D., Papenfuss T. L., McClain M. A., Gienapp I. E., Shawler T. M., Satoskar A. R., and Whitacre C. C. (2005) Cutting edge: macrophage migration inhibitory factor is necessary for progression of experimental autoimmune encephalomyelitis. J. Immunol. 175, 5611–5614 [DOI] [PubMed] [Google Scholar]
- 12.Curtis J. R., Westfall A. O., Allison J., Bijlsma J. W., Freeman A., George V., Kovac S. H., Spettell C. M., and Saag K. G. (2006) Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 55, 420–426 [DOI] [PubMed] [Google Scholar]
- 13.Huscher D., Thiele K., Gromnica-Ihle E., Hein G., Demary W., Dreher R., Zink A., and Buttgereit F. (2009) Dose-related patterns of glucocorticoid-induced side effects. Ann. Rheum. Dis. 68, 1119–1124 [DOI] [PubMed] [Google Scholar]
- 14.Jiang C.-L., Liu L., Li Z., and Buttgereit F. (2015) The novel strategy of glucocorticoid drug development via targeting nongenomic mechanisms. STEROIDS 102, 27–31 [DOI] [PubMed] [Google Scholar]
- 15.Ayroldi E., Macchiarulo A., and Riccardi C. (2014) Targeting glucocorticoid side effects: selective glucocorticoid receptor modulator or glucocorticoid-induced leucine zipper? A perspective. FASEB J. 28, 5055–5070 [DOI] [PubMed] [Google Scholar]
- 16.Schäcke H., Schottelius A., Döcke W.-D., Strehlke P., Jaroch S., Schmees N., Rehwinkel H., Hennekes H., and Asadullah K. (2004) Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc. Natl. Acad. Sci. U.S.A. 101, 227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Bosscher K., Vanden Berghe W., Beck I. M., Van Molle W., Hennuyer N., Hapgood J., Libert C., Staels B., Louw A., and Haegeman G. (2005) A fully dissociated compound of plant origin for inflammatory gene repression. Proc. Natl. Acad. Sci. U.S.A. 102, 15827–15832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wüst S., Tischner D., John M., Tuckermann J. P., Menzfeld C., Hanisch U.-K., van den Brandt J., Lühder F., and Reichardt H. M. (2009) Therapeutic and adverse effects of a non-steroidal glucocorticoid receptor ligand in a mouse model of multiple sclerosis. PLoS ONE 4, e8202–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calandra T., Bernhagen J., Metz C. N., Spiegel L. A., Bacher M., Donnelly T., Cerami A., and Bucala R. (1995) MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377, 68–71 [DOI] [PubMed] [Google Scholar]
- 20.Roger T., Chanson A.-L., Knaup-Reymond M., and Calandra T. (2005) Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur. J. Immunol. 35, 3405–3413 [DOI] [PubMed] [Google Scholar]
- 21.Fan H., Kao W., Yang Y. H., Gu R., Harris J., Fingerle-Rowson G., Bucala R., Ngo D., Beaulieu E., and Morand E. F. (2014) Macrophage migration inhibitory factor inhibits the antiinflammatory effects of glucocorticoids via glucocorticoid-induced leucine zipper. Arthritis Rheumatol. 66, 2059–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrovsky N., Socha L., Silva D., Grossman A. B., Metz C., and Bucala R. (2003) Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter-regulator. Immunol. Cell Biol. 81, 137–143 [DOI] [PubMed] [Google Scholar]
- 23.Flaster H., Bernhagen J., Calandra T., and Bucala R. (2007) The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Mol. Endocrinol. 21, 1267–1280 [DOI] [PubMed] [Google Scholar]
- 24.Vivarelli M., D'Urbano L. E., Insalaco A., Lunt M., Jury F., Tozzi A. E., Ravelli A., Martini A., Donn R., and De Benedetti F. (2007) Macrophage migration inhibitory factor (MIF) and oligoarticular juvenile idiopathic arthritis (o-JIA): association of MIF promoter polymorphisms with response to intra-articular glucocorticoids. Clin. Exp. Rheumatol. 25, 775–781 [PubMed] [Google Scholar]
- 25.Griga T., Wilkens C., Wirkus N., Epplen J., Schmiegel W., and Klein W. (2007) A polymorphism in the macrophage migration inhibitory factor gene is involved in the genetic predisposition of Crohn's disease and associated with cumulative steroid doses. Hepatogastroenterology 54, 784–786 [PubMed] [Google Scholar]
- 26.Senter P. D., Al-Abed Y., Metz C. N., Benigni F., Mitchell R. A., Chesney J., Han J., Gartner C. G., Nelson S. D., Todaro G. J., and Bucala R. (2002) Inhibition of macrophage migration inhibitory factor (MIF) tautomerase and biological activities by acetaminophen metabolites. Proc. Natl. Acad. Sci. U.S.A. 99, 144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crichlow G. V., Cheng K. F., Dabideen D., Ochani M., Aljabari B., Pavlov V. A., Miller E. J., Lolis E., and Al-Abed Y. (2007) Alternative chemical modifications reverse the binding orientation of a pharmacophore scaffold in the active site of macrophage migration inhibitory factor. J. Biol. Chem. 282, 23089–23095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouertatani-Sakouhi H., El-Turk F., Fauvet B., Roger T., Le Roy D., Karpinar D. P., Leng L., Bucala R., Zweckstetter M., Calandra T., and Lashuel H. A. (2009) A new class of isothiocyanate-based irreversible inhibitors of macrophage migration inhibitory factor. Biochemistry 48, 9858–9870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L., Zhang Y., Zheng L., Qiao C., Li Y., Li D., Zhen X., and Hou T. (2014) Discovery of novel inhibitors targeting the macrophage migration inhibitory factor via structure-based virtual screening and bioassays. J. Med. Chem. 57, 3737–3745 [DOI] [PubMed] [Google Scholar]
- 30.Kerschbaumer R. J., Rieger M., Völkel D., Le Roy D., Roger T., Garbaraviciene J., Boehncke W.-H., Müllberg J., Hoet R. M., Wood C. R., Antoine G., Thiele M., Savidis-Dacho H., Dockal M., Ehrlich H., et al. (2012) Neutralization of macrophage migration inhibitory factor (MIF) by fully human antibodies correlates with their specificity for the β-sheet structure of MIF. J. Biol. Chem. 287, 7446–7455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubetsky J. B., Dios A., Han J., Aljabari B., Ruzsicska B., Mitchell R., Lolis E., and Al-Abed Y. (2002) The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents. J. Biol. Chem. 277, 24976–24982 [DOI] [PubMed] [Google Scholar]
- 32.Stathas T., Athanassiou S. D., Drakouli S., Giannopoulou E., Mastronikolis N. S., Naxakis S., and Aletras A. J. (2013) MIF attenuates the suppressive effect of dexamethasone on IL-6 production by nasal polyp. Eur. Rev. Med. Pharmacol. Sci. 17, 1455–1466 [PubMed] [Google Scholar]
- 33.Bacher M., Meinhardt A., Lan H. Y., Mu W., Metz C. N., Chesney J. A., Calandra T., Gemsa D., Donnelly T., Atkins R. C., and Bucala R. (1997) Migration inhibitory factor expression in experimentally induced endotoxemia. Am. J. Pathol. 150, 235–246 [PMC free article] [PubMed] [Google Scholar]
- 34.Harper J. M., Wilkinson J. E., and Miller R. A. (2010) Macrophage migration inhibitory factor-knockout mice are long lived and respond to caloric restriction. FASEB J. 24, 2436–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosengren E., Bucala R., Aman P., Jacobsson L., Odh G., Metz C. N., and Rorsman H. (1996) The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol. Med. 2, 143–149 [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Abed Y., Dabideen D., Aljabari B., Valster A., Messmer D., Ochani M., Tanovic M., Ochani K., Bacher M., Nicoletti F., Metz C., Pavlov V. A., Miller E. J., and Tracey K. J. (2005) ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J. Biol. Chem. 280, 36541–36544 [DOI] [PubMed] [Google Scholar]
- 37.Healy Z. R., Liu H., Holtzclaw W. D., and Talalay P. (2011) Inactivation of tautomerase activity of macrophage migration inhibitory factor by sulforaphane: a potential biomarker for anti-inflammatory intervention. Cancer Epidemiol. Biomarkers Prev. 20, 1516–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouertatani-Sakouhi H., Liu M., El-Turk F., Cuny G. D., Glicksman M. A., and Lashuel H. A. (2010) Kinetic-based high throughput screening assay to discover novel classes of macrophage migration inhibitory factor inhibitors. J. Biomol. Screen. 15, 347–358 [DOI] [PubMed] [Google Scholar]
- 39.Cheng K.-F., and Al-Abed Y. (2006) Critical modifications of the ISO-1 scaffold improve its potent inhibition of macrophage migration inhibitory factor (MIF) tautomerase activity. Bioorg. Med. Chem. Lett. 16, 3376–3379 [DOI] [PubMed] [Google Scholar]
- 40.Al-Abed Y., and VanPatten S. (2011) MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med. Chem. 3, 45–63 [DOI] [PubMed] [Google Scholar]
- 41.Vujicic M., Nikolic I., Krajnovic T., Cheng K.-F., VanPatten S., He M., Stosic-Grujicic S., Stojanovic I., Al-Abed Y., and Saksida T. (2014) Novel inhibitors of macrophage migration inhibitory factor prevent cytokine-induced β cell death. Eur. J. Pharmacol. 740, 683–689 [DOI] [PubMed] [Google Scholar]
- 42.Orita M., Yamamoto S., Katayama N., Aoki M., Takayama K., Yamagiwa Y., Seki N., Suzuki H., Kurihara H., Sakashita H., Takeuchi M., Fujita S., Yamada T., and Tanaka A. (2001) Coumarin and chromen-4-one analogues as tautomerase inhibitors of macrophage migration inhibitory factor: discovery and x-ray crystallography. J. Med. Chem. 44, 540–547 [DOI] [PubMed] [Google Scholar]
- 43.Orita M., Yamamoto S., Katayama N., and Fujita S. (2002) Macrophage migration inhibitory factor and the discovery of tautomerase inhibitors. Curr. Pharm. Des. 8, 1297–1317 [DOI] [PubMed] [Google Scholar]
- 44.Dios A., Mitchell R. A., Aljabari B., Lubetsky J., O'Connor K., Liao H., Senter P. D., Manogue K. R., Lolis E., Metz C., Bucala R., Callaway D. J., and Al-Abed Y. (2002) Inhibition of MIF bioactivity by rational design of pharmacological inhibitors of MIF tautomerase activity. J. Med. Chem. 45, 2410–2416 [DOI] [PubMed] [Google Scholar]
- 45.Al-Abed Y., Metz C. N., Cheng K. F., Aljabari B., VanPatten S., Blau S., Lee H., Ochani M., Pavlov V. A., Coleman T., Meurice N., Tracey K. J., and Miller E. J. (2011) Thyroxine is a potential endogenous antagonist of macrophage migration inhibitory factor (MIF) activity. Proc. Natl. Acad. Sci. U.S.A. 108, 8224–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kudrin A., Scott M., Martin S., Chung C.-W., Donn R., McMaster A., Ellison S., Ray D., Ray K., and Binks M. (2006) Human macrophage migration inhibitory factor: a proven immunomodulatory cytokine? J. Biol. Chem. 281, 29641–29651 [DOI] [PubMed] [Google Scholar]
- 47.Hudson J. D., Shoaibi M. A., Maestro R., Carnero A., Hannon G. J., and Beach D. H. (1999) A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 190, 1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Widera D., Mikenberg I., Elvers M., Kaltschmidt C., and Kaltschmidt B. (2006) Tumor necrosis factor α triggers proliferation of adult neural stem cells via IKK/NF-κB signaling. BMC Neurosci. 7, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mucke H. A. (2012) Iguratimod: a new disease-modifying antirheumatic drug. Drugs Today 48, 577–586 [DOI] [PubMed] [Google Scholar]
- 50.Kohno M., Aikawa Y., Tsubouchi Y., Hashiramoto A., Yamada R., Kawahito Y., Inoue K., Kusaka Y., Kondo M., and Sano H. (2001) Inhibitory effect of T-614 on tumor necrosis factor-α induced cytokine production and nuclear factor-κB activation in cultured human synovial cells. J. Rheumatol. 28, 2591–2596 [PubMed] [Google Scholar]
- 51.Aikawa Y., Yamamoto M., Yamamoto T., Morimoto K., and Tanaka K. (2002) An anti-rheumatic agent T-614 inhibits NF-κB activation in LPS- and TNF-α-stimulated THP-1 cells without interfering with IκBα degradation. Inflamm. Res. 51, 188–194 [DOI] [PubMed] [Google Scholar]
- 52.Tanaka K., Kawasaki H., Kurata K., Aikawa Y., Tsukamoto Y., and Inaba T. (1995) T-614, a novel antirheumatic drug, inhibits both the activity and induction of cyclooxygenase-2 (COX-2) in cultured fibroblasts. Jpn. J. Pharmacol. 67, 305–314 [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto T., Aikawa Y., Funaki J., and Tanaka K. (2007) Immunopharmacological studies of a disease-modifying antirheumatic drug Iguratimod (T-614)–its effect on immunoglobulin production and lymphocyte proliferation. Jpn. Pharmacol. Ther. 35, 561–569 [Google Scholar]
- 54.Zhou L., Somasundaram R., Nederhof R. F., Dijkstra G., Faber K. N., Peppelenbosch M. P., and Fuhler G. M. (2012) Impact of human granulocyte and monocyte isolation procedures on functional studies. Clin. Vaccine Immunol. 19, 1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnston L., Harding S. A., and La Flamme A. C. (2015) Comparing methods for ex vivo characterization of human monocyte phenotypes and in vitro responses. Immunobiology 220, 1305–1310 [DOI] [PubMed] [Google Scholar]
- 56.Greven D., Leng L., and Bucala R. (2010) Autoimmune diseases: MIF as a therapeutic target. Expert Opin. Ther. Targets 14, 253–264 [DOI] [PubMed] [Google Scholar]
- 57.Compston A., and Coles A. (2002) Multiple sclerosis. Lancet 359, 1221–1231 [DOI] [PubMed] [Google Scholar]
- 58.Ciccone A., Beretta S., Brusaferri F., Galea I., Protti A., and Spreafico C. (2008) Corticosteroids for the long-term treatment in multiple sclerosis. Cochrane Database Syst. Rev. 2008, CD006264. [DOI] [PubMed] [Google Scholar]
- 59.Ratzer R., Iversen P., Börnsen L., Dyrby T. B., Romme Christensen J., Ammitzbøll C., Madsen C. G., Garde E., Lyksborg M., Andersen B., Hyldstrup L., Sørensen P. S., Siebner H. R., and Sellebjerg F. (2016) Monthly oral methylprednisolone pulse treatment in progressive multiple sclerosis. Mult. Scler. 22, 926–934 [DOI] [PubMed] [Google Scholar]
- 60.Robinson A. P., Harp C. T., Noronha A., and Miller S. D. (2014) The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb. Clin. Neurol. 122, 173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji N., Kovalovsky A., Fingerle-Rowson G., Guentzel M. N., and Forsthuber T. G. (2015) Macrophage migration inhibitory factor promotes resistance to glucocorticoid treatment in EAE. Neurol. Neuroimmunol. Neuroinflamm. 2, e139-e139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aikawa Y., Tanuma N., Shin T., Makino S., Tanaka K., and Matsumoto Y. (1998) A new anti-rheumatic drug, T-614, effectively suppresses the development of autoimmune encephalomyelitis. J. Neuroimmunol. 89, 35–42 [DOI] [PubMed] [Google Scholar]
- 63.Fleming K. K., Bovaird J. A., Mosier M. C., Emerson M. R., LeVine S. M., and Marquis J. G. (2005) Statistical analysis of data from studies on experimental autoimmune encephalomyelitis. J. Neuroimmunol. 170, 71–84 [DOI] [PubMed] [Google Scholar]
- 64.Wu J. P., Kuo J. S., Liu Y. L., and Tzeng S. F. (2000) Tumor necrosis factor-α modulates the proliferation of neural progenitors in the subventricular/ventricular zone of adult rat brain. Neurosci. Lett. 292, 203–206 [DOI] [PubMed] [Google Scholar]
- 65.Iosif R. E., Ekdahl C. T., Ahlenius H., Pronk C. J., Bonde S., Kokaia Z., Jacobsen S. E., and Lindvall O. (2006) Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J. Neurosci. 26, 9703–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsumoto T., Nakamura I., Miura A., Momoyama G., and Ito K. (2013) New-onset multiple sclerosis associated with adalimumab treatment in rheumatoid arthritis: a case report and literature review. Clin. Rheumatol. 32, 271–275 [DOI] [PubMed] [Google Scholar]
- 67.Kaltsonoudis E., Voulgari P. V., Konitsiotis S., and Drosos A. A. (2014) Demyelination and other neurological adverse events after anti-TNF therapy. Autoimmun. Rev. 13, 54–58 [DOI] [PubMed] [Google Scholar]
- 68.Dooley D., Vidal P., and Hendrix S. (2014) Immunopharmacological intervention for successful neural stem cell therapy: new perspectives in CNS neurogenesis and repair. Pharmacol. Ther. 141, 21–31 [DOI] [PubMed] [Google Scholar]
- 69.Dickerhof N., Schindler L., Bernhagen J., Kettle A. J., and Hampton M. B. (2015) Macrophage migration inhibitory factor (MIF) is rendered enzymatically inactive by myeloperoxidase-derived oxidants but retains its immunomodulatory function. Free Radic. Biol. Med. 89, 498–511 [DOI] [PubMed] [Google Scholar]
- 70.Nguyen M. T., Beck J., Lue H., Fünfzig H., Kleemann R., Koolwijk P., Kapurniotu A., and Bernhagen J. (2003) A 16-residue peptide fragment of macrophage migration inhibitory factor, MIF-(50–65), exhibits redox activity and has MIF-like biological functions. J. Biol. Chem. 278, 33654–33671 [DOI] [PubMed] [Google Scholar]
- 71.Pantouris G., Syed M. A., Fan C., Rajasekaran D., Cho T. Y., Rosenberg E. M. Jr, Bucala R., Bhandari V., and Lolis E. J. (2015) An analysis of MIF structural features that control functional activation of CD74. Chem. Biol. 22, 1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka K., Shimotori T., Makino S., Aikawa Y., Inaba T., Yoshida C., and Takano S. (1992) Pharmacological studies of the new antiinflammatory agent 3-formylamino-7-methylsulfonylamino-6-phenoxy-4H-1-benzopyran-4-one. 1st communication: Antiinflammatory, analgesic, and other related properties. Arzneimittel Forschung 42, 935–944 [PubMed] [Google Scholar]
- 73.Tanaka K., Makino S., Shimotori T., Aikawa Y., Inaba T., and Yoshida C. (1992) Pharmacological studies of the new antiinflammatory agent 3-formylamino-7-methylsulfonylamino-6-phenoxy-4′-1-benzopyran-4-one. 2nd communication: effect on the arachidonic acid cascades. Arzneimittel Forschung 42, 945–950 [PubMed] [Google Scholar]
- 74.Tanaka K., Aikawa Y., Kawasaki H., Asaoka K., Inaba T., and Yoshida C. (1992) Pharmacological studies on 3-formylamino-7-methylsulfonylamino-6-phenoxy-4H-1-benzopyran-4-one (T-614), a novel antiinflammatory agent. 4th communication: inhibitory effect on the production of interleukin-1 and interleukin-6. J. Pharmacobio-dyn. 15, 649–655 [DOI] [PubMed] [Google Scholar]
- 75.Kawakami A., Tsuboi M., Urayama S., Matsuoka N., Yamasaki S., Hida A., Aoyagi T., Furuichi I., Nakashima T., Migita K., Kawabe Y., Nakashima M., Origuchi T., and Eguchi K. (1999) Inhibitory effect of a new anti-rheumatic drug T-614 on costimulatory molecule expression, cytokine production, and antigen presentation by synovial cells. J. Lab. Clin. Med. 133, 566–574 [DOI] [PubMed] [Google Scholar]
- 76.Tanaka K., Yamamoto T., Aikawa Y., Kizawa K., Muramoto K., Matsuno H., and Muraguchi A. (2003) Inhibitory effects of an anti-rheumatic agent T-614 on immunoglobulin production by cultured B cells and rheumatoid synovial tissues engrafted into SCID mice. Rheumatology 42, 1365–1371 [DOI] [PubMed] [Google Scholar]
- 77.Luo Q., Sun Y., Liu W., Qian C., Jin B., Tao F., Gu Y., Wu X., Shen Y., and Xu Q. (2013) A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J. Immunol. 191, 4969–4978 [DOI] [PubMed] [Google Scholar]
- 78.Wei Y., Sun X., Hua M., Tan W., Wang F., and Zhang M. (2015) Inhibitory effect of a novel antirheumatic drug T-614 on the IL-6-induced RANKL/OPG, IL-17, and MMP-3 expression in synovial fibroblasts from rheumatoid arthritis patients. Biomed. Res. Int. 2015, 214683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuriyama K., Higuchi C., Tanaka K., Yoshikawa H., and Itoh K. (2002) A novel anti-rheumatic drug, T-614, stimulates osteoblastic differentiation in vitro and bone morphogenetic protein-2-induced bone formation in vivo. Biochem. Biophys. Res. Commun. 299, 903–909 [DOI] [PubMed] [Google Scholar]
- 80.Gan K., Yang L., Xu L., Feng X., Zhang Q., Wang F., Tan W., and Zhang M. (2016) Iguratimod (T-614) suppresses RANKL-induced osteoclast differentiation and migration in RAW264.7 cells via NF-κB and MAPK pathways. Int. Immunopharmacol. 35, 294–300 [DOI] [PubMed] [Google Scholar]
- 81.Tanaka K. (2009) Iguratimod (T-614): A novel disease modifying anti-rheumatic drug. Rheumatol. Rep. 1, e4 [Google Scholar]
- 82.Eisai Co., Ltd. (2016) Careram® tablets (package insert). Eisai Co. Ltd., Tokyo, Japan [Google Scholar]
- 83.Bacher M., Metz C. N., Calandra T., Mayer K., Chesney J., Lohoff M., Gemsa D., Donnelly T., and Bucala R. (1996) An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl. Acad. Sci. U.S.A. 93, 7849–7854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gore Y., Starlets D., Maharshak N., Becker-Herman S., Kaneyuki U., Leng L., Bucala R., and Shachar I. (2008) Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J. Biol. Chem. 283, 2784–2792 [DOI] [PubMed] [Google Scholar]
- 85.Stojanović I., Cvjetićanin T., Lazaroski S., Stosic-Grujicić S., and Miljković D. (2009) Macrophage migration inhibitory factor stimulates interleukin-17 expression and production in lymph node cells. Immunology 126, 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madeira M. F., Queiroz-Junior C. M., Costa G. M., Santos P. C., Silveira E. M., Garlet G. P., Cisalpino P. S., Teixeira M. M., Silva T. A., and Souza Dda G. (2012) MIF induces osteoclast differentiation and contributes to progression of periodontal disease in mice. Microbes Infect. 14, 198–206 [DOI] [PubMed] [Google Scholar]
- 87.Gu R., Santos L. L., Ngo D., Fan H., Singh P. P., Fingerle-Rowson G., Bucala R., Xu J., Quinn J. M., and Morand E. F. (2015) Macrophage migration inhibitory factor is essential for osteoclastogenic mechanisms in vitro and in vivo mouse model of arthritis. Cytokine 72, 135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mikulowska A., Metz C. N., Bucala R., and Holmdahl R. (1997) Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II-induced arthritis in mice. J. Immunol. 158, 5514–5517 [PubMed] [Google Scholar]
- 89.Santos L., Hall P., Metz C., Bucala R., and Morand E. F. (2001) Role of macrophage migration inhibitory factor (MIF) in murine antigen-induced arthritis: interaction with glucocorticoids. Clin. Exp. Immunol. 123, 309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Onodera S., Nishihira J., Koyama Y., Majima T., Aoki Y., Ichiyama H., Ishibashi T., and Minami A. (2004) Macrophage migration inhibitory factor up-regulates the expression of interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid arthritis patients: common transcriptional regulatory mechanism between interleukin-8 and interleukin-1? Arthritis Rheum. 50, 1437–1447 [DOI] [PubMed] [Google Scholar]
- 91.Morand E. F., Leech M., and Bernhagen J. (2006) MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat. Rev. Drug Discov. 5, 399–410 [DOI] [PubMed] [Google Scholar]
- 92.Lü L.-J., Teng J.-L., Bao C.-D., Han X.-H., Sun L.-Y., Xu J.-H., Li X.-F., and Wu H.-X. (2008) Safety and efficacy of T-614 in the treatment of patients with active rheumatoid arthritis: a double blind, randomized, placebo-controlled and multicenter trial. Chin. Med. J. 121, 615–619 [PubMed] [Google Scholar]
- 93.Lü L.-J., Bao C.-D., Dai M., Teng J.-L., Fan W., Du F., Yang N.-P., Zhao Y.-H., Chen Z.-W., Xu J.-H., He P.-G., Wu H.-X., Tao Y., Zhang M.-J., Han X.-H., et al. (2009) Multicenter, randomized, double-blind, controlled trial of treatment of active rheumatoid arthritis with T-614 compared with methotrexate. Arthritis Rheum. 61, 979–987 [DOI] [PubMed] [Google Scholar]
- 94.Okamura K., Yonemoto Y., Suto T., Okura C., and Takagishi K. (2015) Efficacy at 52 weeks of daily clinical use of iguratimod in patients with rheumatoid arthritis. Mod. Rheumatol. 25, 534–539 [DOI] [PubMed] [Google Scholar]
- 95.Duan X.-W., Zhang X.-L., Mao S.-Y., Shang J.-J., and Shi X.-D. (2015) Efficacy and safety evaluation of a combination of iguratimod and methotrexate therapy for active rheumatoid arthritis patients: a randomized controlled trial. Clin. Rheumatol. 34, 1513–1519 [DOI] [PubMed] [Google Scholar]
- 96.Tanaka K., Urata N., Mikami M., Ogasawara M., Matsunaga T., Terashima N., and Suzuki H. (2007) Effect of iguratimod and other anti-rheumatic drugs on adenocarcinoma colon 26-induced cachexia in mice. Inflamm. Res. 56, 17–23 [DOI] [PubMed] [Google Scholar]
- 97.Lang T., Foote A., Lee J. P., Morand E. F., and Harris J. (2015) MIF: implications in the pathoetiology of systemic lupus erythematosus. Front. Immunol. 6, 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stosic-Grujicic S., Stojanovic I., Maksimovic-Ivanic D., Momcilovic M., Popadic D., Harhaji L., Miljkovic D., Metz C., Mangano K., Papaccio G., Al-Abed Y., and Nicoletti F. (2008) Macrophage migration inhibitory factor (MIF) is necessary for progression of autoimmune diabetes mellitus. J. Cell. Physiol. 215, 665–675 [DOI] [PubMed] [Google Scholar]
- 99.Bloom J., and Al-Abed Y. (2014) MIF: mood improving/inhibiting factor? J. Neuroinflammation 11, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Savaskan N. E., Fingerle-Rowson G., Buchfelder M., and Eyüpoglu I. Y. (2012) Brain miffed by macrophage migration inhibitory factor. Int. J. Cell Biol. 2012, 139573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morrison M. C., and Kleemann R. (2015) Role of macrophage migration inhibitory factor in obesity, insulin resistance, type 2 diabetes, and associated hepatic co-morbidities: a comprehensive review of human and rodent studies. Front. Immunol. 6, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tarnowski M., Grymula K., Liu R., Tarnowska J., Drukala J., Ratajczak J., Mitchell R. A., Ratajczak M. Z., and Kucia M. (2010) Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. Mol. Cancer Res. 8, 1328–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He X. X., Chen K., Yang J., Li X. Y., Gan H. Y., Liu C. Y., Coleman T. R., and Al-Abed Y. (2009) Macrophage migration inhibitory factor promotes colorectal cancer. Mol. Med. 15, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chesney J., Metz C., Bacher M., Peng T., Meinhardt A., and Bucala R. (1999) An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol. Med. 5, 181–191 [PMC free article] [PubMed] [Google Scholar]
- 105.Chesney J. A., and Mitchell R. A. (2015) 25 years on: a retrospective on migration inhibitory factor in tumor angiogenesis. Mol. Med. 21, Suppl. 1, 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sawada T., Hashimoto S., Tohma S., Nishioka Y., Nagai T., Sato T., Ito K., Inoue T., Iwata M., and Yamamoto K. (2000) Inhibition of l-leucine methyl ester mediated killing of THP-1, a human monocytic cell line, by a new anti-inflammatory drug, T614. Immunopharmacology 49, 285–294 [DOI] [PubMed] [Google Scholar]
- 107.Repetto G., del Peso A., and Zurita J. L. (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 3, 1125–1131 [DOI] [PubMed] [Google Scholar]
- 108.Ioannou K., Cheng K.-F., Crichlow G. V., Birmpilis A. I., Lolis E. J., Tsitsilonis O. E., and Al-Abed Y. (2014) ISO-66, a novel inhibitor of macrophage migration, shows efficacy in melanoma and colon cancer models. Int. J. Oncol. 45, 1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]