Abstract
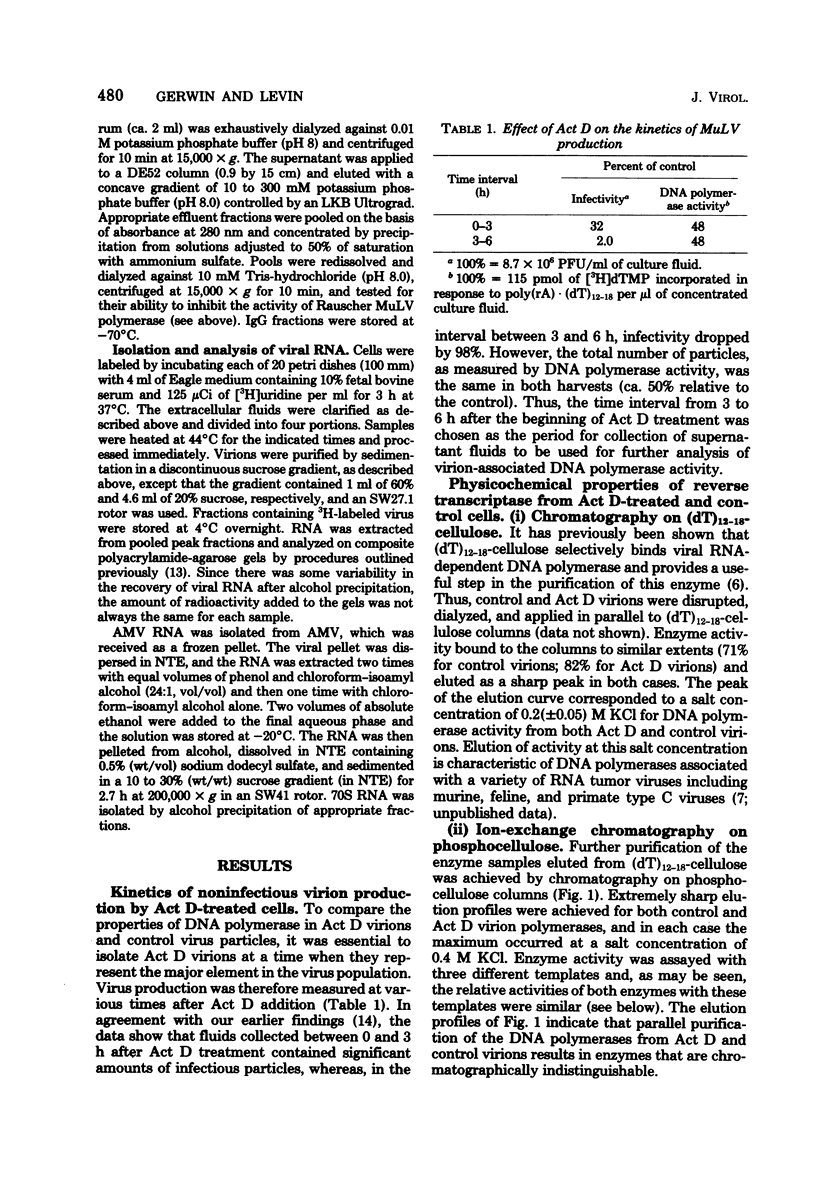
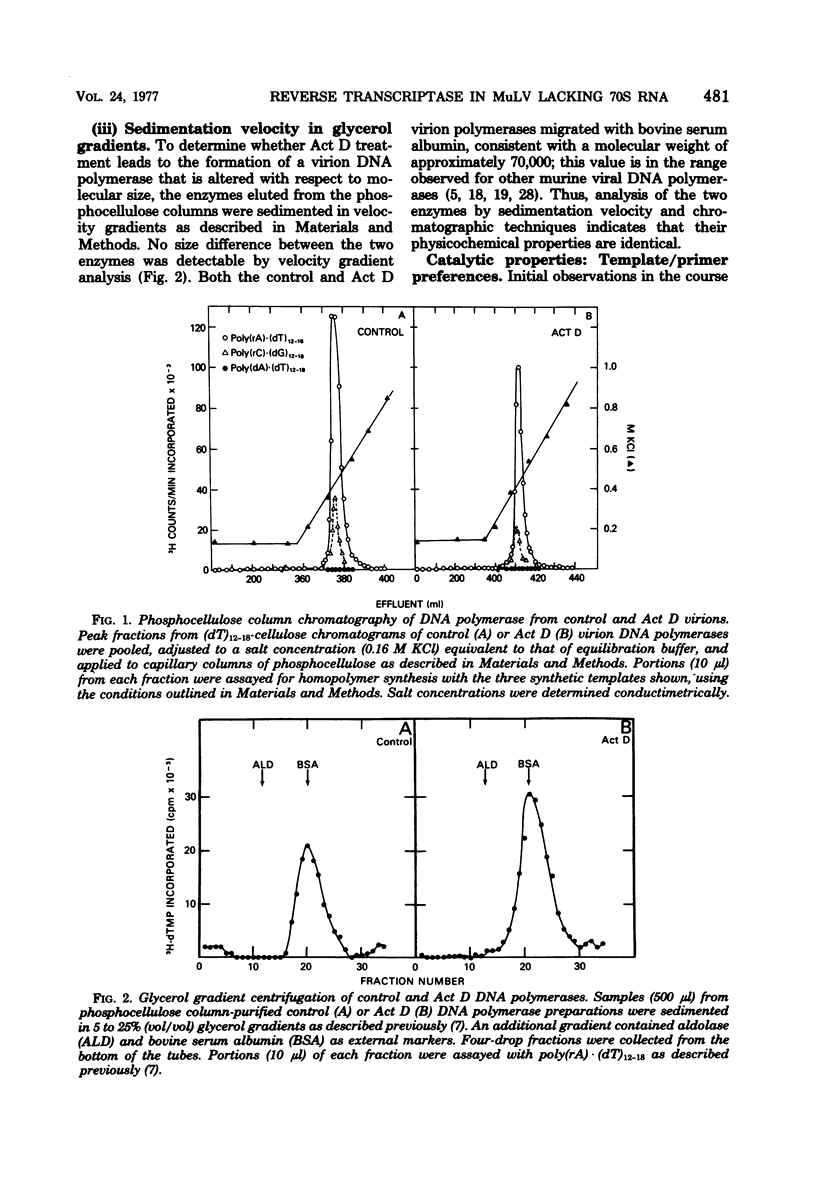
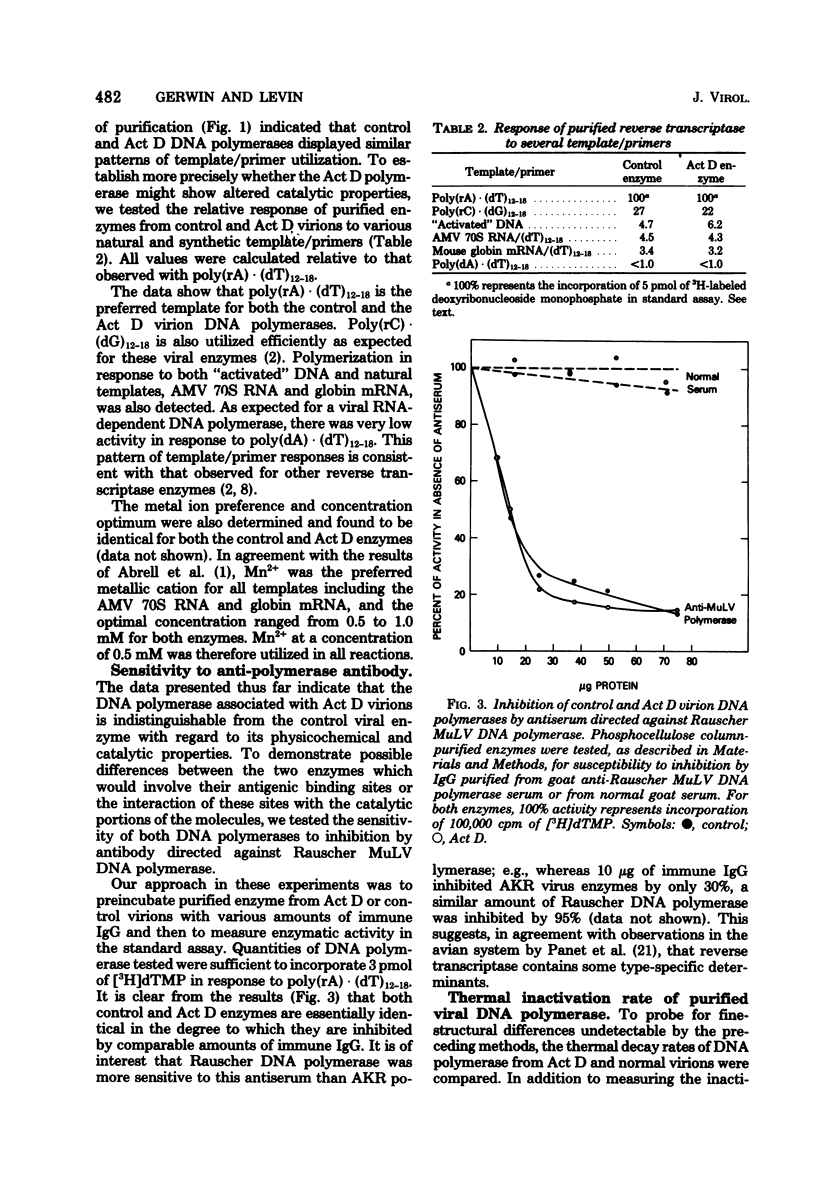
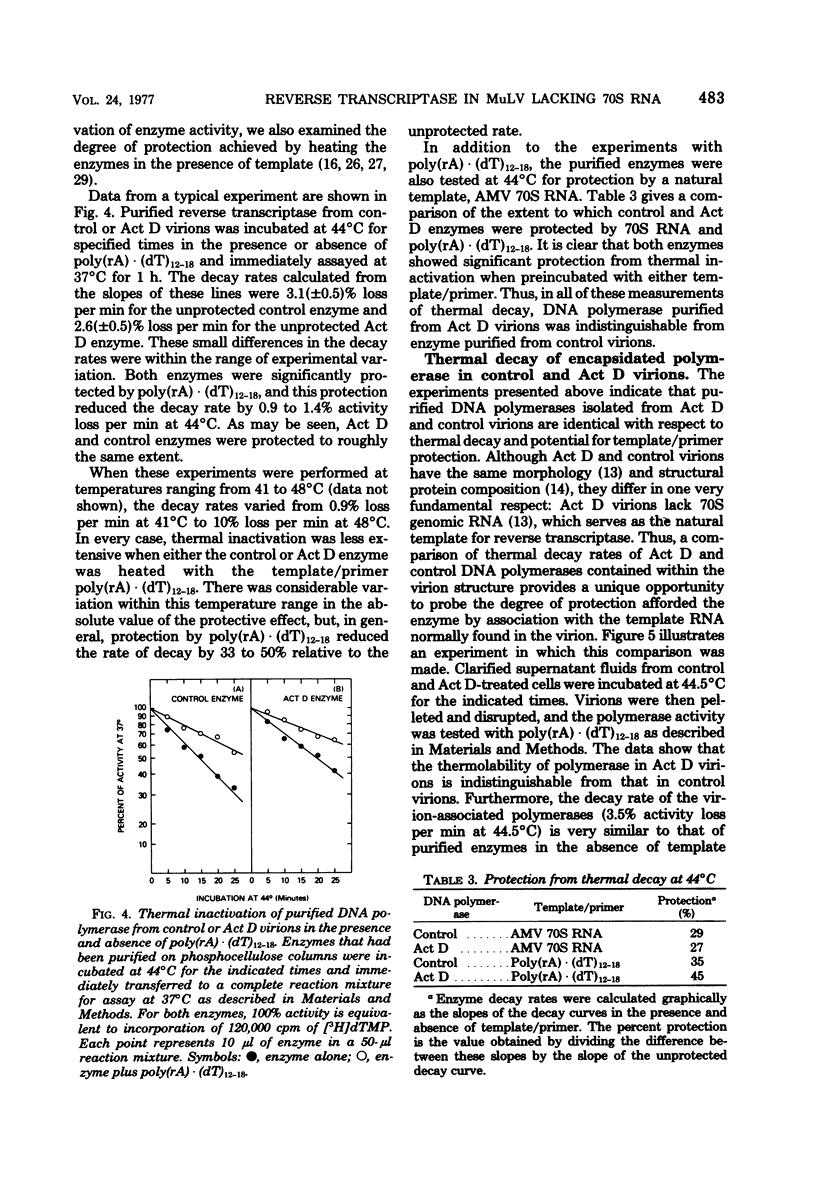
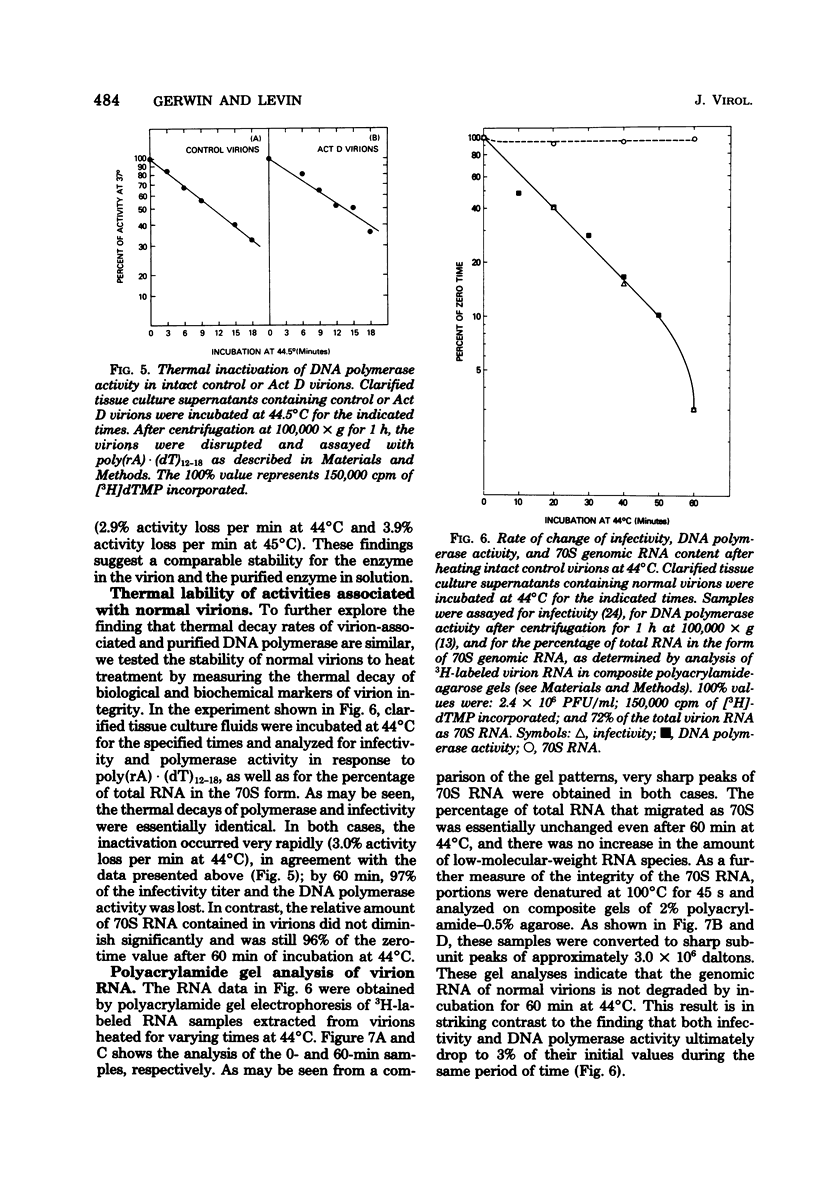
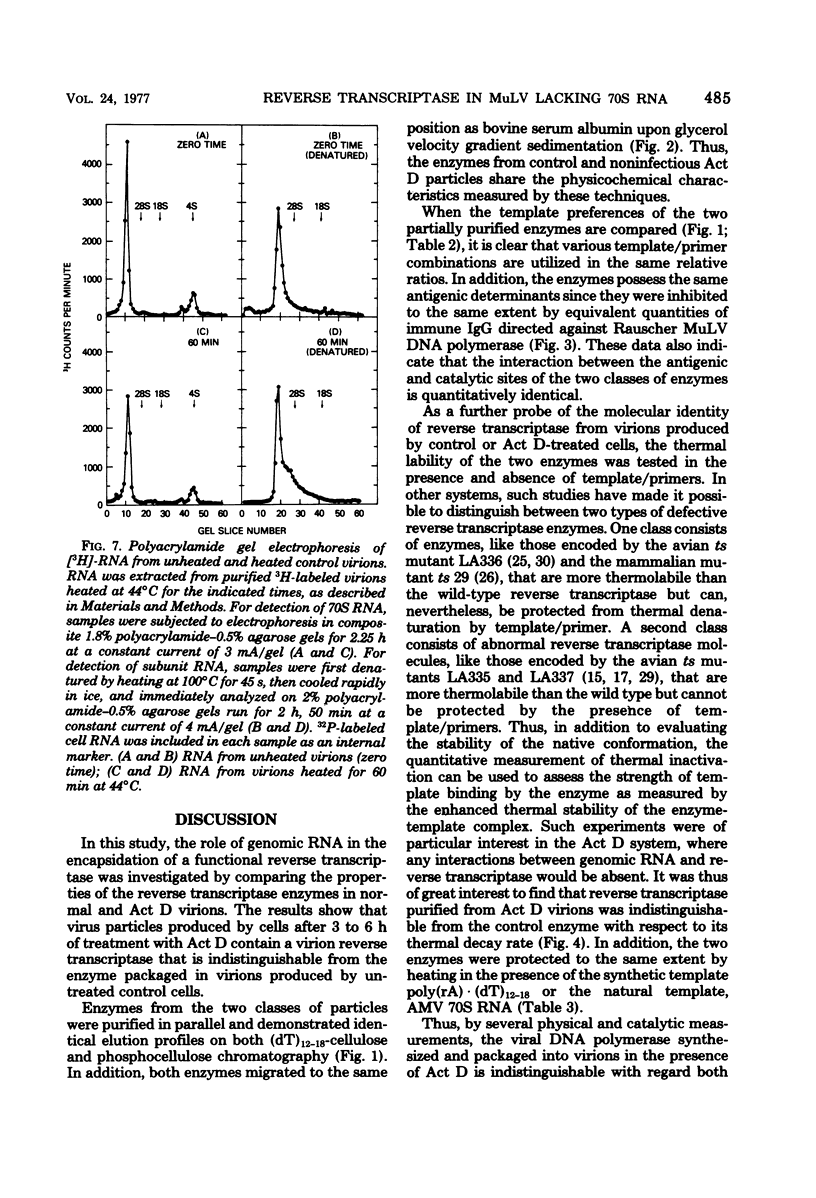
Virions produced by cells in the presence of actinomycin D (Act D virions) contain reverse transcriptase but are deficient in 70S genomic RNA. To assess the role of genomic RNA in encapsidation of a functional reverse transcriptase and to study the interaction of the enzyme and its template in the cores of intact virions, the reverse transcriptase enzymes of normal and Act D virions were compared. The enzymes were indistinguishable by column chromatography, sedimentation velocity, or template/primer preferences. In addition, these enzymes showed equal sensitivity to inactivation by antibodies directed against Rauscher murine leukemia virus DNA polymerase. The enzymes from Act D and normal virions had similar thermal decay rates and were both protected against heat denaturation by natural and synthetic template/primers. By these criteria, the DNA polymerase molecules synthesized and assembled into virions in the absence of genomic RNA are identical to those packaged under normal conditions. Additional studies designed to measure protection of reverse transcriptase by genomic RNA were carried out by comparing the thermal lability of the enzyme in intact Act D and normal virions. The thermal decay rate of reverse transcriptase in Act D virions was identical to that in control virions. In contrast to the lability of the virion-associated enzyme, however, genomic RNA in control virions was stable to heat treatment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Reitz M. S., Gallo R. C. Transcription of 70S RNA by DNA polymerases from mammalian RNA viruses. J Virol. 1975 Dec;16(6):1566–1574. doi: 10.1128/jvi.16.6.1566-1574.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R., Ogura H., Gelderblom H., Halpern M. S. The defective maturation of viral progeny with a temperature-sensitive mutant of avian sarcoma virus. Virology. 1976 Aug;73(1):259–272. doi: 10.1016/0042-6822(76)90079-9. [DOI] [PubMed] [Google Scholar]
- Gerard G. F., Grandgenett D. P. Purification and characterization of the DNA polymerase and RNase H activities in Moloney murine sarcoma-leukemia virus. J Virol. 1975 Apr;15(4):785–797. doi: 10.1128/jvi.15.4.785-797.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Milstien J. B. An oligonucleotide affinity column for RNA-dependent DNA polymerase from RNA tumor viruses. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2599–2603. doi: 10.1073/pnas.69.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Smith S. G., Peebles P. T. Two active forms of RD-114 virus DNA polymerase in infected cells. Cell. 1975 Sep;6(1):45–52. doi: 10.1016/0092-8674(75)90072-0. [DOI] [PubMed] [Google Scholar]
- Goodman N. C., Spiegelman S. Distinguishing reverse transcriptase of an RNA tumor virus from other known DNA polymerases. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2203–2206. doi: 10.1073/pnas.68.9.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms E., Rodhe W., Friis R. R., Bauer H. Influence of defective virion core proteins on RNA maturation with an avian sarcoma virus. J Virol. 1977 Jan;21(1):419–422. doi: 10.1128/jvi.21.1.419-422.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Selective decrease in the rate of cleavage of an intracellular precursor to Rauscher leukemia virus p30 by treatment of infected cells with actinomycin D. J Virol. 1976 Sep;19(3):1054–1072. doi: 10.1128/jvi.19.3.1054-1072.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Grimley P. M., Ramseur J. M., Berezesky I. K. Deficiency of 60 to 70S RNA in murine leukemia virus particles assembled in cells treated with actinomycin D. J Virol. 1974 Jul;14(1):152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Rosenak M. J. Synthesis of murine leukemia virus proteins associated with virions assembled in actinomycin D-treated cells: evidence for persistence of viral messenger RNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1154–1158. doi: 10.1073/pnas.73.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Mason W. S. Characterization of two conditional early mutants of Rous sarcoma virus. Virology. 1973 May;53(1):258–273. doi: 10.1016/0042-6822(73)90484-4. [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Modak M. J., Cavalieri L. F. Evidence for template-specific sites in DNA polymerases. Biochem Biophys Res Commun. 1974 Jan 23;56(2):516–521. doi: 10.1016/0006-291x(74)90873-0. [DOI] [PubMed] [Google Scholar]
- Mason W. S., Friis R. R., Linial M., Vogt P. K. Determination of the defective function in two mutants of Rous sarcoma virus. Virology. 1974 Oct;61(2):559–574. doi: 10.1016/0042-6822(74)90290-6. [DOI] [PubMed] [Google Scholar]
- Modak M. J., Marcus S. L. Purification and properties of Rauscher leukemia virus DNA polymerase and selective inhibition of mammalian viral reverse transcriptase by inorganic phosphate. J Biol Chem. 1977 Jan 10;252(1):11–19. [PubMed] [Google Scholar]
- Moelling K. Further characterization of the Friend murine leukemia virus reverse transcriptase-RNase H complex. J Virol. 1976 May;18(2):418–425. doi: 10.1128/jvi.18.2.418-425.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owada M., Toyoshima K. Analysis on the reproducing and cell-transforming capacities of a temperature sensitive mutant (ts 334) of avian sarcoma virus B77. Virology. 1973 Jul;54(1):170–178. doi: 10.1016/0042-6822(73)90126-8. [DOI] [PubMed] [Google Scholar]
- Panet A., Baltimore D., Hanafusa T. Quantitation of avian RNA tumor virus reverse transcriptase by radioimmunoassay. J Virol. 1975 Jul;16(1):146–152. doi: 10.1128/jvi.16.1.146-152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskind M. P., Weinberg R. A., Baltimore D. Dependence of Moloney murine leukemia virus production on cell growth. Virology. 1975 Sep;67(1):242–248. doi: 10.1016/0042-6822(75)90421-3. [DOI] [PubMed] [Google Scholar]
- Rohrschneider J. M., Diggelmann H., Ogura H., Friis R. R., Bauer H. Defective cleavage of a precursor polypeptide in a temperature-sensitive mutant of avian sarcoma virus. Virology. 1976 Nov;75(1):177–187. doi: 10.1016/0042-6822(76)90016-7. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Temperature sensitive mutants of an avian sarcoma virus. Virology. 1969 Dec;39(4):930–931. doi: 10.1016/0042-6822(69)90030-0. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Stephenson J. R., Verma I. M., Aaronson S. A. Thermolabile reverse transcriptase of a mammalian leukemia virus mutant temperature sensitive in its replication and sarcoma virus helper functions. J Virol. 1975 Dec;16(6):1476–1482. doi: 10.1128/jvi.16.6.1476-1482.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Mason W. S., Drost S. D., Baltimore D. DNA polymerase activity from two temperature-sensitive mutants of Rous sarcoma virus is thermolabile. Nature. 1974 Sep 6;251(5470):27–31. doi: 10.1038/251027a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M. Studies on reverse transcriptase of RNA tumor viruses. I. Localization of thermolabile DNA polymerase and RNase H activities on one polypeptide. J Virol. 1975 Jan;15(1):121–126. doi: 10.1128/jvi.15.1.121-126.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Varmus H. E., Hunter E. Characterization of "early" temperature-sensitive mutants of avian sarcoma viruses: biological properties, thermolability of reverse transcriptase in vitro, and synthesis of viral DNA in infected cells. Virology. 1976 Oct 1;74(1):16–29. doi: 10.1016/0042-6822(76)90124-0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R. Identification of a large polypeptide precursor of avian oncornavirus proteins. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1734–1738. doi: 10.1073/pnas.70.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]


