Abstract
In this report, we investigated the pathogenic mechanism underlying the deafness-associated mitochondrial(mt) tRNAAsp 7551A > G mutation. The m.7551A > G mutation is localized at a highly conserved nucleotide(A37), adjacent (3′) to the anticodon, which is important for the fidelity of codon recognition and stabilization in functional tRNAs. It was anticipated that the m.7551A > G mutation altered the structure and function of mt-tRNAAsp. The primer extension assay demonstrated that the m.7551A > G mutation created the m1G37 modification of mt-tRNAAsp. Using cybrid cell lines generated by transferring mitochondria from lymphoblastoid cell lines derived from a Chinese family into mitochondrial DNA(mtDNA)-less (ρo) cells, we demonstrated the significant decreases in the efficiency of aminoacylation and steady-state level of mt-tRNAAsp in mutant cybrids, compared with control cybrids. A failure in metabolism of mt-tRNAAsp caused the variable reductions in mtDNA-encoded polypeptides in mutant cybrids. Impaired mitochondrial translation led to the respiratory phenotype in mutant cybrids. The respiratory deficiency lowed mitochondrial adenosine triphosphate production and increased the production of oxidative reactive species in mutant cybrids. Our data demonstrated that mitochondrial dysfunctions caused by the m.7551A > G mutation are associated with deafness. Our findings may provide new insights into the pathophysiology of maternally transmitted deafness that was manifested by altered nucleotide modification of mitochondrial tRNA.
INTRODUCTION
The defects in mitochondrial protein synthesis have been associated with both syndromic deafness (hearing loss with other medical problems such as diabetes) and nonsyndromic deafness (hearing loss is the only obvious medical problem) (1–5). The mitochondrial translation machinery composed of 2 rRNAs and 22 tRNAs, encoded by mitochondrial DNA (mtDNA), and more than 150 proteins (ribosomal proteins, ribosomal assembly proteins, aminoacyl–tRNA synthetases, tRNA-modifying enzymes, and several initiation, elongation and termination factors), encoded by nuclear genes (6–8). Mutations in the LARS2 and NARS2 encoding mitochondrial leucyl–tRNA synthetase and asparaginyl–tRNA synthetase have been associated with deafness, respectively (9–10). The mtDNA mutations have been shown to be the important causes of both syndromic and nonsydromic deafness (3–5). Of these, the m.1555A > G and m.1494C > T mutations in the 12S rRNA gene have been associated with both aminoglycoside-induced and nonsyndromic deafness in many families worldwide (3,4,11,12). The most prevalent mtDNA mutations associated with syndromic deafness are the MELAS-associated m.3243A > G mutation in the mt–tRNALeu(UUR) gene (13) and MERRF-associated m.8344A > G mutation in the mt–tRNALys gene (14), while the nonsyndromic deafness-associated mtDNA mutations included the mt–tRNASer(UCN) 7445A > G, 7472insC, 7505T > C and 7511T > C, mt–tRNAHis 12201T > C, mt–tRNAGly 10003T > C and mt–tRNAIle 4295A > G mutations (15–21). These mt–tRNA mutations altered their structures and functions, including the processing of the mt–tRNA from the primary transcripts, stability of the folded secondary structure, the charging of the mt–tRNA, or the codon–anticodon interaction in the process of translation (5,22,23). The m.7445A > G mutation altered the processing of the 3′ end mt–tRNASer(UCN) precursor (24), the m.7511T > C mutations affected the stability of mt-tRNASer(UCN) (25) and m.12201T > C mutation altered the aminoacylation of mt–tRNAHis (20). However, the pathophysiology of these tRNA mutations remains poorly understood.
As the part of a genetic screening program for deafness in a cohort of 2651 Han Chinese affected subjects, we identified the novel m.7551A > G mutation in the mt–tRNAAsp gene in one Han Chinese pedigrees with maternal transmission of nonsyndromic deafness (19,26). As shown in Figure 1, the m.7551A > G mutation is localized at a highly conserved nucleotide (A37), adjacent (3′) to the anticodon of mt–tRNAAsp (22,23). There were no modifications of i6A37 or t6A37 in the human mitochondrial tRNAAsp (27), although the nucleotides at position 37 (A or G) of tRNAs are often modified by methylthiolation (28–29). The modifications at position 37 were shown to contribute to the high fidelity of codon recognition and to the structural formation and stabilization of functional tRNAs (30–33). Thus, the substitution of A with G at position 37 of the mt–tRNAAsp may introduce the m1G37 modification of this tRNA, thereby altering the structure and function of mt–tRNAAsp. In particular, the mutation may affect the aminoacylation capacity and stability of this mt–tRNA and then impair mitochondrial translation. It was also proposed that an impairment of mitochondrial translation caused by the mt–tRNA mutation alters the respiration, production of adenosine triphoshate (ATP) and reactive oxygen species (ROS). To further investigate the pathogenic mechanism of the m.7551A > G mutation, cybrid cell lines were constructed by transferring mitochondria from lymphoblastoid cell lines derived from an affected matrilineal relative in a Chinese family carrying the mtDNA mutation and from a control individual lacking the mtDNA mutation, into human mtDNA-less (ρ°) cells (34–35). First, we examined if the m.7551A > G mutation created the m1G37 modification of mt–tRNAAsp by using primer extension. These resultant cybrid cell lines were then assessed for the effects of the mtDNA mutation on the aminoacylation capacity and stability of this mt–tRNA, mitochondrial translation, respiration and the production of ATP and ROS as well as mitochondrial membrane potential.
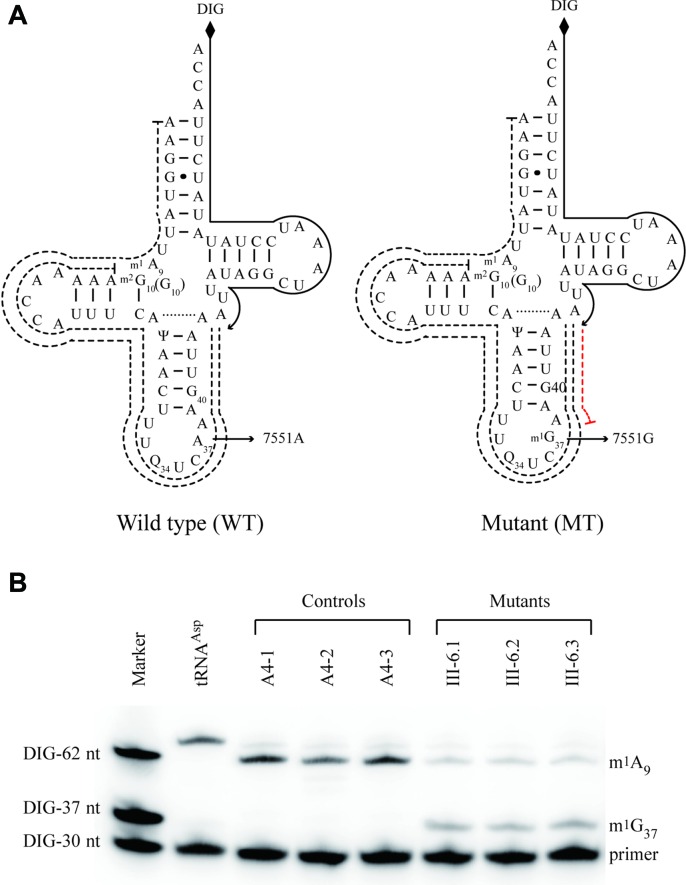
Figure 1.
The m.7551A > G mutation introduced the methylation of G37 in mt-tRNAAsp. (A) Schematic of methylation shown in the cloverleaf structures of human mitochondrial tRNAAsp. An arrow denotes the location of the m.7551A > G mutation. Solid lines represent the DIG-labeled oligonucleotide probe specific for mt–tRNAAsp. Broken lines represent the potential stops of primer extension caused by m1A or m1G modification. (B) Primer extension demonstrated the creation of m1G37 in the mt–tRNAAsp carrying the m.7511A > G mutation. One microgram of mitochondrial RNA from three control cybrids and three mutant cybrids were used for this investigation. DIG-labeled oligonucleotide specific for mt–tRNAAsp was then annealed and the primer extensions were performed. The primer extension termination products caused by m1A or m1G modification are showed.
MATERIALS AND METHODS
Cell lines and culture conditions
Immortalized lymphoblastoid cell lines were generated from one affected matrilineal relative (III-6) of the Chinese family carrying the m.7551A > G mutation (26) and one genetically unrelated Chinese control individual (A4) belonging to the same mtDNA haplogroup but lacking the mutation (Supplementary Table S1) (36). These cell lines were grown in RPMI 1640 medium with 10% fetal bovine serum. The bromodeoxyuridine (BrdU) resistant 143B.TK− cell line was grown in Dulbecco's Modified Eagle Medium (Life Technologies) (containing 4.5 mg of glucose and 0.11 mg pyruvate/ml), supplemented with 100 μg of BrdU/ml and 5% fetal bovine serum. The mtDNA-less ρ°206 cell line, derived from 143B.TK− (34,35) was grown under the same conditions as the parental line, except for the addition of 50 μg of uridine/ml. Transformation by cytoplasts of mtDNA-less ρ°206 cells using one affected subject (III-6) and one control individual (A4) was performed as described elsewhere (34,35,37). All cybrid cell lines constructed with enucleated lymphoblastoid cell lines were maintained in the same medium as the 143B.TK− cell line. An analysis for the presence and level of the m.7551A > G mutation was carried out as described previously (26). The quantification of mtDNA copy number from different cybrids was performed as detailed elsewhere (38).
Detection of m1G37 modification in mt–tRNAAsp using primer extension
To determine the nucleotide positions of m1G modification of mt–tRNAAsp, a primer extension experiment was performed by a modified procedure, as detailed elsewhere (39,40). Mitochondria were isolated from three mutant and three control cybrids (∼2.0 × 108 cells), as described previously (41). Total mitochondrial RNAs were isolated using TOTALLY RNATM kit (Ambion), as described previously. A DNA primer (5′-TGGUAAGATATATAGGATTTAGCCTATAAT-3′) complementary to the 3′ end of the mt-tRNAAsp was 5′ end labeled with non-radioactive digoxin (DIG). Primescript II 1st Strand cDNA Synthesis Kit (TAKARA) was used for reverse transcription with DIG-labeled oligodeoxynucleotide probe specific for the mt–tRNAAsp by using total mt–RNA as templates. Extension reactions were carried out as detailed previously (39,40). Finally, samples were applied onto 15% polyacrylamide-7 M urea gel electrophoresis and electroblotted onto a positively charged nylon membrane. Quantification of density in each band was performed as detailed previously (40).
Mitochondrial tRNA Northern analysis
Two micrograms of total mitochondrial RNA were electrophoresed through a 10% polyacrylamide/7 M urea gel in Tris-borate-EDTA buffer (after heating the sample at 65°C for 10 min). The gels were then electroblotted onto a positively charged nylon membrane (Roche) for the hybridization analysis with DIG-labeled oligodeoxynucleotide probes for mt–tRNAAsp, mt–tRNAThr, mt–tRNALeu(CUN), mt–tRNALys and mt–tRNASer(AGY) were as detailed previously (20,24,25,42,43). DIG-labeled oligodeoxynucleotides were generated by using DIG oligonucleotide Tailing kit (Roche). The hybridization and quantification of density in each band were performed as detailed previously (20,24,25). For the aminoacylation assays, total mitochondrial RNAs were isolated under acid conditions, and 2 μg of total mitochondrial RNAs was electrophoresed at 4°C through an acid (pH 3.0) 10% polyacrylamide–7 M urea gel to separate the charged and uncharged tRNA as detailed elsewhere (43,44). To further distinguish non-aminoacylated tRNA from aminoacylated tRNA, samples of tRNAs were deacylated by being heated for 10 min at 60°C at pH 8.3 and then run in parallel (35,44). The gels were then electroblotted onto a positively charged nylon membrane (Roche) for the hybridization analysis with oligodeoxynucleotide probes as described above. Quantification of density in each band was performed as detailed previously (20).
Western blot analysis
Western blotting analysis was performed as detailed previously (20,43). The antibodies used for this investigation were from Abcam [anti GAPDH (ab72655), p.MT-ND1 (ab74257), p.MT-ND5 (ab92624) and p.MT-ATP6 (ab101908), p.MT-CO2 (ab110258)], Santa Cruz Biotechnology [p.MT-ND4 (sc-20499-R) and p.MT-ND6 (sc-20667)] and Proteintech [p.MT-CYTB (55090-1-AP)]. Peroxidase Affini Pure goat anti-mouse IgG and goat anti-rabbit IgG (Jackson) were used as a secondary antibody and protein signals were detected using the ECL system (CWBIO). Quantification of density in each band was performed as detailed previously (20,43).
Assays of activities of respiratory complexes
The enzymatic activities of complex I, II, III and IV were assayed as detailed elsewhere (43,45,46).
Measurements of oxygen consumption
The rates of oxygen consumption (OCR) in cybrid cell lines were measured with a Seahorse Bioscience XF-96 extracellular flux analyzer (Seahorse Bioscience), as detailed previously (20,43,47).
ATP measurements
The Cell Titer-Glo® Luminescent Cell Viability Assay kit (Promega) was used for the measurement of cellular and mitochondrial ATP levels, according to the modified manufacturer's instructions (20,43).
Assessment of mitochondrial membrane potential
Mitochondrial membrane potential was assessed with JC-10 Assay Kit-Microplate (Abcam) following general manufacturer's recommendations with some modifications, as detailed elsewhere (20,35).
Measurement of ROS production
ROS measurements were performed following the procedures detailed previously (20,43,48,49).
Computer analysis
Statistical analysis was carried out using the unpaired, two-tailed Student's t-test contained in the Microsoft-Excel program or Macintosh (version 2007). Differences were considered significant at a P < 0.05.
RESULTS
The description of one hearing-impaired Chinese pedigree and derived cell lines
The pedigree of the Chinese family with maternally inherited deafness was previously described (19,26). Six of 12 matrilineal relatives suffered from the variable degree of hearing impairment (two with mild hearing loss, two with moderate hearing loss and two with profound hearing loss). The age-at-onset of hearing loss ranged from 5 to 45 years, with the average of 26 years. These matrilineal relatives exhibited no other clinical abnormalities, including cardiac failure, muscular diseases, visual failure and neurological disorders. Immortalized lymphoblastoid cell lines were derived from one affected matrilineal relative carrying the m.7551A > G mutation, with profound hearing loss (III-6; male, 21 years) and from one genetically unrelated hearing normal individual lacking the m.7551A > G mutation belong to the same mtDNA haplogroup (A4, male, 20 years). The lymphoblastoid cells were enucleated, and subsequently fused to a large excess of mtDNA-less human ρ°206 cells, derived from the 143B.TK- cell line (34,35). These cybrid clones were isolated by growing the fusion mixtures in the selective Dulbecco's Modified Eagle Medium, containing BrdU and lacking uridine. Between 25 and 45 days after fusion, 10–15 presumptive mitochondrial cybrids derived from each donor cell line were isolated, and subsequently analyzed for the presence and level of the m.7551A > G mutation (26). The results confirmed the absence of the mtDNA mutation in the control clones and its presence in homoplasmy in all cybrids derived from the mutant cell line (Supplementary Figure S1). Three cybrids derived from each donor cell line with similar mtDNA copy numbers were used for the biochemical characterization described below.
The m.7551A > G mutation created the m1G37 modification of mt–tRNAAsp
There were no modifications of i6A37 or t6A37 detected in the human mitochondrial tRNAAsp (27). To investigate if the m.7551A>G mutation produced the m1G37 modification of mt–tRNAAsp, we subjected mitochondrial RNAs from mutant and control cybrid cell lines to the reverse transcription with DIG-labeled oligonucleotide probe specific for mt–tRNAAsp (Figure 1A). This results in a stop band one residue 3′ to the methylation on 15% polyacrylamide gel. As shown in Figure 1B, the m1G37 modification was present in mt–tRNAAsp derived from three mutant cell lines, while the m1G37 modification was not detected in the mt-tRNAAsp derived from three control cell lines. Furthermore, the m1A9 modifications were detected in mt–tRNAAsp obtained from both control and mutant cell lines.
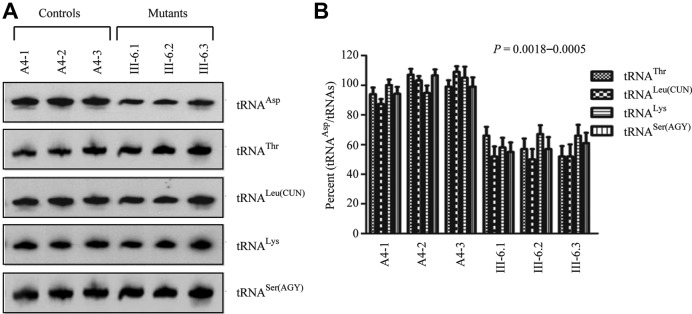
Reduction in the steady-state level of mt–tRNAAsp
To examine if the m.7551A > G mutation affects the stability of mt–tRNAAsp, we subjected mitochondrial RNAs from cybrids cell lines to Northern blots and hybridized them with DIG-labeled oligodeoxynucleotide probes for mt–tRNAAsp, mt–tRNAThr, mt–tRNALeu(CUN), mt–tRNALys and mt–tRNASer(AGY), respectively. As shown in Figure 2A, the amount of mt–tRNAAsp in three mutant cell lines were markedly decreased, compared with those in three control cybrid cell lines. For comparison, the average level of each mt–tRNAAsp in control or mutant cell lines was normalized to the average levels in the same cell line for reference mt–tRNAThr, mt–tRNALeu(CUN), mt–tRNALys and mt–tRNASer(AGY), respectively. As shown in Figure 2B, the average steady-state level of mt–tRNAAsp was significantly decreased in the mutant cells, compared with the wild-type cells. In particular, the average levels of mt–tRNAAsp in the mutant cybrid cell lines were among ∼58%, 52%, 64% and 57% of average values of three control cybrids after normalization to mt–tRNAThr, mt–tRNALeu(CUN), mt–tRNALys and mt–tRNASer(AGY) (P < 0.0018 to 0.0005), respectively.
Figure 2.
Northern blot analysis of mitochondrial tRNA. (A) Equal amounts of total mitochondrial RNA from various cell lines were electrophoresed through a denaturing polyacrylamide gel, electroblotted and hybridized with DIG-labeled oligonucleotide probes specific for the mt–tRNAAsp, mt–tRNAThr, mt–tRNALeu(CUN), mt–tRNALys and mt–tRNASer(AGY), respectively. (B) Quantification of tRNA levels. Average relative mt–tRNAAsp content per cell, was normalized to the average content per cell of mt–tRNAThr, mt–tRNALeu(CUN), mt–tRNALys and mt–tRNASer(AGY) in three cybrid cell lines derived from one affected subject (III-6) carrying the m.7551A > G mutation and three cybrid cell lines derived from one Chinese control subject (A4). The values for the latter are expressed as percentages of the average values for the control cell lines. The calculations were based on three independent determinations of each tRNA content in each cell line and three determinations of the content of reference tRNA marker in each cell line. The error bars indicate two standard errors of the means. P indicates the significance, according to the t-test, of the differences between mutant and control cell lines.
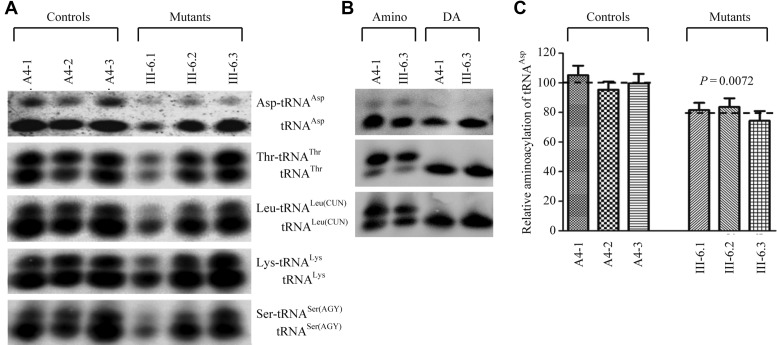
Deficient aminoacylation of mt–tRNAAsp
We evaluated whether the m.7551A > G mutation alters the aminoacylation of mitochondrial tRNAs. For this purpose, the aminoacylation capacities of mt–tRNAAsp, mt–tRNAThr, mt–tRNALeu(CUN), mt–tRNALys and mt–tRNASer(AGY) in control and mutant cell lines were examined by the use of electrophoresis in an acidic polyacrylamide/urea gel system to separate uncharged tRNA species from the corresponding charged tRNA, electroblotting and hybridizing with the tRNA probes described above. As shown in Figure 3A, the upper band represents the charged tRNA, and the lower band represents uncharged tRNA. There were no obvious differences in electrophoretic mobility between the control and mutant cell lines. To further distinguish nonaminoacylated tRNA from aminoacylated tRNA, samples of tRNAs were deacylated by being heated for 10 min at 60°C at pH 8.3 and then run in parallel. As shown in Figure 3B, only one band (uncharged tRNA) was present in both mutant and control cell lines after deacylating. However, the efficiencies of aminoacylated mt–tRNAAsp in these mutant cell lines carrying the m.7551A > G mutation varied from 74 to 84%, with average of 80%, relative to the average values of control cell lines (P = 0.0072) (Figure 3C). However, the levels of aminoacylation in mt–tRNAThr, mt–tRNALeu(CUN), mt–tRNALys and mt–tRNASer(AGY) in the mutant cell lines were comparable with those in the control cell lines (Supplementary Figure S2).
Figure 3.
In vivo aminoacylation assays. (A) Two micrograms of total mitochondrial RNA purified from six cell lines under acid conditions were electrophoresed at 4°C through an acid (pH 3.0) 10% polyacrylamide-7 M urea gel, electroblotted and hybridized with a DIG-labeled oligonucleotide probe specific for the mt–tRNAAsp. The blots were then stripped and rehybridized with mt–tRNAThr, mt–tRNALeu(CUN), mt–tRNALys and mt–tRNASer(AGY), respectively. (B) The samples from one control (A4.1) and mutant (III-6.3) cell lines were deacylated (DA) by heating for 10 min at 60°C at pH.8.3 and electrophoresed as above. Aminoacylation assays for mt–tRNAAsp were carried out in parallel for aminoacylated and deacylated samples. (C) In vivo aminoacylated proportions of mt–tRNAAsp in the mutant and controls. The calculations were based on three independent determinations. Graph details and symbols are explained in the legend to the Figure 2.
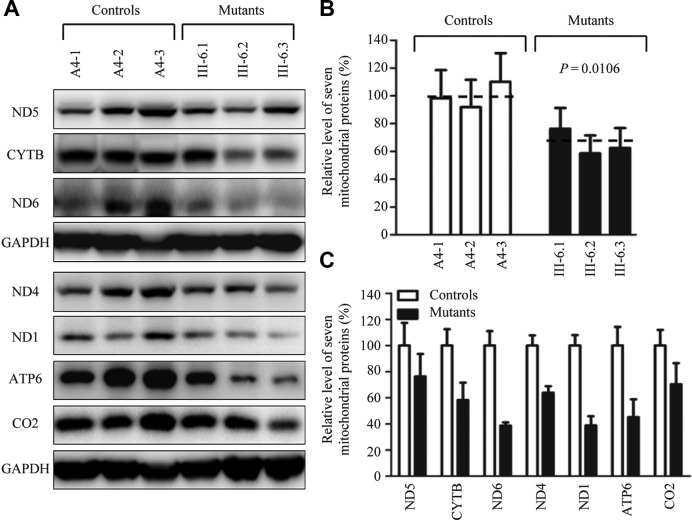
Decreases in the level of mitochondrial proteins
To assess whether a failure in mt–tRNAAsp metabolism caused by the m.7551A > G mutation impaired mitochondrial translation, a Western blot analysis was carried out to examine the steady state levels of seven subunits of respiratory complex in mutant and control cells with GAPDH as a loading control. As shown in Figure 4A, the levels of p.MT-CO1 and p.MT-CO2, subunits I and II of cytochrome c oxidase; p.MT-ND1, p.MT-ND4 and p.MT-ND5, subunits 1, 4 and 5 of NADH dehydrogenase; p.MT-ATP6, subunit 6 of the H±ATPase; p.MT-CYTB, apocytochrome b exhibited the variable reductions in three mutant cell lines, as compared with those of three control cell lines. As shown in Figure 4B, the overall levels of seven mitochondrial translation products in the mutant cell lines ranged from ∼58–76%, with an average of 65% (P = 0.0106), relative to the mean value measured in the control cell lines. Notably, the average levels of p.MT-ND1, p.MT-ND4, p.MT-ND5, p.MT-ND6, p.MT-CO2, p.MT-ATP6 and p.MT-CYTB in the mutant cells were 39%, 64%, 76%, 39%, 70%, 45% and 58% of the average values of control cells, respectively. However, the levels of polypeptide synthesis in mutant cells, relative to those in control cells, showed no significant correlation with either the number of codons or the proportion of aspartic acid residues (Supplementary Table S2).
Figure 4.
Western blot analysis of mitochondrial proteins. (A) Twenty micrograms of total cellular proteins from various cell lines were electrophoresed through a denaturing polyacrylamide gel, electroblotted and hybridized with 7 respiratory complex subunits in mutant and control cells with GAPDH as a loading control. p.MT-COI and p.MT-COII, indicate subunits I and II of cytochrome c oxidase; p.MT-ND1, p.MT-ND4 and p.MT-ND5, subunits 1, 4 and 5 of the reduced nicotinamide–adenine dinucleotide dehydrogenase; p.MT-ATP6, subunit 6 of the H±ATPase; and p.MT-CYTB, apocytochrome b. (B) Quantification of mitochondrial protein levels. Average relative p.MT-COI, p.MT-COII, p.MT-ND1, p.MT-ND4, p.MT-ND5, p.MT-ATP6 and p.MT-CYTB content per cell, normalized to the average content per cell of GAPDH in three mutant cell lines carrying the m.7551A > G mutation and three control cell lines lacking the mutation. The values for the latter are expressed as percentages of the average values for the control cell line. The calculations were based on three independent determinations. Graph details and symbols are explained in the legend to Figure 2.
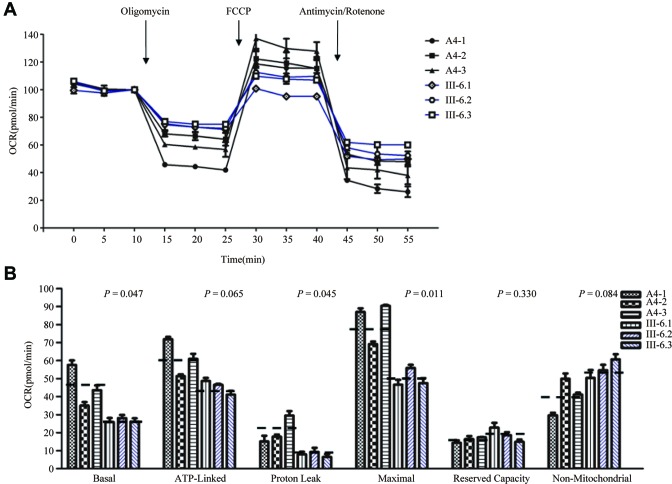
Respiration defects in mutant cells
To evaluate if the m.7551A > G mutation alters cellular bioenergetics, we examined the OCR of three mutant cell lines carrying the m.7551A > G mutation and three control cell lines. As shown in Figure 5, the basal OCR in the mutant cell lines was ∼59% (P = 0.047) relative to the mean value measured in the control cell lines. To investigate which of the enzyme complexes of the respiratory chain was affected in the mutant cell lines, OCR was measured after the sequential addition of oligomycin (to inhibit the ATP synthase), carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (to uncouple the mitochondrial inner membrane and allow for maximum electron flux through the ETC), rotenone (to inhibit complex I) and antimycin A (to inhibit complex III). The difference between the basal OCR and the drug-insensitive OCR yields the amount of ATP-linked OCR, proton leak OCR, maximal OCR, reserve capacity and non-mitochondrial OCR. As shown in Figure 5, the ATP-linked OCR, proton leak OCR, maximal OCR, reserve capacity and non-mitochondrial OCR in mutant cell lines were ∼74%, 38%, 61%, 117% and 137%, relative to the mean value measured in the control cell lines (P = 0.065, 0.045, 0.011, 0.330 and 0.084), respectively.
Figure 5.
Respiration assays. (A) An analysis of O2 consumption in the various cell lines using different inhibitors. The rates of O2 (OCR) were first measured on 2 × 104 cells of each cell line under basal condition and then sequentially added to oligomycin (1.5 μM), carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) (0.5 μM), rotenone (1 μM) and antimycin A (1 μM) at indicated times to determine different parameters of mitochondrial functions. (B) Graphs presented the ATP-linked OCR, proton leak OCR, maximal OCR, reserve capacity and non-mitochondrial OCR in mutant and control cell lines. Non-mitochondrial OCR was determined as the OCR after rotenone/antimycin A treatment. Basal OCR was determined as OCR before oligomycin minus OCR after rotenone/antimycin A. ATP-lined OCR was determined as OCR before oligomycin minus OCR after oligomycin. Proton leak was determined as Basal OCR minus ATP-linked OCR. Maximal was determined as the OCR after FCCP minus non-mitochondrial OCR. Reserve Capacity was defined as the difference between Maximal OCR after FCCP minus Basal OCR. The average of 4 determinations for each cell line is shown, the horizontal dashed lines represent the average value for each group. Graph details and symbols are explained in the legend to Figure 2.
Reduced activities of respiratory chain complexes
To investigate the effect of the m.7551A > G mutation on the oxidative phosphorylation, we measured the activities of respiratory chain complexes by isolating mitochondria from mutant and control cell lines. Complex I (NADH ubiquinone oxidoreductase) activity was determined by following the oxidation of NADH with ubiquinone as the electron acceptor (47,48). Complex III (ubiquinone cytochrome c oxidoreductase) activity was measured as the reduction of cytochrome c (III) using d-ubiquinol-2 as the electron donor. The activity of complex IV (cytochrome c oxidase) was monitored by following the oxidation of cytochrome c (II). As shown in Figure 6, the activity of complexes I, II, III and IV in the mutant cells carrying m.7551A > G mutation were 49%, 104%, 71% and 69% of the mean value measured in three control cell lines, respectively.
Figure 6.
Enzymatic activities of respiratory chain complexes. The activities of respiratory complexes were investigated by enzymatic assays on complexes I, II, III and IV in mitochondria isolated from three mutant and three control cybrid cell lines.The calculations were based on four independent determinations. Graph details and symbols are explained in the legend to Figure 2.
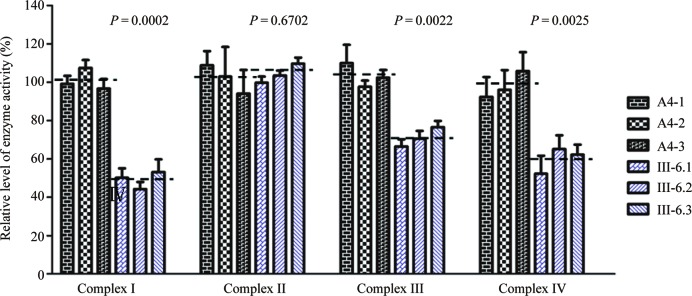
Reduced levels in mitochondrial ATP production
The capacity of oxidative phosphorylation in mutant and wild-type cell lines was examined by measuring the levels of cellular and mitochondrial ATP using a luciferin/luciferase assay. Populations of cells were incubated in the media in the presence of glucose, and 2-deoxy-D-glucose with pyruvate (20,50). As shown in Figure 7A, the levels of ATP production in mutant cells in the presence of glucose (total cellular levels of ATP) were 80% of those measured in the control cell lines. By contrast, as shown in Figure 7B, the levels of ATP production in mutant cell lines, in the presence of pyruvate and 2-deoxy-D-glucose to inhibit glycolysis (mitochondrial levels of ATP) ranged from 54 to 63%, with an average of 57% relative to the mean value measured in the control cell lines (P = 0.0012).
Figure 7.
Measurement of cellular and mitochondrial ATP levels using bioluminescence assay. Cells were incubated with 10 mM glucose or 5 mM 2-deoxy-d-glucose plus 5 mM pyruvate to determine ATP generation under mitochondrial ATP synthesis. Average rates of ATP level per cell line and are shown: (A) ATP level in total cells; (B) ATP level in mitochondria. Six to seven determinations were made for each cell line. Graph details and symbols are explained in the legend to Figure 2.
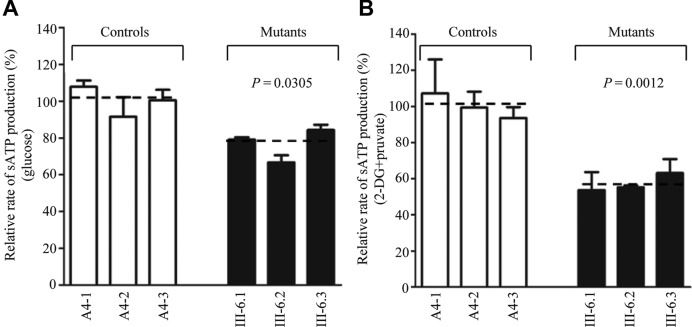
Mild decreases in mitochondrial membrane potentials
The mitochondrial membrane potential (ΔΨm) changes were measured in mutant and control cell lines using the fluorescence probe JC-10 assay system. The ratios of fluorescence intensities Ex/Em = 490/590 and 490/530 nm (FL590/FL530) were recorded to delineate the ΔΨm level of each sample. The relative ratios of FL590/FL530 geometric mean between mutant and control cell lines were calculated to represent the level of ΔΨm. As shown in Figure 8, the levels of the ΔΨm in the mutant cell lines carrying the m.7551A > G mutation ranged from 77.2% and 96.8%, with an average 86.2% (P = 0.182) of the mean value measured in the control cell lines. In contrast, the levels of ΔΨm in mutant cells in the presence of carbonyl cyanide-p-(trifluoromethoxy)phenylhydrazone were comparable with those measured in the control cell lines (P = 0.766).
Figure 8.
Mitochondrial membrane potential analysis. The mitochondrial membrane potential (ΔΨm) was measured in three mutant and three control cell lines using a fluorescence probe JC-10 assay system. The ratio of fluorescence intensities Ex/Em = 490/590 nm and 490/530 nm (FL590/FL530) were recorded to delineate the ΔΨm level of each sample. The relative ratios of FL590/FL530 geometric mean between mutant and control cell lines were calculated to reflect the level of ΔΨm. Relative ratio of JC-10 fluorescence intensities at Ex/Em = 490/530 nm and 490/590 nm in (A) absence and (B) presence of 10 μM of FCCP. The average of 3–5 determinations for each cell line is shown. Graph details and symbols are explained in the legend to Figure 2.
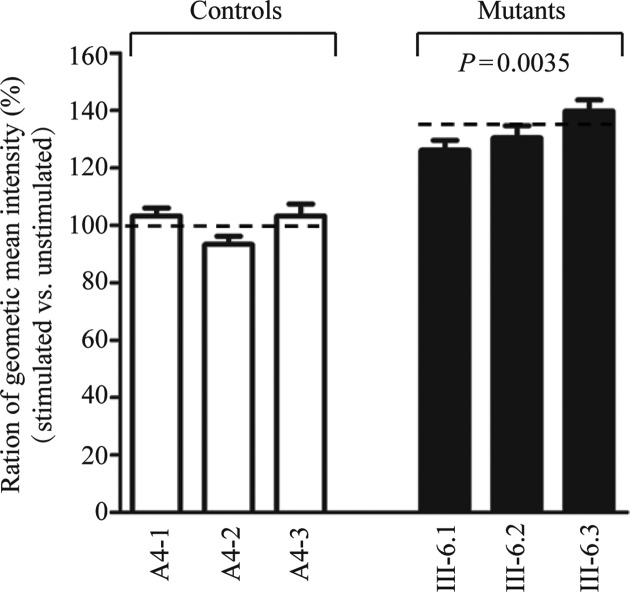
The increase of ROS production
The levels of the ROS generation in the vital cells derived from three mutant cybrid cell lines carrying the m.7551A > G mutation and three control cybrid cell lines lacking the mutation were measured with flow cytometry under normal conditions and following H2O2 stimulation (20,49). Geometric mean intensity was recorded to measure the rate of ROS of each sample. The ratio of geometric mean intensity between unstimulated and stimulated with H2O2 in each cell line was calculated to delineate the reaction upon increasing level of ROS under oxidative stress. As shown in Figure 9, the levels of ROS generation in the mutant cell lines carrying the m.7551A > G mutation ranged from 126 and 140%, with an average 132% (P = 0.0035) of the mean value measured in the control cell lines.
Figure 9.
Ratio of geometric mean intensity between levels of the ROS generation in the vital cells with or without H2O2 stimulation. The rates of production in ROS from three mutant cell lines and three control cell lines were analyzed by BD–LSR II flow cytometer system with or without H2O2 stimulation. The relative ratio of intensity (stimulated versus unstimulated with H2O2) was calculated. The average of three determinations for each cell line is shown. Graph details and symbols are explained in the legend to Figure 2.
DISCUSSION
In the present study, we investigated the pathogenetic mechanism of the deafness-associated m.7551A > G mutation in the mt–tRNAAsp gene. The mutation is localized at a highly conserved nucleotide (A37), adjacent to the 3′ end of the anticodon of the mt–tRNAAsp (22,23). The nucleotide at position 37 of the tRNAs are often modified by methylthiolation (28,29). Modification at position A37 of some mitochondrial tRNAs such as mt–tRNALys and mt–tRNASer(UCN) was catalyzed by the modifying enzymes TRIT1 (MiaA in bacterial ortholog), while the modification of G37 in other tRNAs such as mt–tRNALeu(CUN) and mt–tRNAGln was catalyzed by the TRMT5 (TrmD in bacterial ortholog) (28–30). Modification at position 37 contributes to the high fidelity of codon recognition and to the structural formation and stabilization of functional tRNAs (28–31). However, the modification of A37 was not detected in human mt–tRNAAsp, mt–tRNAMet and mt–tRNALeu(UUR) (27,28). Thus, it was anticipated that the m.7551A > G mutation created the m1G37 modification, thereby altering the structure and function of mt–tRNAAsp. In this study, the primer extension experiment demonstrated that the m.7551A > G mutation produced the m1G37 modification of mt–tRNAAsp. The primary defect in this mutation appeared to affect the stability and aminoacylation of mutant mt–tRNAAsp (50,51). Unlike the m.12201T > C mutation in the mt–tRNAHis and m.15927A > G mutation in the mt–tRNAThr (20,50), the m.7551A > G mutation did not change conformation of mt–tRNAAsp. However, the aminoacylation level of mt–tRNAAsp but no other tRNAs were mildly decreased in mutant cell lines, as compared with controls. In fact, the A-to-G substitution at position 37 resulted in a 10-fold reduction in the amount of bacterial tRNA at the aminoacyl–tRNA binding site (52). A failure to aminoacylate tRNA properly then makes the mutant mt–tRNAAsp to be metabolically less stable and more subject to degradation, thereby lowering the level of this tRNA, as in the case of m.3243A > G mutation in the mt–tRNALeu(UUR) (42,53). Alternatively, the m.7551A > G mutation likely affects the formation of functional structure of tRNAs and thus makes the tRNA be more unstable (22,23). In the present study, 42% reduction in the steady-state level of mt–tRNAAsp observed in mutant cybrids was consistent with the previous observations in the lymphoblastoid cell lines carrying the m.4435A > G mutation in the tRNAMet gene (32,33). However, the reduced level of mt–tRNAAsp in mutant cells harboring the m.7551A > G mutation is indeed above the proposed threshold to produce a clinical phenotype associated with a mitochondrial tRNA mutation (20,53,54). This suggest that the m.7551A > G mutation alone is not sufficient to produce a clinical phenotype, as in the case of the deafness-associated m.1555A > G and m.1494C > T mutations in the 12S rRNA gene (37,55).
A failure in tRNA metabolism including inefficient aminoacylation or shortage of mt–tRNAAsp could be the source of impaired mitochondrial translation of mtDNA encoding polypeptides and subsequent respiratory chain defects. In the present study, reduced levels of mitochondrial proteins (an average decrease of ∼35%) were observed in mutant cybrid cell lines, as compared to the average levels in control cell lines. The reduced levels of variable mitochondrial protein detected in cybrids carrying the m.7551A > G mutation were comparable with the reduced rates of mitochondrial protein synthesis observed in lymphoblastoid cell lines carrying the m.4435A > G mutation (32,33). In mutant cell lines, a variable decrease in level of 7 mtDNA-encoded polypeptides was observed in each protein. However, polypeptide levels in mutant cell lines, relative to those in control cell lines, did not significantly correlate with either the number or proportion of aspartic acid codons, in contrast to what was previously shown in cell lines carrying the m.7445A > G mutation in the precursor of mt–tRNASer(UCN) (24) or the m.8344A > G mutation in mt–tRNALys (54). The lower level of mt–tRNAAsp may contribute to the variable decrease of each polypeptide in mutant cell lines. Thus, the impaired synthesis of these mtDNA-encoding polypeptides may affect the activities of complex I, complex III and complex IV, respectively. Furthermore, impairment of mitochondrial protein synthesis were apparently responsible for the reduced rates in the basal OCR, or ATP-linked OCR, reserve capacity and maximal OCR among the control and mutant cell lines. These observations were clearly consistent with the critical role of mt–tRNAAsp metabolic failure in producing their respiration defects, as in the cases of mutant cell lines carrying the deafness-associated mt–tRNASer(UCN) 7445A > G, 7511T > C and mt–tRNAHis 12201T > C mutations (20,24,25).
The respiratory deficiency then affects the efficiency of mitochondrial ATP synthesis. In this investigation, 43% drop in mitochondrial ATP production in mutant cybrids carrying the m.7551A > G mutation may be caused by the defective activities of complexes I, III and IV. The reducing level was comparable with those in cells carrying the deafness-associated m.12201T > C and LHON-associated m.11778G > A mutations (20,43), but the decreasing level was much lower than those in cells carrying the mt-tRNALys 8344A > G and mt–tRNALeu(UUR) 3243A > G mutations (56,57). Alternatively, the reduction in mitochondrial ATP production in mutant cells was likely a consequence of the decrease in the proton electrochemical potential gradient of mutant mitochondria (56). As a result, the hair cells carrying the mtDNA mutation may be particularly sensitive to increased ATP demand (3,4,58). Furthermore, the deficient activities of respiratory chain complexes caused by tRNA mutations often alter mitochondrial membrane potentials (59). In this study, only relatively mild reductions in mitochondrial membrane potential were observed in mutant cell lines carrying the m.7551A > G mutation, in contrast with those in the cell lines carrying the m.12201T > C mutation (20). The impairment of oxidative phoshorylation can lead to more electron leakage from electron transport chain, and in turn, elevate the production of ROS in mutant cells (60), thereby damaging mitochondrial and cellular proteins, lipids and nuclear acids (61). The hair cells and cochlear neurons may be preferentially involved because they are somehow exquisitely sensitive to subtle imbalance in cellular redox state or increased level of free radicals (61–63). However, the incomplete penetrance of deafness and mild biochemical defects indicated that the m.7551A > G mutation may be necessary evident but not sufficient to produce a clinical phenotype. The other genetic or epigenetic factors may contribute to the development of clinical phenotype in subjects carrying the m.7551A > G mutation (64,65). In particular, the hearing specific phenotype of this tRNA mutation may be attributed to the tissue-specificity of OXPHOS via tRNA modification or involvement of nuclear modifier genes (64,66–68).
In summary, our findings convincingly demonstrate the pathogenic mechanism leading to an impaired oxidative phosphorylation in cybrid cell lines carrying the deafness-associated m.7551A > G mutation in the mt–tRNAAsp gene. The m.7551A > G mutation created the m1G37 modification of mt-tRNAAsp, but reduced the efficiency of aminoacylation and stability of this tRNA. A failure in tRNAAsp metabolism led to the decreased synthesis of mtDNA encoding polypeptides and respiration. As a result, this respiratory deficiency reduced mitochondrial ATP production and increased the production of oxidative reactive species. The subsequent hearing loss may be involved in the participation of other genetic, epigenetic and environmental modifier factors. Thus, our findings may provide the new insights into the understanding of pathophysiology of maternally inherited deafness that was manifested by altered nucleotide modification of mitochondrial tRNA.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Basic Research Priorities Program of China (2014CB541700) and Chinese National Science Foundation grant 81330024 to M.X.G.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Basic Research Priorities Program of China [2014CB541700]; Chinese National Science Foundation [81330024 to M.X.G.]. Funding for open access charge: National Basic Research Priorities Program of China [2014CB541700]; Chinese National Science Foundation [81330024].
Conflict of interest statement. None declared.
REFERENCE
- 1.Jacobs H. Disorders of mitochondrial protein synthesis. Hum. Mol. Genet. 2003;12:R293–R301. doi: 10.1093/hmg/ddg285. [DOI] [PubMed] [Google Scholar]
- 2.Rötig A. Human diseases with impaired mitochondrial protein synthesis. Biochim. Biophys. Acta. 2011;1807:1198–1205. doi: 10.1016/j.bbabio.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Fischel-Ghodsian N. Mitochondrial deafness mutations reviewed. Hum. Mutat. 1999;13:261–270. doi: 10.1002/(SICI)1098-1004(1999)13:4<261::AID-HUMU1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Guan M.X. Mitochondrial 12S rRNA mutations associated with aminoglycoside ototoxicity. Mitochondrion. 2011;11:237–245. doi: 10.1016/j.mito.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Zheng J., Ji Y., Guan M.X. Mitochondrial tRNA mutations associated with deafness. Mitochondrion. 2012;12:406–413. doi: 10.1016/j.mito.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Andrews R.M., Kubacka I., Chinnery P.F., Lightowlers R.N., Turnbull D.M., Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 7.Calvo S. E., Mootha V. K. The mitochondrial proteome and human disease. Annu. Rev. Genomics Hum. Genet. 2010;11:25–44. doi: 10.1146/annurev-genom-082509-141720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Area-Gomez E., Schon E.A. Mitochondrial genetics and disease. J. Child Neurol. 2014;29:1208–1215. doi: 10.1177/0883073814539561. [DOI] [PubMed] [Google Scholar]
- 9.Simon M., Richard E.M., Wang X., Shahzad M., Huang V.H., Qaiser T.A., Potluri P., Mahl S.E., Davila A., Nazli S., et al. Mutations of human NARS2, encoding the mitochondrial asparaginyl-tRNA synthetase, cause nonsyndromic deafness and Leigh syndrome. PLoS Genet. 2015;11:e1005097. doi: 10.1371/journal.pgen.1005097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierce S.B., Gersak K., Michaelson-Cohen R., Walsh T., Lee M.K., Malach D., Klevit R.E., King M.C., Levy-Lahad E. Mutations in LARS2, encoding mitochondrial leucyl-tRNA synthetase, lead to premature ovarian failure and hearing loss in Perrault syndrome. Am. J. Hum. Genet. 2013;92:614–620. doi: 10.1016/j.ajhg.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prezant T.R., Agapian J.V., Bohlman M.C., Bu X., Oztas S., Qiu W.Q., Arnos K.S., Cortopassi G.A., Jaber L., Rotter J.I., et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat. Genet. 1993;4:289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H., Li R., Wang Q., Yan Q., Deng J.H., Han D., Bai Y., Young W.Y., Guan M.X. Maternally inherited aminoglycoside-induced and non-syndromic deafness is associated with the novel C1494T mutation in the mitochondrial 12S rRNA gene in a large Chinese family. Am. J. Hum. Genet. 2004;74:139–152. doi: 10.1086/381133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto Y., Nonaka I., Horai S. A mutation in the tRNALeu(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 14.Mancuso M., Orsucci D., Angelini C., Bertini E., Carelli V., Comi G.P., Minetti C., Moggio M., Mongini T., Servidei S., et al. Phenotypic heterogeneity of the 8344A>G mtDNA ‘MERRF’ mutation. Neurology. 2013;80:2049–2054. doi: 10.1212/WNL.0b013e318294b44c. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez Cortes N., Pertuiset C., Dumon E., Borlin M., Hebert-Chatelain E., Pierron D., Feldmann D., Jonard L., Marlin S., Letellier T., et al. Novel mitochondrial DNA mutations responsible for maternally inherited nonsyndromic hearing loss. Hum. Mutat. 2012;33:681–689. doi: 10.1002/humu.22023. [DOI] [PubMed] [Google Scholar]
- 16.Fischel-Ghodsian N., Prezant T.R., Fournier P., Stewart I.A., Maw M. Mitochondrial mutation associated with nonsyndromic deafness. Am. J. Otolaryngol. 1995;16:403–408. doi: 10.1016/0196-0709(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 17.Tiranti V., Chariot P., Carella F., Toscano A., Soliveri P., Girlanda P., Carrara F., Fratta G.M., Reid F.M., Mariotti C., et al. Maternally inherited hearing loss, ataxia and myoclonus associated with a novel point mutation in mitochondrial tRNASer(UCN) gene. Hum. Mol. Genet. 1995;4:1421–1427. doi: 10.1093/hmg/4.8.1421. [DOI] [PubMed] [Google Scholar]
- 18.Sue C.M., Tanji K., Hadjigeorgiou G., Andreu A.L., Nishino I., Krishna S., Bruno C., Hirano M., Shanske S., Bonilla E., et al. Maternally inherited hearing loss in a large kindred with a novel T7511C mutation in the mitochondrial DNA tRNASer(UCN) gene. Neurology. 1999;52:1905–1908. doi: 10.1212/wnl.52.9.1905. [DOI] [PubMed] [Google Scholar]
- 19.Tang X., Zheng J., Ying Z., Cai Z., Gao Y., He Z., Yu H., Yao J., Yang Y., Wang H., et al. Mitochondrial tRNASer(UCN) variants in 2651 Han Chinese subjects with hearing loss. Mitochondrion. 2015;23:17–24. doi: 10.1016/j.mito.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Gong S., Peng Y., Jiang P., Wang M., Fan M., Wang X., Zhou H., Li H., Yan Q., Huang T., et al. A deafness-associated tRNAHis mutation alters the mitochondrial function, ROS production and membrane potential. Nucleic Acids Res. 2014;42:8039–8048. doi: 10.1093/nar/gku466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H., Li R., Li W., Wang M., Ji J., Zheng J., Mao Z., Mo J.Q., Jiang P., Lu J., et al. Maternally inherited diabetes is associated with a homoplasmic T10003C mutation in the mitochondrial tRNAGly gene. Mitochondrion. 2015;21:49–57. doi: 10.1016/j.mito.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T., Nagao A., Suzuki T. Human mitochondrial tRNAs: Biogenesis, function, structural aspects, and diseases. Annu. Rev. Genet. 2011;45:299–329. doi: 10.1146/annurev-genet-110410-132531. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X.L., Wang ED. Transfer RNA: a dancer between charging and mis-charging for protein biosynthesis. Sci. China Life Sci. 2013;56:921–932. doi: 10.1007/s11427-013-4542-9. [DOI] [PubMed] [Google Scholar]
- 24.Guan M.X., Enriquez J.A., Fischel-Ghodsian N., Puranam R.S., Lin C.P., Maw M.A., Attardi G. The deafness-associated mitochondrial DNA mutation at position 7445, which affects tRNASer(UCN) precursor processing, has long-range effects on NADH dehydrogenase subunit ND6 gene expression. Mol. Cell. Biol. 1998;18:5868–5879. doi: 10.1128/mcb.18.10.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Fischel-Ghodsian N., Schwartz F., Yan Q., Friedman R.A., Guan M.X. Biochemical characterization of the mitochondrial tRNASer(UCN) T7511C mutation associated with nonsyndromic deafness. Nucleic. Acids Res. 2004;32:867–877. doi: 10.1093/nar/gkh226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y., Liang L.Z., Xiao H.L., Yang Y.L., Yu X., Zheng J., Fang F., Zheng B.J., Tang X.W, Jin L.J., et al. Hearing loss may be associated with the novel mitochondrial tRNAAsp A7551G mutation in a Chinese family. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;48:978–984. [PubMed] [Google Scholar]
- 27.Messmer M., Pütz J., Suzuki T., Suzuki T., Sauter C., Sissler M., Catherine F. Tertiary network in mammalian mitochondrial tRNAAsp revealed by solution probing and phylogeny. Nucleic Acids Res. 2009;37:6881–6895. doi: 10.1093/nar/gkp697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki T., Suzuki T. A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2014;42:7346–7357. doi: 10.1093/nar/gku390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allnér O., Nilsson L. Nucleotide modifications and tRNA anticodon-mRNA codon interactions on the ribosome. RNA. 2011;17:2177–2188. doi: 10.1261/rna.029231.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Björk G.R., Hagervall T.G. Transfer RNA modification: presence, synthesis, and function. EcoSal Plus. 2014;6 doi: 10.1128/ecosalplus.ESP-0007-2013. doi:10.1128/ecosalplus.ESP-0007-2013. [DOI] [PubMed] [Google Scholar]
- 31.Wei F.Y., Zhou B., Suzuki T., Miyata K., Ujihara Y., Horiguchi H., Takahashi N., Xie P., Michiue H., Fujimura A., et al. Cdk5rap1-mediated 2-methylthio modification of mitochondrial tRNAs governs protein translation and contributes to myopathy in mice and humans. Cell Metab. 2015;21:428–442. doi: 10.1016/j.cmet.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Qu J., Li R., Tong Y., Lu F., Qian Y., Hu Y., Mo J.Q., West C.E., Guan M.X. The novel A4435G mutation in the mitochondrial tRNAMet may modulate the phenotypic expression of the LHON-associated ND4 G11778A mutation. Invest. Ophthalmol. Vis. Sci. 2006;47:475–483. doi: 10.1167/iovs.05-0665. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y., Li R., Li Z., Wang X.J., Yang L., Wang S., Guan M.X. Mitochondrial transfer RNAMet 4435A>G mutation is associated with maternally inherited hypertension in a Chinese pedigree. Hypertension. 2009;53:1083–1090. doi: 10.1161/HYPERTENSIONAHA.109.128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King M.P., Attadi G. Mitochondria-mediated transformation of human rho(0) cells. Methods Enzymol. 1996;264:313–334. doi: 10.1016/s0076-6879(96)64030-0. [DOI] [PubMed] [Google Scholar]
- 35.Jiang P., Wang M., Xue L., Xiao Y., Yu J., Wang H., Yao J., Liu H., Peng Y., Liu H., et al. A hypertension-associated tRNAAla mutation alters the tRNA metabolism and mitochondrial function. Mol. Cell. Biol. 2016;36:1920–1930. doi: 10.1128/MCB.00199-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc. Natl. Acad. Sci. U.S.A. 1973;70:190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guan M.X., Fischel-Ghodsian N., Attardi G. Nuclear background determines biochemical phenotype in the deafness-associated mitochondrial 12S rRNA mutation. Hum. Mol. Genet. 2001;10:573–580. doi: 10.1093/hmg/10.6.573. [DOI] [PubMed] [Google Scholar]
- 38.Guan M.X., Fischel-Ghodsian N., Attardi G. Biochemical evidence for nuclear gene involvement in phenotype of non-syndromic deafness associated with mitochondrial 12S rRNA mutation. Hum. Mol. Genet. 1996;5:963–971. doi: 10.1093/hmg/5.7.963. [DOI] [PubMed] [Google Scholar]
- 39.Ofengand J., Del Campo M., Kaya Y. Mapping pseudouridines in RNA molecules. Methods. 2001;25:365–373. doi: 10.1006/meth.2001.1249. [DOI] [PubMed] [Google Scholar]
- 40.Qian Y., Guan M.X. Interaction of aminoglycosides with human mitochondrial 12S rRNA carrying the deafness-associated mutation. Antimicrob. Agents Chemother. 2009;53:4612–4618. doi: 10.1128/AAC.00965-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King M.P., Attardi G. Post-transcriptional regulation of the steady-state levels of mitochondrial tRNAs in HeLa cells. J. Biol. Chem. 1993;268:10228–10237. [PubMed] [Google Scholar]
- 42.Li R., Guan M.X. Human mitochondrial leucyl-tRNA synthetase corrects mitochondrial dysfunctions due to the tRNALeu(UUR) A3243G mutation, associated with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like symptoms and diabetes. Mol. Cell. Biol. 2010;30:2147–2154. doi: 10.1128/MCB.01614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang P., Jin X., Peng Y., Wang M., Liu H., Liu X., Zhang Z., Ji Y., Zhang J., Liang M., et al. The exome sequencing identified the mutation in YARS2 encoding the mitochondrial tyrosyl-tRNA synthetase as a nuclear modifier for the phenotypic manifestation of Leber's hereditary optic neuropathy-associated mitochondrial DNA mutation. Hum. Mol. Genet. 2016;25:584–596. doi: 10.1093/hmg/ddv498. [DOI] [PubMed] [Google Scholar]
- 44.Enriquez J.A., Attardi G. Analysis of aminoacylation of human mitochondrial tRNAs. Methods Enzymol. 1996;264:183–196. doi: 10.1016/s0076-6879(96)64019-1. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., D'Aurelio M., Deng J.H., Park J.S., Manfredi G., Hu P., Lu J., Bai Y. An assembled complex IV maintains the stability and activity of complex I in mammalian mitochondria. J. Biol. Chem. 2007;282:17557–17562. doi: 10.1074/jbc.M701056200. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J., Jiang P., Jin X., Liu X., Zhang M., Xie S., Gao M., Zhang S., Sun Y.H., Zhu J., et al. Leber's hereditary optic neuropathy caused by the homoplasmic ND1 m.3635G>A mutation in nine Han Chinese families. Mitochondrion. 2014;18:18–26. doi: 10.1016/j.mito.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Dranka B.P., Benavides G.A., Diers A.R., Giordano S., Zelickson B.R., Reily C., Zou L., Chatham J.C., Hill B.G., Zhang J., et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic. Biol. Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X., Qian Y., Zhang J., Tong Y., Jiang P., Liang M., Dai X., Zhou H., Zhao F., Ji Y., et al. Leber's hereditary optic neuropathy is associated with the T3866C mutation in mitochondrial ND1 gene in three Han Chinese Families. Invest. Ophthalmol. Vis. Sci. 2012;53:4586–4594. doi: 10.1167/iovs.11-9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahfouz R., Sharma R., Lackner J., Aziz N., Agarwal A. Evaluation of chemiluminescence and flow cytometry as tools in assessing production of hydrogen peroxide and superoxide anion in human spermatozoa. Fertil. Steril. 2009;92:819–827. doi: 10.1016/j.fertnstert.2008.05.087. [DOI] [PubMed] [Google Scholar]
- 50.Jia Z., Wang X., Qin Y., Xue L., Jiang P., Meng Y., Shi S., Wang Y., Qin Mo J., Guan M.X. Coronary heart disease is associated with a mutation in mitochondrial tRNA. Hum. Mol. Genet. 2013;22:4064–4073. doi: 10.1093/hmg/ddt256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones C.N., Jones C.I., Graham W.D., Agris P.F., Spremulli L.L. A disease-causing point mutation in human mitochondrial tRNAMet results in tRNA misfolding leading to defects in translational initiation and elongation. J. Biol. Chem. 2008;283:34445–34456. doi: 10.1074/jbc.M806992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yarus M., Cline S.W., Wier P., Breeden L., Thompson R.C. Actions of the anticodon arm in translation on the phenotypes of RNA mutants. J. Mol. Biol. 1986;192:235–255. doi: 10.1016/0022-2836(86)90362-1. [DOI] [PubMed] [Google Scholar]
- 53.Chomyn A., Enriquez J.A., Micol V., Fernandez-Silva P., Attardi G. The mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episode syndrome-associated human mitochondrial tRNALeu(UUR) mutation causes aminoacylation deficiency and concomitant reduced association of mRNA with ribosomes. J. Biol. Chem. 2000;275:19198–19209. doi: 10.1074/jbc.M908734199. [DOI] [PubMed] [Google Scholar]
- 54.Enriquez J.A., Chomyn A., Attardi G. MtDNA mutation in MERRF syndrome causes defective aminoacylation of tRNALys and premature translation termination. Nat. Genet. 1995;10:47–55. doi: 10.1038/ng0595-47. [DOI] [PubMed] [Google Scholar]
- 55.Zhao H., Young W.Y., Yan Q., Li R., Cao J., Wang Q., Li X., Peters J.L., Han D., Guan M,X. Functional characterization of the mitochondrial 12S rRNA C1494T mutation associated with aminoglycoside-induced and non-syndromic hearing loss. Nucleic Acids Res. 2005;33:1132–1139. doi: 10.1093/nar/gki262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.James A.M., Sheard P.W., Wei Y.H., Murphy M.P. Decreased ATP synthesis is phenotypically expressed during increased energy demand in fibroblasts containing mitochondrial tRNA mutations. Eur. J. Biochem. 1999;259:462–469. doi: 10.1046/j.1432-1327.1999.00066.x. [DOI] [PubMed] [Google Scholar]
- 57.Pallotti F., Baracca A., Hernandez-Rosa E., Walker W.F., Solaini G., Lenaz G., Melzi D'Eril G.V., Dimauro S., Schon E.A., Davidson M.M. Biochemical analysis of respiratory function in cybrid cell lines harbouring mitochondrial DNA mutations. Biochem. J. 2004;384:287–293. doi: 10.1042/BJ20040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan M.X. Molecular pathogenetic mechanism of maternally inherited deafness. Ann. N. Y. Acad. Sci. 2004;1011:259–271. doi: 10.1007/978-3-662-41088-2_25. [DOI] [PubMed] [Google Scholar]
- 59.de Andrade P.B., Rubi B., Frigerio F., van den Ouweland J.M., Maassen J.A., Maechler P. Diabetes-associated mitochondrial DNA mutation A3243G impairs cellular metabolic pathways necessary for beta cell function. Diabetologia. 2006;49:1816–1826. doi: 10.1007/s00125-006-0301-9. [DOI] [PubMed] [Google Scholar]
- 60.Lenaz G., Baracca A., Carelli V., D'Aurelio M., Sgarbi G., Solaini G. Bioenergetics of mitochondrial diseases associated with mtDNA mutations. Biochim. Biophys. Acta. 2004;1658:89–94. doi: 10.1016/j.bbabio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi G., Cortopassi G. Oxidative stress in inherited mitochondrial diseases. Free Radic. Biol. Med. 2015;88:10–17. doi: 10.1016/j.freeradbiomed.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raimundo N., Song L., Shutt T.E., McKay S.E., Cotney J., Guan M.X., Gilliland T.C., Hohuan D., Santos-Sacchi J., Shadel G.S. Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness. Cell. 2012;148:716–726. doi: 10.1016/j.cell.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guan M. X., Yan Q., Li X., Bykhovskaya Y., Gallo-Teran J., Hajek P., Umeda N., Zhao H., Garrido G., Mengesha E., et al. Mutation in TRMU related to transfer RNA modification modulates the phenotypic expression of the deafness-associated mitochondrial 12S ribosomal RNA mutations. Am. J. Hum. Genet. 2006;79:291–302. doi: 10.1086/506389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen C., Chen Y., Guan M.X. A peep into mitochondrial disorder: multifaceted from mitochondrial DNA mutations to nuclear gene modulation. Protein Cell. 2015;6:862–870. doi: 10.1007/s13238-015-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tischner C., Hofer A., Wulff V., Stepek J., Dumitru I., Becker L., Haack T., Kremer L., Datta A.N., Sperl W., et al. MTO1 mediates tissue specificity of OXPHOS defects via tRNA modification and translation optimization, which can be bypassed by dietary intervention. Hum. Mol. Genet. 2015;24:2247–2266. doi: 10.1093/hmg/ddu743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen D., Li F., Yang Q., Tian M., Zhang Z., Zhang Q., Chen Y., Guan M.X. The defective expression of gtpbp3 related to tRNA modification alters the mitochondrial function and development of zebrafish. Int. J. Biochem. Cell. Biol. 2016;77:1–9. doi: 10.1016/j.biocel.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 68.Dittmar K.A., Goodenbour J.M., Pan T. Tissue-specific differences in human transfer RNA expression. PLoS Genet. 2006;2:e221. doi: 10.1371/journal.pgen.0020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.