Abstract
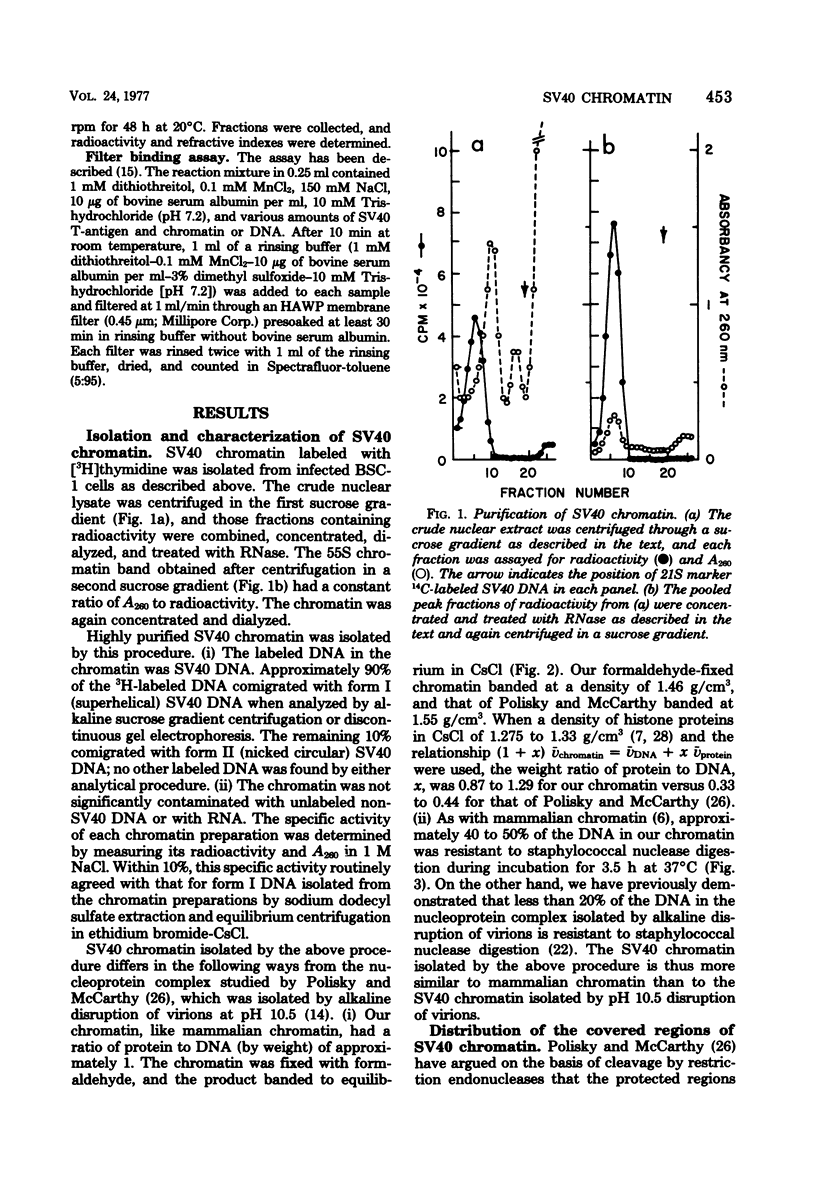
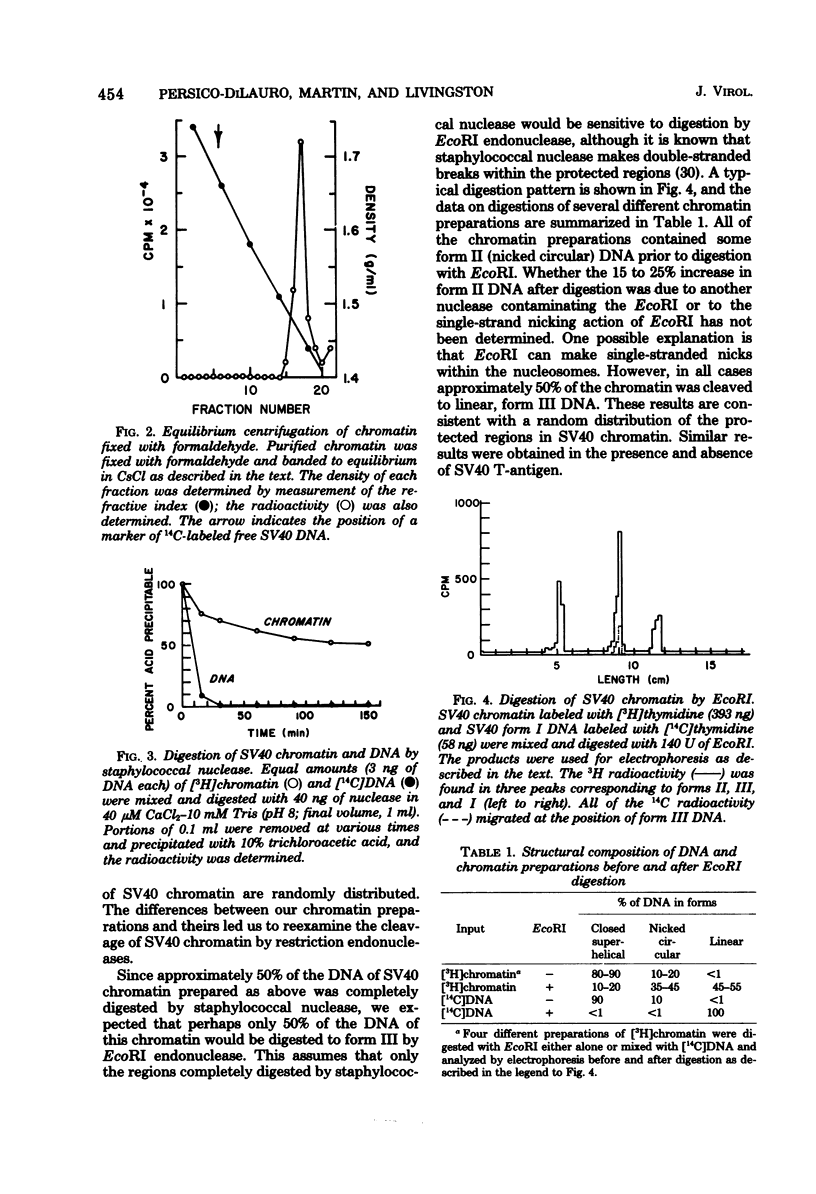
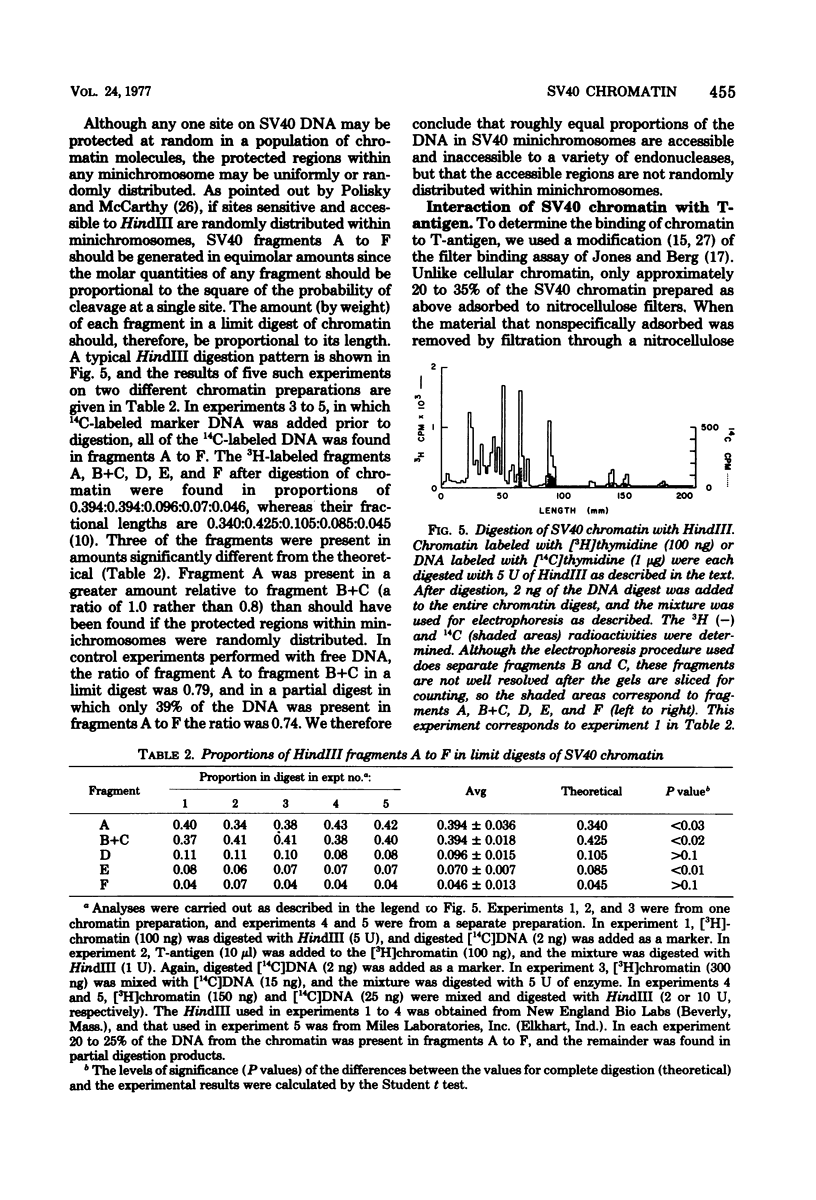
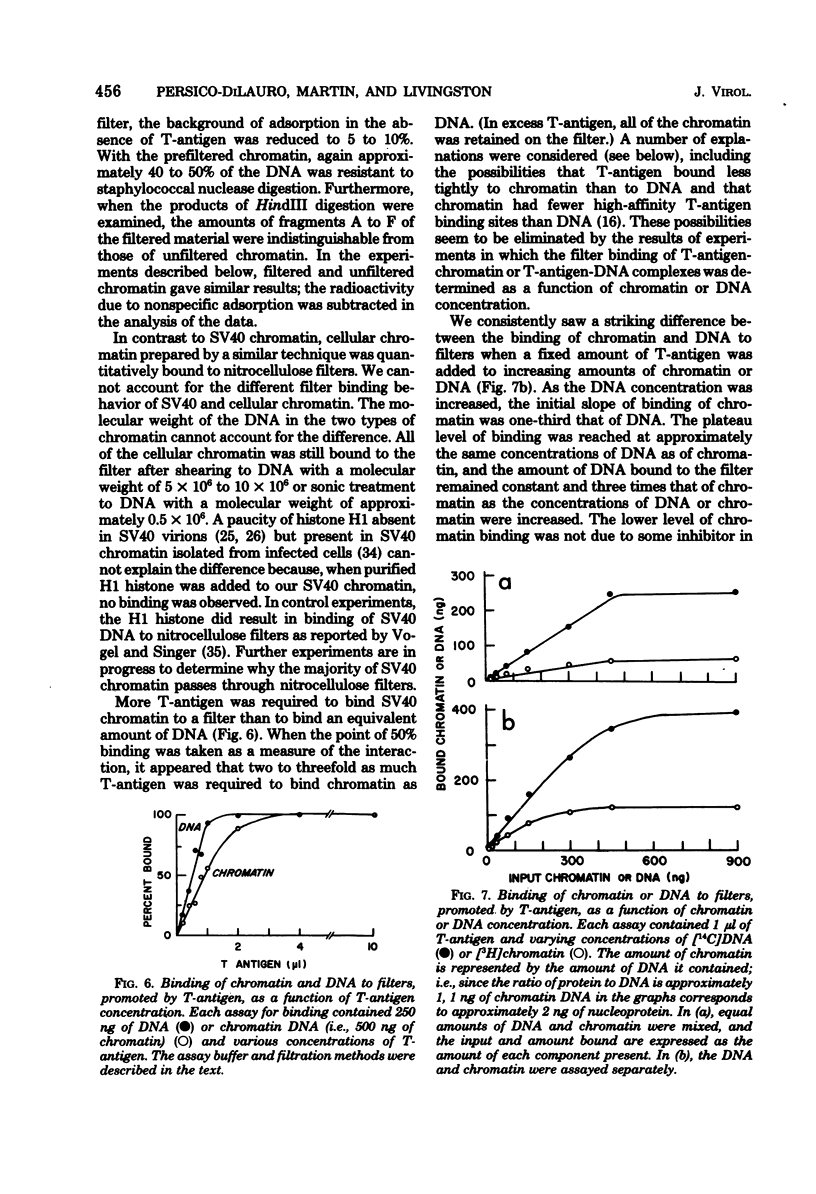
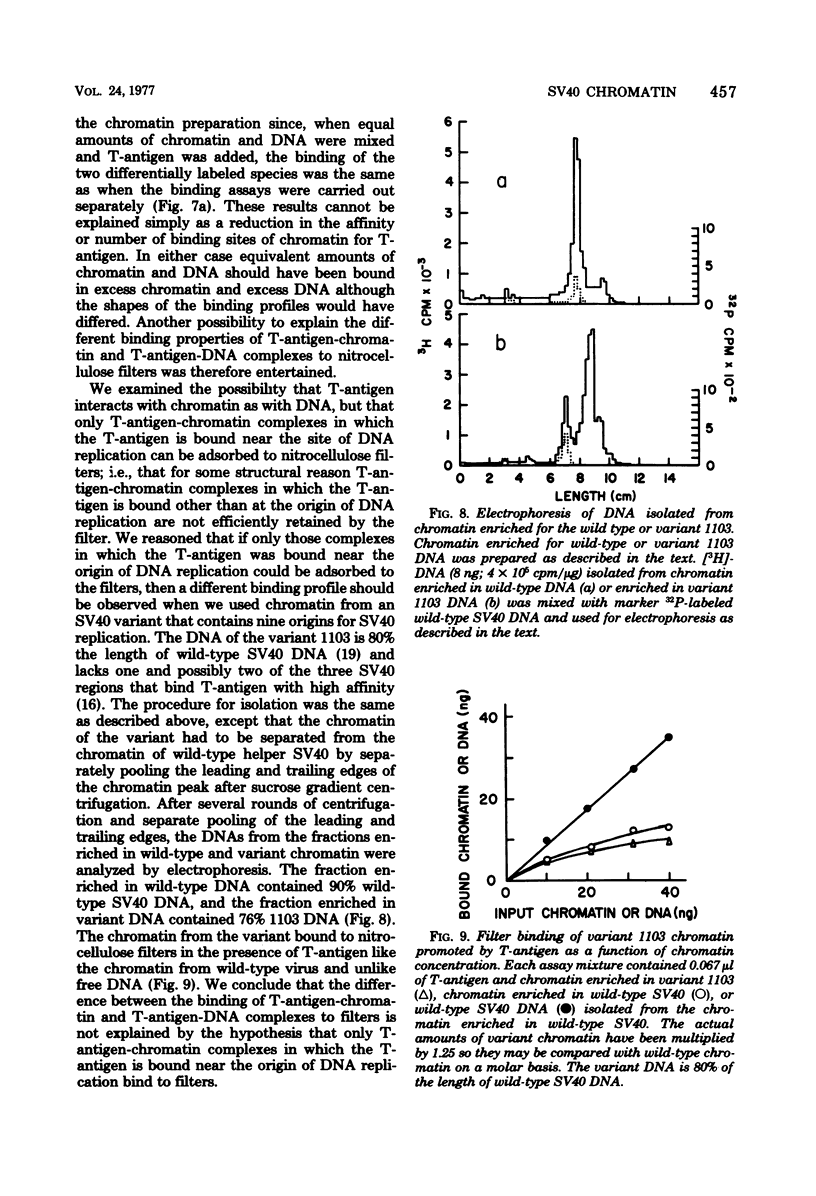
We have studied the binding of the tumor antigen (T-antigen) of simian virus 40 to simian virus 40 chromatin (minichromosomes). The minichromosomes isolated from infected cells by a modification of standard techniques were relatively free of contaminating RNA and cellular DNA and had a ratio (by weight) of protein to DNA of approximately 1; their DNA was 50 to 60% digestible to an acid-soluble form by staphylococcal nuclease. Cleavage of this chromatin with restriction endonucleases indicated that the nuclease-resistant regions were randomly distributed in the population of minichromosomes, but were not randomly distributed within minichromosomes. Only 20 to 35% of these minichromosomes adsorbed nonspecifically to nitrocellulose filters, permitting binding studies between simian virus 40 T-antigen and chromatin to be performed. Approximately two to three times as much T-antigen was required to bind chromatin as to bind an equivalent amount of free DNA. When T-antigen was present in excess, both chromatin and free DNA were quantitatively retained on the filters. On the other hand, when DNA or chromatin was present in excess, only one-third as much chromatin as DNA was retained. We suggest that T-antigen-chromatin complexes may be formed by the cooperative binding of T-antigen to chromatin, whereas T-antigen-DNA complexes may be formed by simple bimolecular interactions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Melchior W., Jr, Sollner-Webb B., Felsenfeld G. Specific sites of interaction between histones and DNA in chromatin. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4101–4105. doi: 10.1073/pnas.71.10.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Hager L., Dulbecco R. Simian virus 40 T antigen binds to DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3754–3757. doi: 10.1073/pnas.71.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Avila J., Martin R. G. Viral DNA synthesis in cells infected by temperature-sensitive mutants of simian virus 40. J Virol. 1974 Jul;14(1):116–124. doi: 10.1128/jvi.14.1.116-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Association of arginine-rich histones with G-C-rich regions of DNA in chromatin. Nat New Biol. 1972 Dec 20;240(103):226–229. doi: 10.1038/newbio240226a0. [DOI] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Structure of chromatin. Nat New Biol. 1971 Jan 27;229(4):101–106. doi: 10.1038/newbio229101a0. [DOI] [PubMed] [Google Scholar]
- Compton J. L., Bellard M., Chambon P. Biochemical evidence of variability in the DNA repeat length in the chromatin of higher eukaryotes. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4382–4386. doi: 10.1073/pnas.73.12.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremisi C., Pignatti P. F., Croissant O., Yaniv M. Chromatin-like structures in polyoma virus and simian virus 10 lytic cycle. J Virol. 1975 Jan;17(1):204–211. doi: 10.1128/jvi.17.1.204-211.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Estes M. K., Pagano J. S. Structure and function of the polypeptides in simian virus 40. I. Existence of subviral deoxynucleoprotein complexes. J Virol. 1972 Jun;9(6):923–929. doi: 10.1128/jvi.9.6.923-929.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessel D., Hudson J., Landau T., Tenen D., Livingston D. M. Interaction of partially purified simian virus 40 T antigen with circular viral DNA molecules. Proc Natl Acad Sci U S A. 1975 May;72(5):1960–1964. doi: 10.1073/pnas.72.5.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessel D., Landau T., Hudson J., Lalor T., Tenen D., Livingston D. M. Identification of regions of the SV40 genome which contain preferred SV40 T antigen-binding sites. Cell. 1976 Aug;8(4):535–545. doi: 10.1016/0092-8674(76)90222-1. [DOI] [PubMed] [Google Scholar]
- Jones O. W., Berg P. Studies on the binding of RNA polymerase to polynucleotides. J Mol Biol. 1966 Dec 28;22(2):199–209. doi: 10.1016/0022-2836(66)90126-4. [DOI] [PubMed] [Google Scholar]
- Kuchino T., Yamaguchi N. Characterization of T antigen in cells infected with a temperature-sensitive mutant of simian virus 40. J Virol. 1975 Jun;15(6):1302–1307. doi: 10.1128/jvi.15.6.1302-1307.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee T. N., Brockman W. W., Nathans D. Evolutionary variants of simian virus 40: cloned substituted variants containing multiple initiation sites for DNA replication. Virology. 1975 Jul;66(1):53–69. doi: 10.1016/0042-6822(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Henderson I. C., Hudson J. SV40 T antigen: partial purification and properties. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):283–289. doi: 10.1101/sqb.1974.039.01.037. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Martin R. G. Nuclease digestion of the DNA-protein complex from simian virus 40 virions. Biochim Biophys Acta. 1975 Jul 7;395(3):280–283. doi: 10.1016/0005-2787(75)90198-7. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. SV40: T antigen, the A function and transformation. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):267–276. doi: 10.1101/sqb.1974.039.01.035. [DOI] [PubMed] [Google Scholar]
- Oxman M. N., Takemoto K. K., Eckhart W. Polyoma T antigen synthesis by temperature-sensitive mutants of polyoma virus. Virology. 1972 Sep;49(3):675–682. doi: 10.1016/0042-6822(72)90524-7. [DOI] [PubMed] [Google Scholar]
- Pett D. M., Estes M. K., Pagano J. S. Structural proteins of simian virus 40. I. Histone characteristics of low-molecular-weight polypeptides. J Virol. 1975 Feb;15(2):379–385. doi: 10.1128/jvi.15.2.379-385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., McCarthy B. Location of histones on simian virus 40 DNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2895–2899. doi: 10.1073/pnas.72.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Sen A., Hancock R., Levine A. J. The properties and origin of the proteins in the SV40 nucleoprotein complex. Virology. 1974 Sep;61(1):11–21. doi: 10.1016/0042-6822(74)90237-2. [DOI] [PubMed] [Google Scholar]
- Shaw B. R., Herman T. M., Kovacic R. T., Beaudreau G. S., Van Holde K. E. Analysis of subunit organization in chicken erythrocyte chromatin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):505–509. doi: 10.1073/pnas.73.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., Felsenfeld G. A comparison of the digestion of nuclei and chromatin by staphylococcal nuclease. Biochemistry. 1975 Jul;14(13):2915–2920. doi: 10.1021/bi00684a019. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenen D. G., Baygell P., Livingston D. M. Thermolabile T (tumor) antigen from cells transformed by a temperature-sensitive mutant of simian virus 40. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4351–4355. doi: 10.1073/pnas.72.11.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenen D. G., Martin R. G., Anderson J., Livingston D. M. Biological and biochemical studies of cells transformed by simian virus 40 temperature-sensitive gene A mutants and A mutant revertants. J Virol. 1977 Apr;22(1):210–218. doi: 10.1128/jvi.22.1.210-218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Chumackov P. M., Georgiev G. P. Minichromosome of simian virus 40: presence of histone HI. Nucleic Acids Res. 1976 Aug;3(8):2101–2113. doi: 10.1093/nar/3.8.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T., Singer M. The interaction of histones with simian virus 40 supercoiled circular deoxyribonucleic acid in vitro. J Biol Chem. 1975 Jan 25;250(2):796–798. [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Simpson R. T. Removal of histone H1 exposes a fifty base pair DNA segment between nucleosomes. Biochemistry. 1976 Jul 27;15(15):3307–3314. doi: 10.1021/bi00660a022. [DOI] [PubMed] [Google Scholar]



