Abstract
Raffaelea (Ophiostomatales) is a genus of more than 20 ophiostomatoid fungi commonly occurring in symbioses with wood-boring ambrosia beetles. We examined ambrosia beetles and plant hosts in the USA and Taiwan for the presence of these mycosymbionts and found 22 isolates representing known and undescribed lineages in Raffaelea. From 28S rDNA and β-tubulin sequences, we generated a molecular phylogeny of Ophiostomatales and observed morphological features of seven cultures representing undescribed lineages in Raffaelea s. lat. From these analyses, we describe five new species in Raffaelea s. lat.: R. aguacate, R. campbellii, R. crossotarsa, R. cyclorhipidia, and R. xyleborina spp. nov. Our analyses also identified two plant-pathogenic species of Raffaelea associated with previously undocumented beetle hosts: (1) R. quercivora, the causative agent of Japanese oak wilt, from Cyclorhipidion ohnoi and Crossotarsus emancipatus in Taiwan, and (2) R. lauricola, the pathogen responsible for laurel wilt, from Ambrosiodmus lecontei in Florida. The results of this study show that Raffaelea and associated ophiostomatoid fungi have been poorly sampled and that future investigations on ambrosia beetle mycosymbionts should reveal a substantially increased diversity.
Keywords: entomogenous fungi, insect-fungus interactions, Japanese oak wilt, laurel wilt, molecular phylogenetics, mycosymbioses
INTRODUCTION
Raffaelea (Arx & Hennebert 1965) is a genus of primarily asexual fungi including more than 20 species in Ophiostomatales (Harrington et al. 2010, de Beer et al. 2013, Musvuugwa et al. 2015). These fungi commonly occur in symbioses with wood-boring ambrosia beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae). Ambrosia beetles propagate these and other fungi, which obtain nutrients from plant tissues and provide the beetles with a food source, throughout galleries in their plant hosts. When female beetles leave the parental gallery to establish a new generation, they transport inocula of one or several mycosymbionts in specialized cavities in various parts of their bodies, to be grown in the subsequently developed galleries (Hubbard 1897, Beaver 1989).
The asexual morphological characteristics of Raffaelea are rather simple: hyaline, rarely-branching, commonly single-celled conidiophores are arranged singly or aggregated in sporodochia; conidiogenous cells are precurrently or sympodially proliferating, which may leave denticles, annellations, or inconspicuous scarring; conidia range from elliptical to globose, with some exceptions, and may reproduce by yeast-like budding (Harrington et al. 2010, Musvuugwa et al. 2015). De Beer & Wingfield (2013) recognized two sexually reproducing species of Ophiostoma, O. seticolle and O. deltoideosporum, in Raffaelea s. str. based on DNA sequence phylogenies, but they did not transfer these species to Raffaelea. Subsequently, Musvuugwa et al. (2015) described a Raffaelea species, R. vaginata, with an observed sexual morph, similar to those of O. seticolle and O. deltoideosporum. The latter authors consequently emended the circumscription of the genus to include both asexual and sexual morphs, and transferred the two Ophiostoma species to Raffaelea as R. seticollis and R. deltiodeospora, consistent with the one fungus-one name rule (Hawksworth 2011).
Some molecular phylogenies of Raffaelea and additional genera within Ophiostomatales have suggested that Raffaelea is monophyletic (e.g. Harrington et al. 2010). However, more recent and comprehensive analyses (de Beer & Wingfield 2013, Dreaden et al. 2014, Musvuugwa et al. 2015) have shown that Raffaelea species constitute three clades in the order, Raffaelea s. str., the R. lauricola complex, and the R. sulphurea complex. Of ecological interest, the two clades exterior to Raffaelea s. str. each include plant pathogens that have been spread in the last decade by their respective insect vectors. Raffaelea lauricola, the causative agent of laurel wilt in the southeastern USA, is associated with the ambrosia beetle Xyleborus glabratus (Harrington et al. 2008, Ploetz et al. 2013), among others (Carrillo et al. 2014). Raffaelea lauricola, the eponymous member of the R. lauricola complex (de Beer & Wingfield 2013, Musvuugwa et al. 2015), is sometimes placed as sister to Raffaelea s str. in molecular phylogenies of individual rDNA loci (Musvuugwa et al. 2015) and additional coding genes (Dreaden et al. 2014). Raffaelea quercivora, which is responsible for Japanese oak wilt and associated with Platypus quercivorus (Kubono & Ito 2002, Kusumoto et al. 2014), lies within the R. sulphurea complex in Leptographium s. lat. (de Beer & Wingfield 2013, Dreaden et al. 2014, Musvuugwa et al. 2015).
During domestic (Campbell et al. 2016) and international studies to investigate the diversity of ambrosia beetles and their fungal symbionts, raffaelea-like isolates from the southeastern USA and Taiwan were collected; preliminary molecular analyses indicated that some of these isolates represent novel lineages within Raffaelea s. lat. In this study, we use nine isolates to describe five new species in Raffaelea from collections of plant hosts and ambrosia beetles. We have also characterized 13 additional Raffaelea isolates based on DNA sequence data.
MATERIALS AND METHODS
DNA extraction, PCR amplification and sequencing
Twenty-two Raffaelea cultures and DNA extracts were aggregated from the Forest Entomology laboratory at the University of Florida (Gainesville, FL) and the University of Florida’s Tropical Research and Education Center (Homestead, FL). Cultures from ambrosia beetle hosts were isolated by dilution plating of mycangial contents, as described by Li et al. (2015). Cultures of newly described species are deposited in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa (Table 1).
Table 1.
Cultures of Raffaelea examined in our molecular phylogenetic analyses, with ex-type cultures of new species in bold.
| Species | Isolate | CMW culture number (Other identifier) | Beetle host | Plant host | Country | Region (Locality) | 28S rDNA | BTUB | 18S rDNA | ITS DNA |
|---|---|---|---|---|---|---|---|---|---|---|
| R. aguacate | PL1004 | CMW38067 (272) | Persea americana | USA | Florida (Miami-Dade Co.) | KJ909296 | KJ909297 | KF026302 | ||
| R. campbellii | 103p2 | CMW44800 | Persea americana | USA | Florida (Miami-Dade Co., Homestead) | KR018414 | KR018442 | KR018402 | ||
| R. campbellii | 110p2 | CMW44801 | Persea americana | USA | Florida (Miami-Dade Co., Homestead) | KR018430 | KR018444 | KR018403 | ||
| R. cf. campbellii | Hulcr7355 | Euplatypus compositus | USA | Florida | KX267101 | KX267112 | ||||
| R. cf. subalba | Hulcr7375 | Euplatypus compositus | USA | Florida | KX267102 | KX267113 | ||||
| R. crossotarsa | Hulcr7182 | CMW44793 | Crossotarsus emancipatus | Lithocarpus sp. | Taiwan | Fushan | KX267103 | KX267114 | KX267129 | KX267135 |
| R. cyclorhipidia | Hulcr7168 | CMW44790 | Cyclorhipidion ohnoi | Lithocarpus sp. | Taiwan | Fushan | KX267104 | KX267115 | KX267130 | KX267136 |
| R. lauricola | Hulcr4530 | (PL1007) | Ambrosiodmus lecontei | Persea borbonia | USA | Florida (Lake Kissimmee) | KX267116 | |||
| R. lauricola | Hulcr7161 | Xyleborinus glabratus | Taiwan | KX267105 | KX267117 | |||||
| R. lauricola | Hulcr7164 | Xyleborinus glabratus | Taiwan | KX267106 | KX267118 | |||||
| R. quercivora | Hulcr7167 | Cyclorhipidion ohnoi | Lithocarpus sp. | Taiwan | Fushan | KX267107 | KX267119 | KX267131 | ||
| R. quercivora | Hulcr7176 | Crossotarsus emancipatus | Lithocarpus sp. | Taiwan | Fushan | KX267108 | KX267120 | KX267132 | ||
| R. subfusca | Hulcr4520 | (C2335) | Xyleborus glabratus | USA | South Carolina (Hunting Island State Park) | KX267121 | KX267137 | |||
| R. subfusca | Hulcr4717 | Euwallacea validus | USA | Virginia (Albemarle Co., Batesville) | KX267122 | KX267133 | KX267138 | |||
| R. subfusca | Hulcr4719 | Euwallacea validus | USA | Pennsylvania (Huntingdon Co. Raystown Lake) | KX267109 | KX267123 | KX267134 | |||
| R. xyleborina | Hulcr6099 | CMW45859 | Xyleborinus andrewesii | USA | Florida (Highlands Co., Venus) | KX267110 | KX267124 | |||
| R. xyleborina | Hulcr6100 | Xyleborinus andrewesii | USA | Florida (Highlands Co., Venus) | KX267111 | KX267125 | ||||
| R. xyleborina | Hulcr6406 | Xyleborinus andrewesii | USA | Florida (Highlands Co., Venus) | KX267126 | KX267139 | ||||
| R. xyleborina | Hulcr6408 | Xyleborinus andrewesii | USA | Florida (Highlands Co., Venus) | KX267127 | KX267140 | ||||
| Raffaelea sp. | Hulcr5951 | (PL1635) | Xyleborus pinicola | Pinus keysei | Thailand | Mae Chaem | KJ909308 | KJ909310 | KJ909309 | |
| Raffaelea sp. | Hulcr7507 | Xyleborinus gracilis | USA | Florida | KX267128 | KX267141 | ||||
| Raffaelea sp. | PL1001 | CMW38062 | Persea americana | USA | California | KJ909293 | KJ909295 | KJ909294 |
Fungal DNA was isolated with Extract-N-Amp PCR kits (Sigma-Aldrich), as described by Li et al. (2015). Final concentrations of PCR reagent solutions in 25 μL were: (1) 1× ClonTech-TaKaRa Ex Taq Buffer; (2) 5 % DMSO; (3) 0.2 mM each dNTP; (4) 0.5 μM each primer; (5) 0.625 U Ex Taq polymerase; and (6) 0.01–0.1 ng extracted DNA. Primer combinations used for amplifications were: (1) LR0R/LR5 (Vilgalys & Hester 1990, Rehner & Samuels 1994) for nuclear large subunit (28S) ribosomal DNA (rDNA); (2) T10 or Bt2a/Bt2b (Glass & Donaldson 1995, O’Donnell & Cigelnik 1997) for β-tubulin (βT); (3) NS1/NS4 for nuclear small subunit (18S) rDNA; and (4) either ITS3/LR3 or ITS1F/ITS4 (White et al. 1990, Gardes & Bruns 1993) for portions of the ITS1-5.8S-ITS2 (ITS) rDNA locus. The PCR conditions for βT and ITS rDNA were the same as those used by Yin et al. (2015) and for 18S and 28S rDNA by Dreaden et al. (2014). Amplified products were visualized and purified as described by Li et al. (2015), and these were submitted to the University of Florida Interdisciplinary Center for Biotechnology Research for Sanger sequencing. Chromatograms were assembled and inspected with Geneious v. 9.0.5.
Phylogenetic analyses
Sequences of 28S rDNA and βT (introns 3/4/5 removed) were aligned and visually inspected in Geneious for phylogenetic reconstruction. The alignment was divided into four partitions for phylogenetic consideration: one partition for the 28S rDNA alignment and for each of the three codon positions in the protein encoding βT. The Akaike information criterion in jModeltest 0.1.1 (Guindon & Gascuel 2003, Posada 2008) was used to select the nucleotide substitution model for each partition. Maximum likelihood (ML) phylogenetic analyses were conducted in GARLI 2.01 (Zwickl 2006) with the recommended partition parameters to determine the best tree topology (Fig. 1) and bootstrap support values from 500 search replicates, which were summarized in SumTrees (Sukumaran & Holder 2010). Bayesian posterior probabilities (BPP) were estimated with the same partition parameters in an analysis conducted in MrBayes 3.1.2 (Ronquist & Huelsenbeck 2003), in which two runs of four chains each were executed simultaneously for 5 000 000 generations, with sampling every 500 generations. SumTrees was used to compute BPP from a summary of 7501 trees retained after a burn-in of the first 2500 trees collected.
Fig. 1.
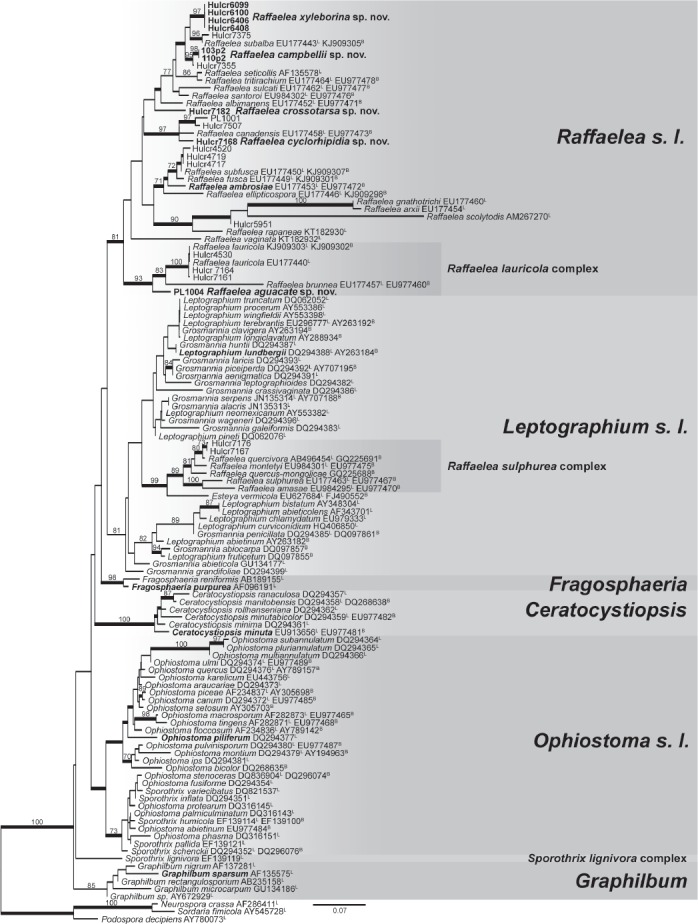
Best ML tree from GARLI analysis of 28S rDNA and βT data matrix of Ophiostomatales genera with Sordariales as outgroup (Musvuugwa et al. 2015). Values at nodes represent ML bootstrap percentages ≥ 70 % from a summary of 500 replicates, and branches in bold represent BPP ≥ 95 %. L denotes GenBank accession number of 28S rDNA sequence for taxon; B denotes GenBank accession number of βT sequence for taxon. Types of genera and new species of Raffaelea in bold.
Growth trials and morphological characterization
To determine optimal growth rates of each new species of Raffaelea, discs of agar (7 mm diam) covered with mycelium were aseptically removed from 1-wk-old cultures growing on BD Difco™ MEA and used to inoculate plates incubated at 10–35 °C, in 5 °C intervals. After 9 d, colony growth was calculated as by Musvuugwa et al. (2015). Morphological features were examined by inoculating sterile slide mounts of BD Difco™ MEA with propagules collected by running a sterile needle along the surface of cultures growing on BD Difco™ MEA. Once reproductive structures were observed using a dissecting microscope (24–48 h), slides were examined on an Olympus BX53 equipped with a Canon EOS Rebel T3i using EOS Utility 2 software. For each new species, measurements of conidiophores (n=5) and conidia (n=10) were made to the nearest 0.5 μm, and means (± standard deviation) were calculated to the nearest 0.1 μm.
RESULTS
All isolates we examined resided in Raffaelea s. str., the R. lauricola complex, or the R. sulphurea complex in the phylogenetic analyses of the 28S rDNA and βT data matrices (Fig. 1). The R. lauricola complex was sister to the Raffaelea s. str. clade with 81 % ML bootstrap and 100 % BPP support, and the well-supported R. sulphurea clade resolved within Leptographium s. lat. The ITS and 18S rDNA sequences were not included in the phylogenetic analyses, but these sequences were used for molecular identification (Table 1). The data matrix for the 28S rDNA and βT regions has been deposited in TreeBASE as submission 19323.
The new species in Raffaelea s. str. and the R. lauricola complex (Table 1) possessed all βT introns (3/4/5). Isolates Hulcr7167 and Hulcr7176 possessed two introns (3/4/-). These patterns of intron presence were the expected conditions for the majority of species in each clade (de Beer & Wingfield 2013). Although they were not isolated from Platypus quercivorus, isolates Hulcr7167 and Hulcr7176 resolved in the R. sulphurea complex with R. quercivora, and were 99 % (396/400 bp) and 98 % (392/400 bp) similar, respectively, to the βT sequence (including introns) of R. quercivora. The 28S rDNA sequences for isolates Hulcr7167 and Hulcr7176 were 98 % (492–493/499 bp) similar to R. quercivora, but the representative R. quercivora sequence (GenBank accession AB496454) had six ambiguous bases that increased the level of dissimilarity with our isolates.
TAXONOMY
Raffaelea aguacate D.R. Simmons, Dreaden & Ploetz, sp. nov.
MycoBank MB817170
(Fig. 2)
Fig. 2.
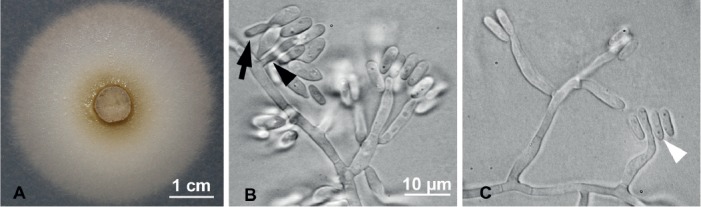
Raffaelea aguacate (PL1004) morphological features in pure culture on MEA. A. Colony growth after 9 d at 25 °C. B. Hyphae bearing long, tapering conidiogenous cells with conidia at apex, and occasional sessile lateral conidia (black arrowhead); elongated conidia may bud yeast-like daughter cell (black arrow). C. Hyphae with long and slightly irregular conidiogenous cells, with conidia truncated at base (white arrowhead). Bar in B applies also to C.
Etymology: The epithet “aguacate” refers to the Spanish for avocado (Persea americana), from which this isolate was cultured.
Diagnosis: Conidiogenous cells 13 (±2) × 2.7 (±0.3) μm, hyaline, sometimes irregular; conidia at conidiogenous cell apex or sessile and lateral; conidia 7.2 (±0.6) × 2.6 (±0.5) μm, elongate, truncated at base, hyaline, rarely with yeast-like budding.
Type: USA: Florida: Miami-Dade Co., Homestead, from bioassay of Persea americana, 2009, C. L. Harmon (BPI 910154 – holotype; 272 = PL1004 = CMW38067 – ex-type cultures).
Description: Colonies initially cream, turning light green to olivaceous, aging to dark green on MEA; reverse subhyaline. Optimal colony diameter after 9 d at 25 °C in the dark was 70.2 (±3.9) mm; 46.0 (±2.6) mm at 10 °C; no growth at 35 °C. Conidiogenous cells hyaline, sometimes irregular, tapering at ends, 13 (±2) × 2.7 (±0.3) μm. Conidia forming from apex of conidiogenous cells, hyaline, occasionally sessile and lateral. Conidia produced singly, aseptate, elongate, and occasionally truncated at the base, 7.2 (±0.6) × 2.6 (±0.5) μm. Conidia rarely budding. Sexual morph unknown.
Raffaelea campbellii D.R. Simmons, A. Campbell & Ploetz, sp. nov.
MycoBank MB817171
(Fig. 3)
Fig. 3.
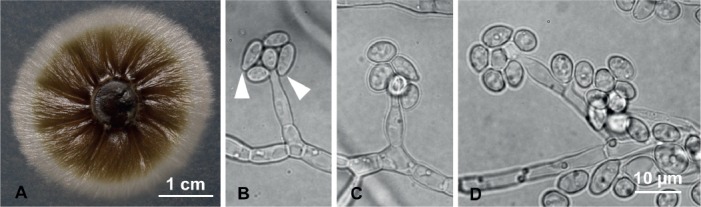
Raffaelea campbellii (103p2) morphological features in pure culture on MEA. A. Colony growth after 9 d at 25 °C. B–D. Hyphae bearing flask-shaped conidiogenous cells with ovoid to elliptical conidia, often truncated at the base (white arrowheads). Bar in D applies also to B–C.
Etymology: The epithet “campbellii” is in honor of Donald and Princesa Campbell, parents of Alina S. Campbell, collector of the specimen, for their guidance and support.
Diagnosis: Conidiogenous cells 13.7 (±1.6) × 3.7 (±0.3) μm, hyaline, flask-shaped; conidia at conidiogenous cell apex; conidia 6.7 (±1.2) × 3.6 (±0.5) μm, ovoid to elliptical, truncated at base, hyaline.
Type: USA: Florida: Miami-Dade Co., cultured from Xyleborus glabratus that infected Persea palustris, Jun. 2013, A. S. Campbell (BPI 910156 – holotype; 103p2 = CMW44800 – ex-type culture).
Additional specimen examined: Loc. cit (110p2 = CMW44801).
Description: Colonies initially cream, turning olivaceous to blackish on MEA, surface tough and wrinkled; reverse subhyaline. Optimal colony diameter after 9 d at 25 °C in the dark 25.7 (±1.3) mm; no growth at 10 °C or 35 °C. Conidiogenous cells hyaline, flask-shaped, tapering towards the apex, 13.7 (±1.6) × 3.7 (±0.3) μm. Conidia forming from apex of conidiogenous cells, hyaline. Conidia produced singly, accumulating at tip of conidiogenous cells, aseptate, ovoid to elliptical, sometimes fusiform, and often truncate at the base, 6.7 (±1.2) × 3.6 (±0.5) μm. Sexual morph unknown.
Raffaelea crossotarsa D.R. Simmons & Y.T. Huang, sp. nov.
MycoBank MB817172
(Fig. 4)
Fig. 4.
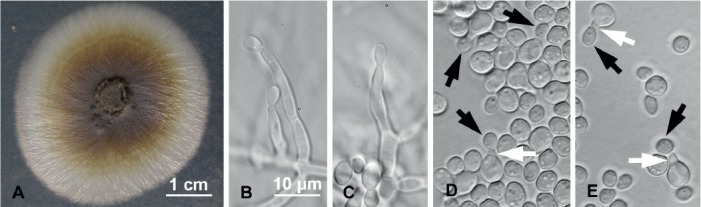
Raffaelea crossotarsa (Hulcr7182) morphological features in pure culture on MEA. A. Colony growth after 9 d at 25 °C. B–C. Hyphae bearing long, tapering conidiogenous cells with conidia. D–E. Globose to ovoid conidia budding yeast-like daughter cells (black arrows), which protrude from prominent isthmuses (white arrows). Bar in B applies also to C–E.
Etymology: The epithet “crossotarsa” refers to the genus of the host beetle (Crossotarsus emancipatus), the mycangium of which yielded this fungus.
Diagnosis: Conidiogenous cells 15.2 (±2.1) × 3 (±0.3) μm, hyaline, slender; conidia at conidiogenous cell apex; conidia 6 (±0.4) × 4.9 (±0.3) μm, globose to ovoid, hyaline, yeast-like budding from prominent isthmus.
Type: Taiwan: Fushan, cultured from Crossotarsus emancipatus collected from Lithocarpus sp., Mar. 2015, J. Hulcr, A. Black & D. R. Simmons (BPI 910157 – holotype; Hulcr7182 = CMW44793 – ex-type culture).
Description: Colonies initially cream, aging from golden olivaceous to dark green or dark ruddy brown on MEA, surface tough; reverse subhyaline. Optimal colony diameter after 9 d at 25 °C in the dark was 39.2 (±1.2) mm; 9.0 (±0.5) mm at 10 °C; no growth at 35 °C. Conidiogenous cells hyaline, slender, tapering at ends, 15.2 (±2.1) × 3 (±0.3) μm. Conidia forming from apex of conidiogenous cells, hyaline. Conidia produced singly, aseptate, globose to ovoid, 6 (±0.4) × 4.9 (±0.3) μm. Conidia producing budding cells from prominent isthmus, 1–2 μm long. Sexual morph unknown.
Raffaelea cyclorhipidia D.R. Simmons & Y.T. Huang, sp. nov.
MycoBank MB817173
(Fig. 5)
Fig. 5.
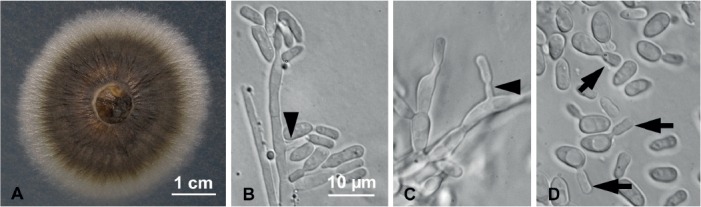
Raffaelea cyclorhipidia (Hulcr7168) morphological features in pure culture on MEA. A. Colony growth after 9 d at 25 °C. B–C. Hyphae bearing typical flask-shaped conidiogenous cells with conidia at apex, and occasional lateral sessile conidia (black arrowheads). D. Elliptical to elongate conidia budding yeast-like daughter cells (black arrows). Bar in B applies also to C–D.
Etymology: The epithet “cyclorhipidia” refers to the genus of the host beetle (Cyclorhipidion ohnoi), the mycangium of which yielded this fungus.
Diagnosis: Conidiogenous cells 12 (±1.7) × 3.6 (±0.3) μm, hyaline, flask-shaped; conidia at conidiogenous cell apex or sessile and lateral; conidia 7.3 (±1.0) × 3.5 (±0.7) μm, elliptical to elongate, hyaline, yeast-like budding.
Type: Taiwan: Fushan, cultured from Cyclorhipidion ohnoi collected infesting Lithocarpus sp., Mar. 2015, J. Hulcr, A. Black & D. R. Simmons (BPI 910158 – holotype; Hulcr7168 = CMW44790 – ex-type culture).
Description: Colonies initially cream, aging from olivaceous to golden brown or blackish on MEA, surface tough and wrinkled; reverse subhyaline. Optimal colony diameter after 9 d at 25 °C in the dark was 47.5 (±1.9) mm; 20.6 (±1.8) mm at 10 °C; no growth at 35 °C. Conidiogenous cells hyaline, flask-shaped, tapering towards the apex, 12 (±1.7) × 3.6 (±0.3) μm. Conidia forming at apex of conidiogenous cells, occasionally sessile and lateral, hyaline. Conidia produced singly, aseptate, elliptical to elongate, occasionally truncate at base, 7.3 (±1.0) × 3.5 (±0.7) μm. Conidia produce budding cells. Sexual morph unknown.
Raffaelea xyleborina D.R. Simmons & C. Bateman, sp. nov.
MycoBank MB817174
(Fig. 6)
Fig. 6.
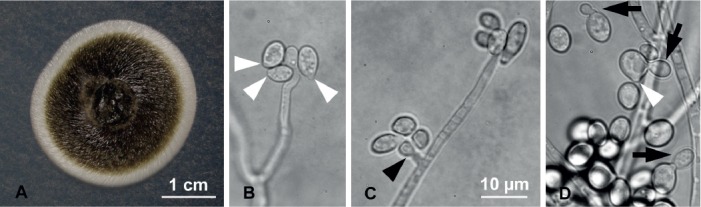
Raffaelea xyleborina (Hulcr6099) morphological features in pure culture on MEA. A. Colony growth after 9 d at 25 °C. B. Micronematous conidiogenous cells with ovoid conidia truncated at base (white arrowhead). C. Micronematous conidiophore with short conidiogenous cell sessile at side (black arrowhead) and at apex. D. Globose to ovoid conidia truncated at the base (white arrowhead) and budding yeast-like daughter cells (black arrows). Bar in C applies also to B–D.
Etymology: The epithet “xyleborina” refers to the genus of the host beetle (Xyleborinus andrewesii), the mycangium of which yielded this fungus.
Diagnosis: Conidiophores micronematous, hyaline; conidia at conidiogenous cell apex or lateral and sessile; conidia 6.5 (±0.7) × 4.9 (±0.8) μm, globose to ovoid, truncated at base, hyaline, yeast-like budding.
Type: USA: Florida: Highlands Co., Venus, cultured from Xyleborinus andrewesii collected from bait trap, 3 Jan. 2013, C. Bateman, C. Gibbard & L. L. Stelinski (BPI 910159 – holotype; Hulcr6099 = CMW45859 – ex-type culture;
Additional specimens examined: Loc. cit (Hulcr6100, Hulcr6406, Hulcr6408).
Description: Colonies initially cream, varying with age from cream to dark green to blackish on MEA, surface tough and spiral in appearance; reverse subhyaline. Optimal colony diameter after 9 d at 35 °C in the dark was 26.8 (±3.0) mm; 14.9 (±1.3) mm at 25 °C; no growth at 10 °C. Conidiogenous cells hyaline, micronematous, with conidia forming at apex, occasionally sessile and lateral. Conidia produced singly, aseptate, hyaline, globose to ovoid, sometimes elongate, and often truncated at base, 6.5 (±0.7) × 4.9 (±0.8) μm. Conidia produce budding cells. Sexual morph unknown.
DISCUSSION
Considering the damage that ambrosia fungi and their vectors cause (Ploetz et al. 2013), there is an urgent need to determine not only the diversity of these fungi globally but also to gain an enhanced knowledge of the host vector range for these potentially devastating species. Comparison of fungal isolates in this study with known species of Raffaelea revealed that two isolates from Taiwan, Hulcr7167 and Hulcr7176, grouped with R. quercivora. Raffaelea quercivora has been isolated from Platypus quercivorus in Japan, where it is responsible for ongoing epidemics of Japanese oak wilt (Kubono & Ito 2002), as well as in Taiwan (Kusumoto et al. 2014). Our isolates of R. quercivora were not isolated from the mycangia or fungal galleries of P. quercivorus, however, but rather from the mycangia of Cyclorhipidion ohnoi and the fungal galleries of Crossotarsus emancipatus from Taiwan. Though the latter two beetle species have not been implicated in oak wilt, these symbioses suggest that other vectors of R. quercivora exist. These isolates were collected from the same beetle host populations in Taiwan from which two of the species newly described in this study, R. cyclorhipidia and R. crossotarsa, were recovered. Therefore, these beetle-associated species display a degree of promiscuity with fungi within and exterior to Raffaelea s. str.
Raffaelea lauricola, the causative agent of laurel wilt, was found in Taiwan, from the documented host Xyleborus glabratus, and in Florida, from the previously unrecorded host Ambrosiodmus lecontei. Carrillo et al. (2014) reported that R. lauricola was laterally transferred to additional ambrosia beetle hosts, other than X. glabratus, when these species co-inhabit trees infected with this fungal pathogen. This finding demonstrates that the pathogen is a relatively promiscuous symbiont of ambrosia beetles, raising its importance from the biosecurity perspective. Despite Carrillo et al. (2014) having examined 41 adult A. lecontei females emerging from laurel wilt-affected swamp bay bolts, they failed to isolate R. lauricola from this host species. However, we recovered R. lauricola from A. lecontei infesting Persea borbonia near Lake Kissimmee (FL). The presence of Raffaelea with Ambrosiodmus may be phoretic or facultative, because Ambrosiodmus species examined to date carry a highly specific ambrosial basidiomycetous species (Li et al. 2015).
Besides information on known ambrosia fungi, results of this study suggest that under-explored regions of the world contain a large diversity of undescribed ambrosia fungi. Phylogenetic analyses of DNA sequence data for 22 isolates of Raffaelea-like fungi led to the discovery of the five new species described here. Some additional isolates resolved in lineages that would generally support their description as novel taxa (i.e. Hulcr5951; Hulcr7355; Hulcr7507 and PL1001), but these cultures could not be revived for morphological characterization after cryopreservation. Four of the new species described in this study were isolated from mycangia of ambrosia beetle hosts. Although sampling efforts that provided the foundation for this study included many different parts of the world, three of the novel taxa were from the eastern US. Whether this is a true reflection of an unexamined area of Raffaelea species diversity, or due to sampling bias, is unknown but deserves future consideration.
Results from this study indicate that Raffaelea s. str. and the R. lauricola complex are monophyletic (Fig. 1; Raffaelea s. lat.). This is consistent with previous analyses using rDNA and βT sequences (Dreaden et al. 2014). Analyses of 28S rDNA across Ophiostomatales have shown the same association with some support (Musvuugwa et al. 2015) or that these clades are disparate (de Beer & Wingfield 2013). Until a more accurate determination of their relationship is conducted with additional genetic loci, we conclude that these two clades are distinct.
Fungal symbioses with ambrosia beetles have become especially fertile topics for research, and further study will likely identify an increasingly large diversity of fungal associates. Indeed, Bateman et al. (2016) described a new genus in Ophiostomatales from Premnobius cavipennis (Scolytinae; Ipini), an independently evolved ambrosia beetle lineage largely confined to Africa. Furthermore, ambrosia beetles’ mycosymbionts are not limited to the ascomycetous Ophiostomatales. Li et al. (2015) found a new basidiomycetous Polyporales fungus, Flavodon ambrosius (Simmons et al. 2016), in symbiosis with Ambrosiodmus species, and Kasson et al. (2016) found the same mycosymbiont associated with another genus, Ambrosiophilus, which is sister to Ambrosiodmus (Hulcr & Cognato 2010). Thus, as investigations into these insects increase in number, additional fungal genera in unexpected lineages may be found in symbioses with ambrosia beetles.
Acknowledgments
We thank Stephen Taerum and Joshua Konkol for assistance in culturing fungi, Miranda Erasmus and Tuan Duong for taxonomic and phylogenetic assistance, Masato Torii and Naoto Kamata for document procurement, and Ching-Shan Lin and Roger Beaver for beetle identifications. This project was funded by the United States Department of Agriculture (USDA) Forest Service (FS)-SRS Coop agreement 14-CA-11330130-032, USDA-FS-FHP Coop agreement 12-CA-11420004-042, USDA Farm Bill agreement 12-8130-0377-CA, and National Science Foundation grant DEB 1256968. We also acknowledge the financial support of the Department of Science and Technology/ National Research Foundation Centre of Excellence in Tree Health Biotechnology (CTHB), South Africa.
REFERENCES
- Arx JA von, Hennebert GL. (1965) Deux champignons ambrosia. Mycopathologia et Mycologia Applicata 25: 309–315. [Google Scholar]
- Bateman CC, Huang Y-T, Simmons DR, Kasson MT, Stanley EL, Hulcr J. (2016) Ambrosia beetle Premnobius cavipennis (Scolytinae: Ipini) carries highly divergent ascomycotan ambrosia fungus Afroraffaelea ambrosiae gen. nov. sp. nov. (Ophiostomatales). Fungal Ecology: in press. [Google Scholar]
- Beaver RA. (1989) Insect-fungus relationship in the bark and ambrosia beetles. In: Insect-fungus Interactions (Wilding N, Collins NM, Hammond PM, Webber JF, eds): 121–143. San Diego: Elsevier. [Google Scholar]
- Campbell AS, Ploetz RC, Dreaden TJ, Kendra PE, Montgomery WS. (2016) Geographic variation in mycangial communities of Xyleborus glabratus. Mycologia 108: 657–667. [DOI] [PubMed] [Google Scholar]
- Carrillo D, Duncan RE, Ploetz JN, Campbell AF, Ploetz RC, Peña JE. (2014) Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathology 63: 54–62. [Google Scholar]
- de Beer ZW, Seifert KA, Wingfield MJ. (2013) A nomenclator for ophiostomatoid genera and species in the Ophiostomatales and Microascales. In: Ophiostomatoid Fungi: expanding frontiers (Seifert KA, de Beer ZW, Wingfield MJ, eds): 245-322. [CBS Biodiversity Series 12.] Utrecht: CBS-KNAW Fungal Biodiversity Centre. [Google Scholar]
- de Beer ZW, Wingfield MJ. (2013) Emerging lineages in the Ophiostomatales. In: Ophiostomatoid Fungi: expanding frontiers (Seifert KA, de Beer ZW, Wingfield MJ, eds): 21–46. [CBS Biodiversity Series 12.] Utrecht: CBS-KNAW Fungal Biodiversity Centre. [Google Scholar]
- Dreaden TJ, Davis JM, de Beer ZW, Ploetz RC, Soltis PS, et al. (2014) Phylogeny of ambrosia beetle symbionts in the genus Raffaelea. Fungal Biology 118: 970–978. [DOI] [PubMed] [Google Scholar]
- Gardes M, Bruns TD. (1993) ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Harrington TC, Aghayeva DN, Fraedrich SW. (2010) New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the Redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 111: 337–361. [Google Scholar]
- Harrington TC, Fraedrich SW, Aghayeva DN. (2008) Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon 104: 399–404. [Google Scholar]
- Hawksworth DL. (2011) A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. MycoKeys 1: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard HG. (1897) The ambrosia beetles of the United States. USDA, Division of Entomology, Bulletin, n.s. 7: 9–30. [Google Scholar]
- Hulcr J, Cognato AI. (2010) Repeated evolution of crop theft in fungus-farming ambrosia beetles. Evolution 64: 3205–3212. [DOI] [PubMed] [Google Scholar]
- Kasson MT, Wickert KL, Stauder CM, Macias AM, Berger MC, et al. (2016) Mutualism with aggressive wood-degrading Flavodon ambrosius (Polyporales) facilitates niche expansion and sub-sociality in Ambrosiophilus ambrosia beetles. Fungal Ecology 23: 86–96. [Google Scholar]
- Kubono T, Ito S. (2002) Raffaelea quercivora sp. nov. associated with mass mortality of Japanese oak, and the ambrosia beetle (Platypus quercivorus). Mycoscience 43: 255–260. [Google Scholar]
- Kusumoto D, Masuya H, Hirao T, Goto H, Hamaguchi K, et al. (2014) Discoloration induced by Raffaelea quercivora isolates in Quercus serrata logs and its relation to phylogeny: a comparison among isolates with and without the Japanese oak wilt incidence including outside of Japan. Journal of Forest Research 19: 404–410. [Google Scholar]
- Li Y, Simmons DR, Bateman CC, Short DPG, Kasson MT, Rabaglia RJ, Hulcr J. (2015) New fungus-insect symbiosis: culturing, molecular, and histological methods determine saprophytic Polyporales mutualists of Ambrosiodmus ambrosia beetles. PLoS ONE 10: e0137689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musvuugwa T, de Beer ZW, Duong TA, Dreyer LL, Oberlander KC, Roets F. (2015) New species of Ophiostomatales from Scolytinae and Platypodinae beetles in the Cape Floristic Region, including the discovery of the sexual state of Raffaelea. Antonie Van Leeuwenhoek 108: 933–950. [DOI] [PubMed] [Google Scholar]
- O’Donnell KL, Cigelnik E. (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- Ploetz RC, Hulcr J, Wingfield MJ, de Beer ZW. (2013) Destructive tree diseases associated with ambrosia and bark beetles: black swan events in tree pathology? Plant Disease 97: 856–872. [DOI] [PubMed] [Google Scholar]
- Posada D. (2008) jModeltest: phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ. (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634. [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Simmons DR, Li Y, Bateman CC, Hulcr J. (2016) Flavodon ambrosius sp. nov., a basidiomycetous mycosymbiont of Ambrosiodmus ambrosia beetles. Mycotaxon 131: 277–285. [Google Scholar]
- Sukumaran J, Holder MT. (2010) DendroPy: a python library for phylogenetic computing. Bioinformatics. 26: 1569–1571. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns TD, Lee SB, Taylor JW. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds): 315–322. San Diego: Academic Press. [Google Scholar]
- Yin M, Duong TA, Wingfield MJ, Zhou X, de Beer ZW. (2015) Taxonomy and phylogeny of the Leptographium procerum complex, including Leptographium sinense sp. nov. and Leptographium longiconidiophorum sp. nov. Antonie Van Leeuwenhoek 107: 547–563. [DOI] [PubMed] [Google Scholar]
- Zwickl DJ. (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD dissertation, University of Texas at Austin. [Google Scholar]


