Abstract
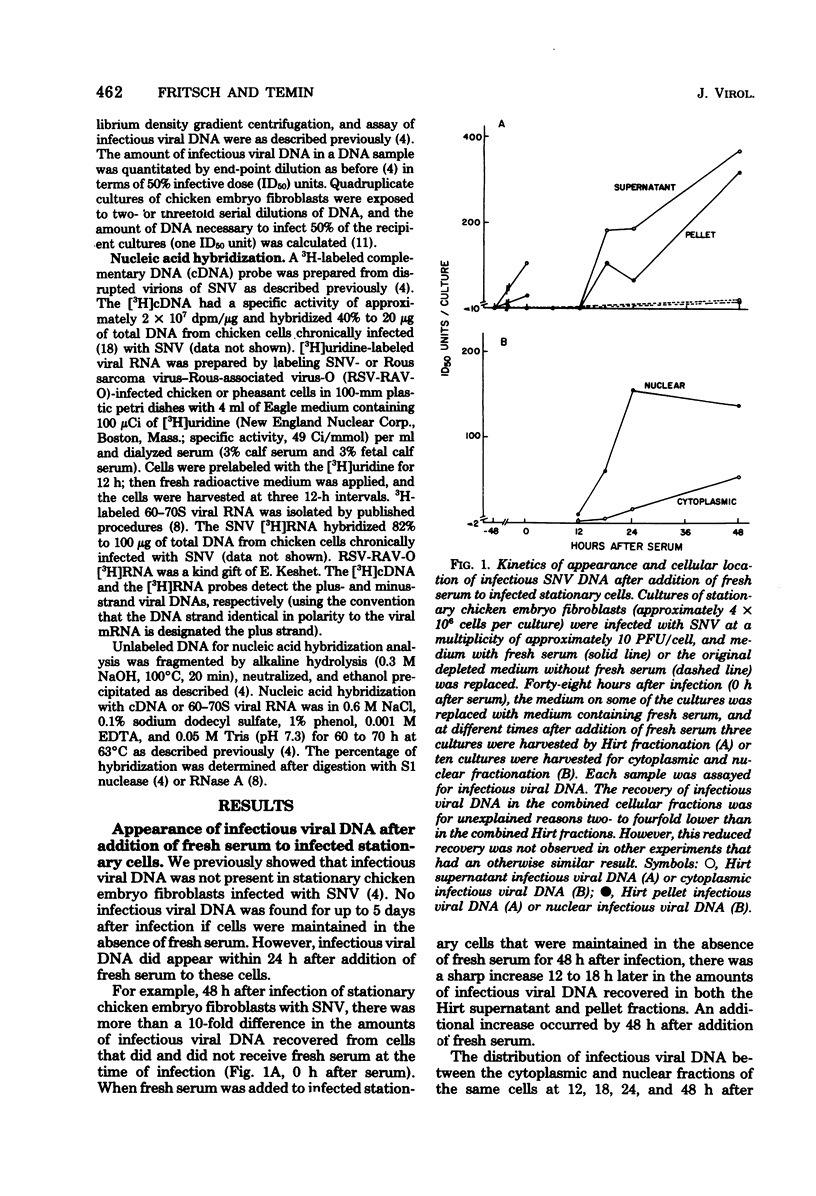
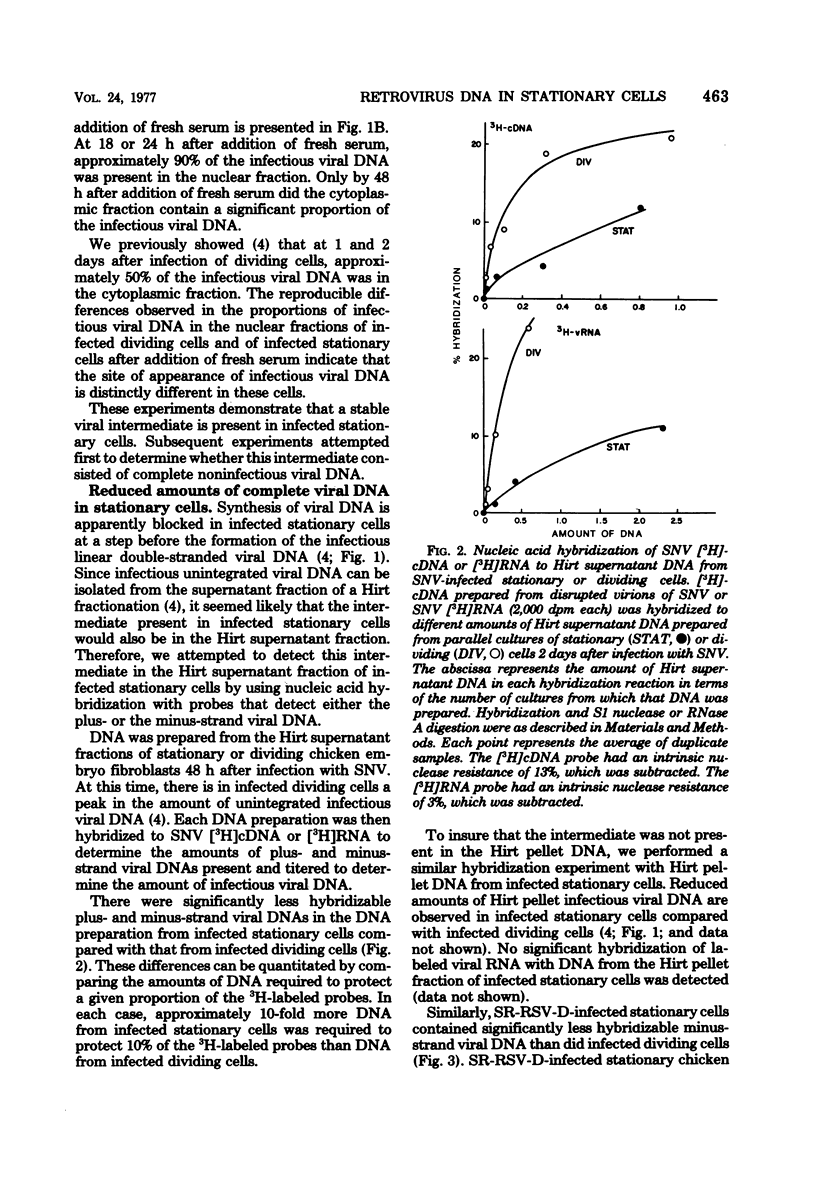
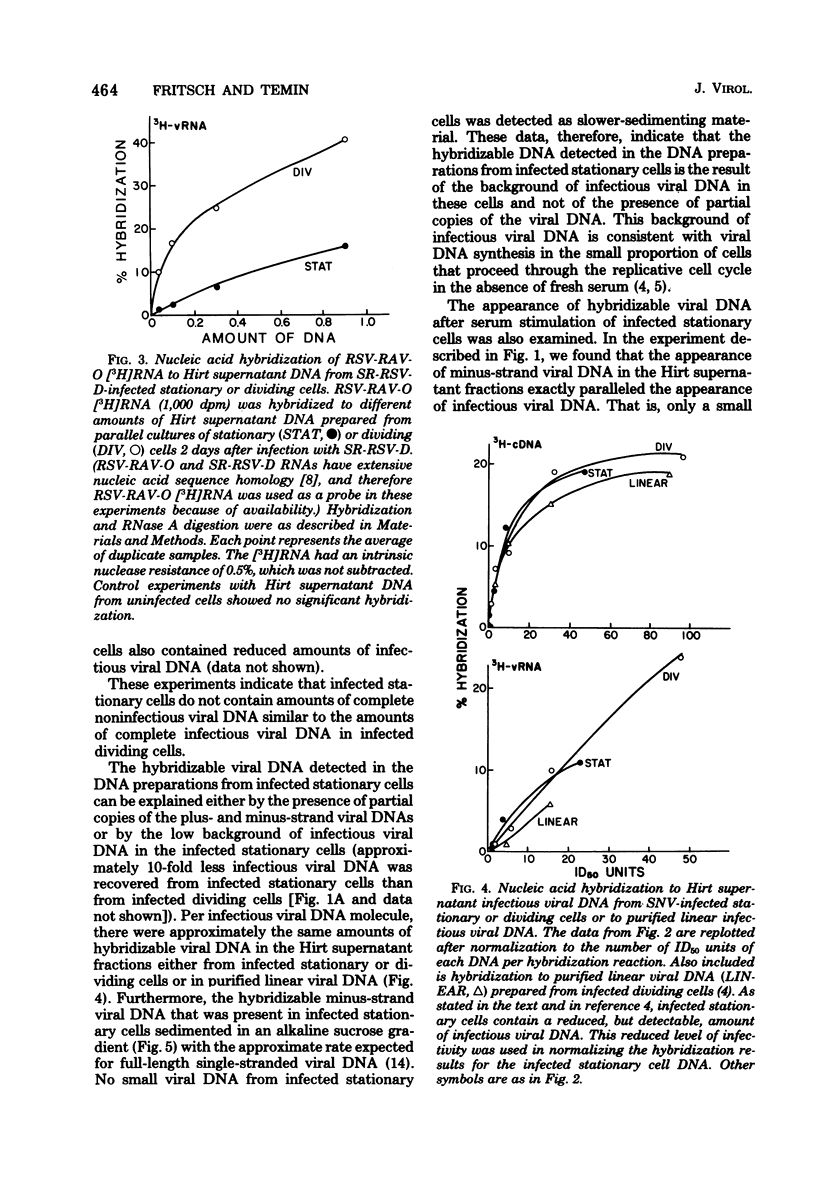
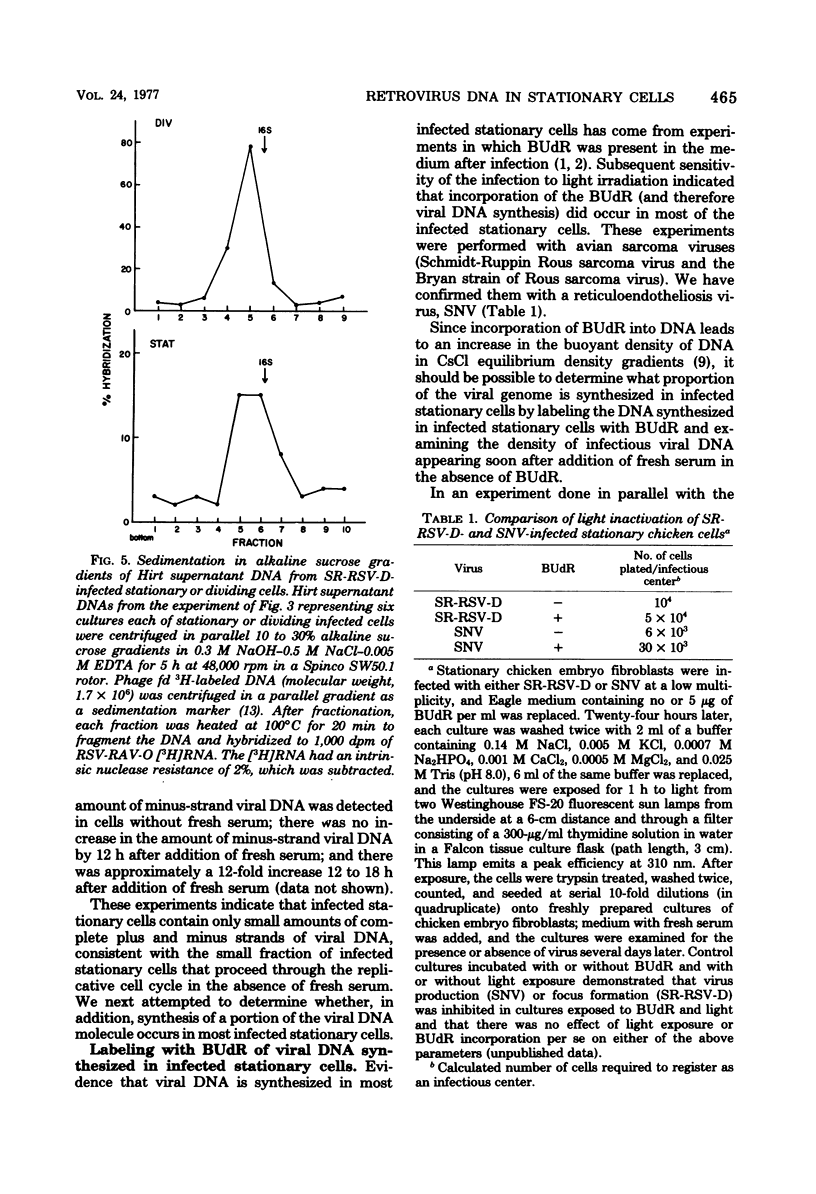
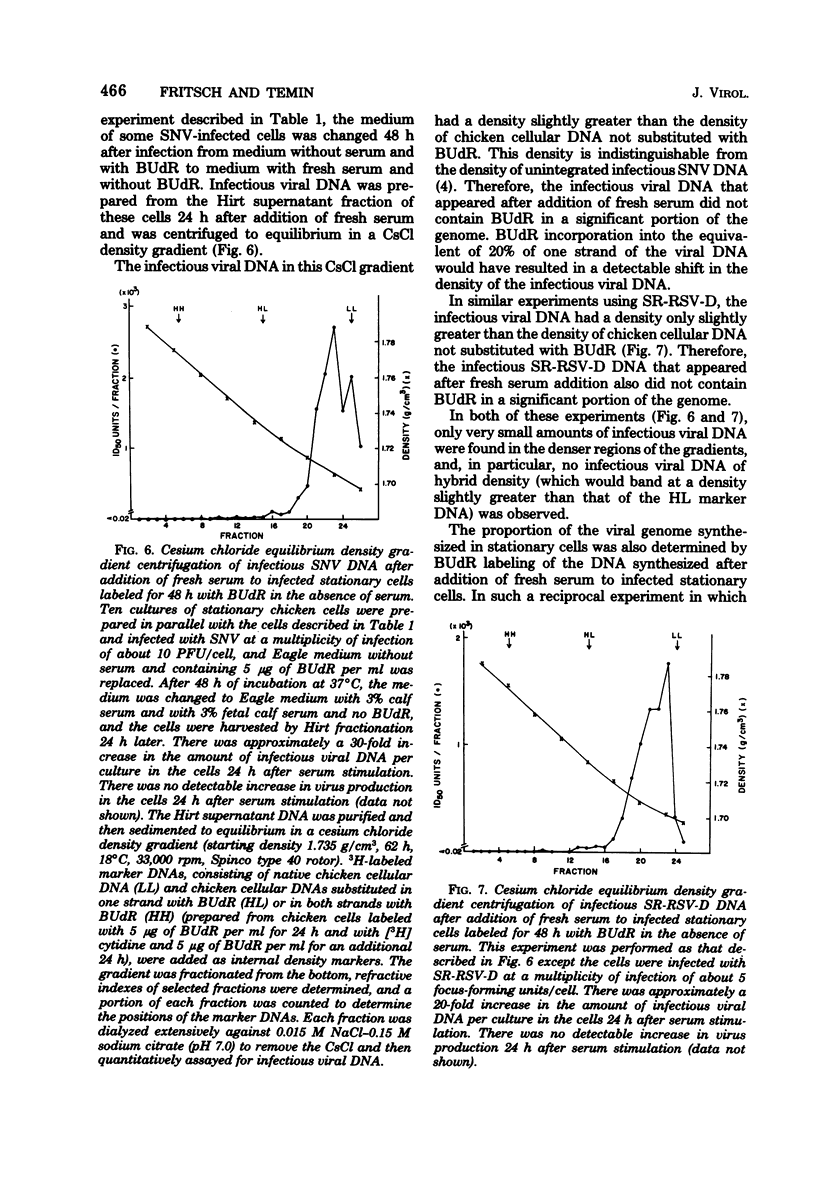
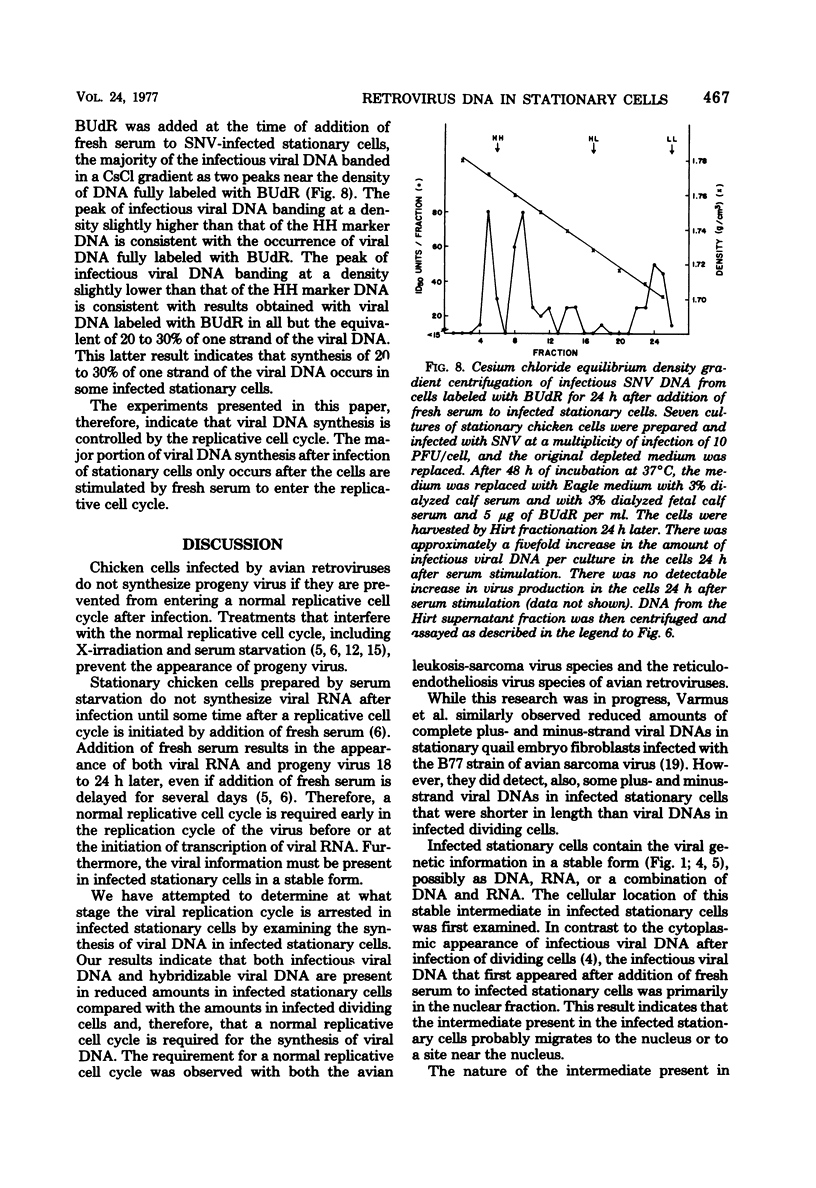
Previously, we reported (Fritsch and Temin, J. Virol. 21:119-130, 1977) that infectious viral DNA was not present in spleen necrosis virus-infected stationary chicken cells. However, a stable intermediate was present in such infected stationary cells as evidenced by the appearance of infectious viral DNA shortly after serum stimulation of these cells. After serum stimulation of infected stationary cells, the infectious viral DNA appeared first in the nucleus. In contrast, in infected dividing cells the infectious viral DNA appeared first in the cytoplasm. Significantly reduced amounts of complete plus- or minus-strand viral DNAs were detected by nucleic acid hybridization in stationary chicken cells infected with spleen necrosis virus or Schmidt-Ruppin Rous sarcoma virus compared with the amounts detected in infected dividing cells. These experiments indicated that infected stationary cells did not contain complete noninfectious copies of viral DNA. Furthermore, 5-bromodeoxyuridine labeling and cesium chloride density gradient centrifugation analysis of the infectious viral DNA that appeared after serum stimulation of infected stationary cells indicated that most viral DNA synthesis occurred after addition of fresh serum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balduzzi P., Morgan H. R. Mechanism of oncogenic transformation by Rous sarcoma virus. I. Intracellular inactivation of cell-transforming ability of Rous sarcoma virus by 5-bromodeoxyuridine and light. J Virol. 1970 Apr;5(4):470–477. doi: 10.1128/jvi.5.4.470-477.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D., Temin H. M. Light inactivation of focus formation by chicken embryo fibroblasts infected with avian sarcoma virus in the presence of 5-bromodeoxyuridine. Nature. 1970 Nov 14;228(5272):622–624. doi: 10.1038/228622a0. [DOI] [PubMed] [Google Scholar]
- Colby C., Edlin G. Nucleotide pool levels in growing, inhibited, and transformed chick fibroblast cells. Biochemistry. 1970 Feb 17;9(4):917–920. doi: 10.1021/bi00806a029. [DOI] [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Cell cycle-dependent activation of rous sarcoma virus-infected stationary chicken cells: avian leukosis virus group-specific antigens and ribonucleic acid. J Virol. 1972 Jul;10(1):82–87. doi: 10.1128/jvi.10.1.82-87.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Temin H. M. Requirement for cell division for initiation of transcription of Rous sarcoma virus RNA. J Virol. 1974 Sep;14(3):531–546. doi: 10.1128/jvi.14.3.531-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Kenney F. T., Tennant R. W. Evidence for a stable intermediate in leukemia virus activation in AKR mouse embryo cells. J Virol. 1974 Sep;14(3):451–456. doi: 10.1128/jvi.14.3.451-456.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- RUBIN H., TEMIN H. M. A radiological study of cell-virus interaction in the Rous sarcoma. Virology. 1959 Jan;7(1):75–91. doi: 10.1016/0042-6822(59)90178-3. [DOI] [PubMed] [Google Scholar]
- Rush M. G., Warner R. C. Alkali denaturation of covalently closed circular duplex deoxyribonucleic acid. J Biol Chem. 1970 May 25;245(10):2704–2708. [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Carcinogenesis by avian sarcoma viruses. Cancer Res. 1968 Sep;28(9):1835–1838. [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: acute infection. J Virol. 1974 Feb;13(2):291–297. doi: 10.1128/jvi.13.2.291-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: chronic infection. J Gen Virol. 1975 Jun;27(3):267–274. doi: 10.1099/0022-1317-27-3-267. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]



