Hepatitis C virus (HCV) direct-acting antivirals has renewed discussions as to whether these therapies could be used to prevent infection. We review transmission of HCV in the healthcare setting and provide an argument against postexposure prophylaxis based on a simple cost analysis.
Keywords: hepatitis C virus, occupational exposure, direct acting antivirals, postexposure prophylaxis, cost-analysis
Abstract
Currently, 380 000–400 000 occupational exposures to blood-borne pathogens occur annually in the United States. The management for occupational HIV or hepatitis B virus exposures includes postexposure prophylaxis (PEP) when necessary; however, PEP is not recommended for hepatitis C virus (HCV) exposures. Recent approval of HCV direct-acting antivirals (DAAs) has renewed discussions as to whether these therapies could be used to prevent infection after exposure. There are no published studies addressing this question, but the prescribing of DAAs for PEP has been reported. We will discuss the differences in transmission of the 3 most common blood-borne pathogens, the natural history of early HCV infection, and the scientific rationale for PEP. In particular, we will discuss how the low feasibility of conducting an adequately powered clinical trial of DAA use for PEP and the low cost-effectiveness of such an intervention is not supportive of targeting limited resources for such use.
(See the Editorial Commentary by Barocas and Linas on pages 100–1.)
Occupational exposure to blood-borne pathogens is a recognized risk for all healthcare workers (HCWs). A total of 380 000–400 000 occupational exposures occur annually in the United States [1, 2]. Three blood-borne pathogens account for the majority of cases: human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) [3]. Specific management for HIV or HBV exposures includes postexposure prophylaxis (PEP) and, in the case of HBV, vaccination [4–6]. Currently, PEP is not recommended for HCV exposures. We will discuss the differences in transmission, the natural history of early HCV infection, and the scientific rationale for and against PEP. In particular, we will discuss what role, if any, direct-acting antivirals (DAAs) for HCV should play in PEP. Due to the rapidly changing standard of care of HCV treatment, we will not focus on specific DAA therapies, but the principle of DAAs for HCV PEP.
OCCUPATIONAL TRANSMISSION OF HCV
The occupational transmission of HCV is well documented, although the variation in reported rates is wide (0%–10%) [7–18] (Table 1). The majority of reports support a low estimated transmission rate, and pooled longitudinal data following parenteral exposure to blood from HCV-infected source patients reported an estimated incidence of 1.9% per exposure [19]. This is compared to a 0.32% risk (approximately 1 infection for every 325 documented exposures) and 19%–37% risk (approximately 1 infection for every 3–5 documented exposures among HCWs without protective immunity from HBV vaccination) per percutaneous exposure to blood from HIV-infected and HBV-infected source patients, respectively [20–23].
Table 1.
Selected Studies of Hepatitis C Virus Infection Following Occupational Exposure
| Source, Year | Size of Study, Exposures, No. | Incident HCV, No. (%) | Comments |
|---|---|---|---|
| Hernandez et al, 1992 [7] | 81 | 0 (0) | Retrospective, needlestick injury, anti-HCV confirmation of source |
| Mitsui et al, 1992 [8] | 68 | 7 (10) | Retrospective, needlestick injury, only analyzed source exposures with detectable HCV RNA |
| Sodeyama et al, 1993 [9] | 92 | 3 (3.3) | Retrospective, needlestick injury, only analyzed source exposures with detectable HCV RNA |
| Lanphear et al, 1994 [10] | 50 | 3 (6) | Retrospective, needlestick injury, anti-HCV confirmation of source |
| Puro et al, 1995 [11] | 331 | 4 (1.2) | Prospective, needlestick injury, anti-HCV confirmation of source |
| Aria et al, 1996 [12] | 56 | 3 (5.4) | Prospective, needlestick injury |
| Takagi et al, 1998 [13] | 251 | 4 (1.6) | Retrospective, multiple injury types (87.7% needlestick or suture/surgical) |
| Hasan et al, 1999 [14] | 25 | 0 (0) | Prospective, needlestick injury, all source patients with detectable HCV RNA |
| Baldo et al, 2002 [15] | 68 | 0 (0) | Prospective, all injuries included, only analyzed source exposures with detectable HCV RNA |
| Chung et al, 2003 [16] | 405 | 1 (0.2) | Retrospective, needlestick injury, anti-HCV confirmation of source |
| De Carli et al, 2003 [17] | 1876 | 14 (0.74) | Prospective, needlestick, anti-HCV confirmation of source |
| Tomkins et al, 2012 [18] | 626 | 14 (2.2) | Retrospective, all injuries included, anti-HCV confirmation of source |
Abbreviations: HCV, hepatitis C virus; RNA, ribonucleic acid.
These data conform to the conceptual model that transmission risk is directly proportional to the infectivity of the body fluid and the susceptibility of the tissue exposed [24]. The infectivity of the body fluid is assumed to correlate with both the concentration of viral particles in the body fluid and the volume of inoculation. Supporting this model is the observation that transmission is high with hollow-bore needlesticks that can transfer a larger inoculum and greatest with deep penetration of a scalpel into muscle [18, 22].
While HCV RNA has been detected in other body fluids including saliva, semen, and vaginal secretions, HCV RNA levels are consistently higher in serum [25–27]. Existing data suggest that a higher level of HCV RNA in serum correlates to higher risk of transmission [22, 28–30]. Chimpanzee challenge studies have suggested that there is an infectious titer (chimpanzee infective dose) required to transmit infection, and that this level of inoculum is different in other animal models (humanized liver–mouse models) [31]. Whereas these studies have unequivocally established the infectivity of blood, it is possible that RNA detected in other body fluids might not correspond as directly with infectious virions.
ACUTE HCV INFECTION AND SPONTANEOUS CLEARANCE
Clinical Presentation and Diagnosis
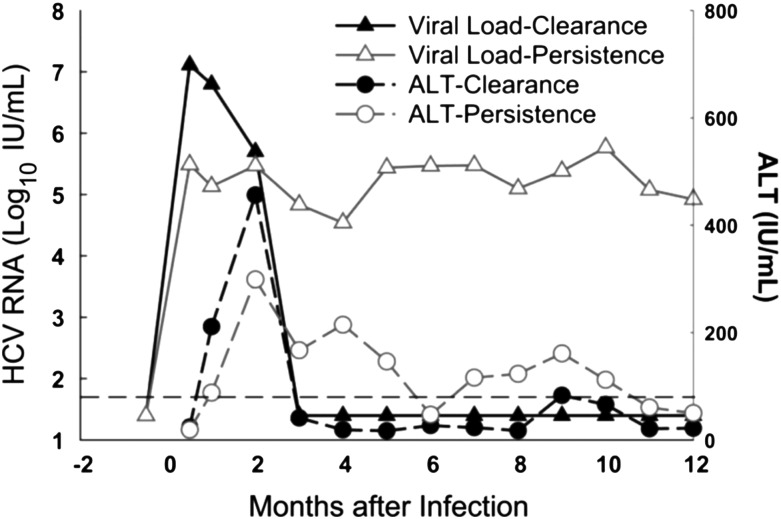
Following an occupational exposure, a minority (estimated 1.9%) of HCWs will develop acute HCV infection [32]. Initial infection with HCV is characterized by detection of HCV RNA in the blood (8–10 days following exposure) followed by a rapid increase in serum liver enzymes (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), which occurs during the plateau phase of infection (days 40–60) [33, 34] (Figure 1). A majority of acutely infected patients are asymptomatic, and for the 15%–30% of patients experiencing symptoms, the presentation can be mild and consistent with a nonspecific viral syndrome [35]. Approximately 25% of patients will go on to spontaneously clear the viral infection, defined as persistent undetectable levels of HCV RNA (below the lower limit of quantification, target not detected) in the blood, while the majority will develop viral persistence and chronic infection [36]. For the exposed HCW, the most reliable early marker of infection is the HCV RNA in the blood, which should be detectable by day 14 postexposure (Table 2).
Figure 1.
Laboratory presentation of acute hepatitis C infection. Hepatitis C virus (HCV) ribonucleic acid (RNA) (open and closed triangles) and alanine aminotransferase (ALT) (open and closed circles) over time with infection in months. Reprinted from “Spontaneous Clearance of Primary Acute Hepatitis C Virus Infection Correlated with High Initial Viral RNA Level and Rapid HVR1 Evolution” by L. Liu, 2012, Hepatology, 55(6):1684–91. Copyright 2012 by John Wiley & Sons Ltd. Reprinted with permission.
Table 2.
Testing for Hepatitis C Virus Infection Following Exposure
| Timing After Exposure | Laboratory Testing |
Comment | ||
|---|---|---|---|---|
| HCV EIA | HCV RNA | ALT | ||
| Source patient Immediate | Yes | If HCV EIA positive: Yes If HCV EIA negative: Recommend only if source is at risk for false-negative test |
No | Although HCV RNA testing is not routinely recommended, it may be useful in immunocompromised source patients who may have false-negative serology. |
| Healthcare worker (if source patient has evidence of HCV infection) | ||||
| Immediate | Yes | If HCV EIA positive: Yes | Yes | Healthcare worker does not require follow-up if source patient is HCV negative; however, baseline testing of HCW is prudent. |
| 4–6 wk | Yes | Yes | Consider | If earlier diagnosis of HCV infection is desired, testing for HCV RNA may be performed to help guide treatment decision making. Due to the intermittent nature of HCV viremia in acute HCV infection, RNA testing should not be the sole screening test. |
| 4–6 mo | Yes | Yes | Yes | HCV antibody testing 4–6 mo postexposure is considered the optimal means of detecting infection, although seronegative infections have been reported. |
Abbreviations: ALT, alanine aminotransferase; EIA, enzyme immunoassay; HCV, hepatitis C virus; HCW, healthcare worker; RNA, ribonucleic acid.
Multiple factors have been reported as predictive of spontaneous clearance including female sex, lack of HIV infection, positive hepatitis B surface antigen status, host genetic factors including the IL28B genotype, and early favorable HCV RNA kinetics [37–42]. There are limited long-term natural history follow-up studies of acute HCV infection, which report variability in the timing of natural clearance of the virus [41, 43–45]. While it is accepted that the majority of patients will spontaneously clear the infection in the first 24 weeks, there can be significant variability in HCV RNA in the early stages of infection with interposed detectable and undetectable levels [41, 45, 46]. Thus, confirmation of HCV RNA clearance is recommended, a minimum of 6 months apart.
Pathogenesis
The pathogenesis of acute infection is poorly understood because of the absence of small animal models and due to the asymptomatic nature of the infection. To this end, much of our knowledge of the initial phase of infection is derived from the chimpanzee model, which is no longer used. We do not know what occurs at the site of inoculation or in the first 72 hours of exposure; most studies of early infection have investigated the innate immune response in the host and the early viral kinetics. The timing of hepatocyte entry and extent of entry are unknown. The early innate response is attenuated by countermeasures from HCV including expression of NS3/4A that appears to diminish downstream signaling [47].
One of the hallmarks of acute HCV infection is the delayed adaptive immune response, which is not detectable until weeks 5–9 after infection [48]. Defective T- and B-cell priming has been proposed as the mechanism for this delay, although how or why this occurs is poorly understood. What we do know is that clearance of HCV is strongly associated with CD4+ T-cell responses, and reduced breadth and strength of the specific CD4+ T-cell response results in persistence of HCV infection [48–50]. In fact, a recent study in HCWs reports that subclinical transmission, determined by proliferative T-cell responses targeting nonstructural HCV proteins, is common despite undetectable systemic viremia and lack of serologic evidence of infection [51]. Neutralizing antibodies generally are produced too late to play a critical role in viral clearance [52].
SCIENTIFIC RATIONALE FOR POSTEXPOSURE CHEMOPROPHYLAXIS
The rationale for PEP chemoprophylaxis is based on several core principles: (1) the pathogenesis and time course of early infection; (2) the biological plausibility that infection could be prevented with antiviral drugs; (3) evidence of antiviral efficacy of the drugs being used for PEP; and (4) the risk to the HCWs from exposure to PEP [32]. The impact of the failure to prevent the development of a chronic infection also drives the clinical need for exploring PEP for infectious pathogens. For example, in the case of HBV and HIV, there is no cure for chronic infection and the long-term impact of infection may be substantial; on the other hand, chronic HCV infection is curable in the vast majority of patients. As such, the impact on the HCW of the failure to prevent chronic infection is less for HCV compared with HIV or HBV infection.
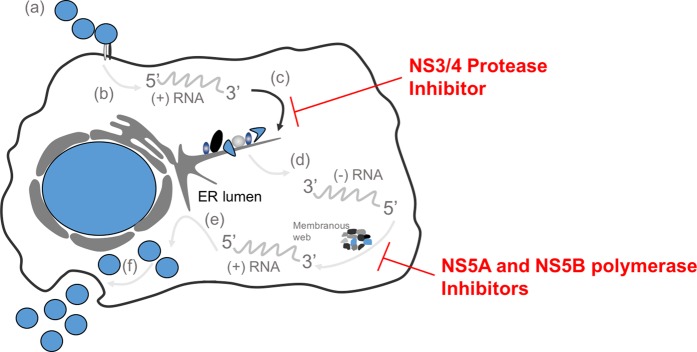
Our ability to rationalize the role of PEP in the first few days of infection is limited by the lack of understanding of the pathogenesis of early HCV infection. To use HIV as a correlate, primate models of simian immunodeficiency virus (SIV) infection suggest that systemic infection does not occur until postexposure day 3–5; thus, it is theoretically possible to prevent or inhibit systemic infection by blocking viral replication in the initial target cells or lymph nodes [53]. This was followed by primate studies confirming that a 4-week regimen with tenofovir administered 48 hours before, 4 hours after, or 24 hours after intravenous inoculation of SIV prevented infection [54–56]. We lack such a detailed understanding of the kinetics of acute HCV infection. Viruses would be expected to pass through the liver within hours of reaching the blood. There they attach to and enter susceptible hepatocytes through a series of at least 5 distinct molecular encounters [55]. Within the cell the positive strand is released and associated with a ribosome, and a single large polyprotein is made and initially cleaved using host enzymes (Figure 2). The virus-encoded proteins then complete replication including production of a negative strand that is repeatedly copied as new virions are produced in the cytoplasm. There is no known nuclear phase nor any permanent archive of HCV infection which has to be sustained by ongoing replication.
Figure 2.
Life Cycle of Hepatitis C Viral Infection and Targets for Mechanism of Action for Direct Acting Antivirals. (a) virus particle-receptor binding and endocytosis; (b) cytoplasmic release and uncoating; (c) translation and polyprotein processing with structural and non-structural proteins shown at the endoplasmic reticulum (ER) - this is the site for the mechanism of action of NS3/4 protease inhibitors; (d) ribonucleic acid (RNA) replication occurring in the membranous web - this is the site for the mechanism of action of the NS5A inhibitors and NS5B polymerase inhibitors; (e) virion packaging and assembly; and (f) virion maturation and release.
Biologic Plausibility of Prevention
Based on what we know about the early phase of HCV infection, what mechanism would be most crucial to prevent infection? Presumably, prevention of infection would require blocking of early de novo infection of susceptible cells or spread of the infection to the critical number of hepatocytes required to achieve persistence. However, currently approved DAAs target postentry processes and would not be predicted to prevent initial hepatocyte entry. Necessary steps of protease cleavage, replication complex assembly, and reproduction of the positive strand would be inhibited by approved medications (Figure 2). Thus, the key factors may be how many cells harbor the positive strand genome and the relative stability of the RNAs. Since a small number of “founder viruses” initially establish infection [57] and viremia isn't detectable for more than a week, it is also possible PEP might prevent the early amplification and spread of infection. However, there is no in vivo information to answer how long the downstream processes would need to be inhibited before those RNAs lost the ability to initiate infection. To date, there are no proof-of-principle studies investigating the efficacy of PEP using direct-acting antivirals (DAAs), although there was a registered study assessing the safety and tolerability of telaprevir (NS3/4A protease inhibitor) dosed 750 mg 3 times daily for 4 weeks for occupational PEP for HCV (Clinical Trials.gov identifier NCT01766115). That study has since been withdrawn.
Antiviral Efficacy of DAAs
US Food and Drug Administration (FDA)–approved DAAs target the NS3/4A protease, the NS5B polymerase, and the NS5A protein (Figure 2). The most recently approved DAAs exhibit picomolar antiviral potency in vitro, and when used in combination have shown high efficacy for the treatment of chronic HCV infection [58–66] (Supplementary Table 1). When used as monotherapy in persons with established high-level infection, failure rates are high, and for DAA with low barriers to resistance (NS3/4A protease inhibitors and NS5A inhibitors), the rapid selection of resistance mutations is universal at the time of on-treatment failure [67]. Similar to HIV, the expected approach to PEP in HCV involves combination therapy of multiple mechanistic targets, which is the same as the approach to the treatment of chronic HCV infection. Also like HIV, the longer the delay to delivering medications, the more similar PEP is to treatment of chronic infection (vs preexposure prophylaxis).
Risk and Benefit of HCW Exposure to PEP
The final consideration influencing the rationalization for PEP is the risk and benefit of PEP to the exposed HCW, and to extend this out to the population level, the cost of PEP. It is unclear what length of treatment would be required for HCV PEP; the use of 4 weeks of PEP for systemic HIV infection was based on animal model data suggesting that 4 weeks was superior to 3 or 10 days [53]. We do not have such data in HCV, although the ability to cure select patients with chronic infection in as little as 6 weeks with potent all-oral DAA combinations suggests that such a shortened course for prevention may be reasonable for early viral eradication [68]. While all antivirals have been associated with adverse effects, interferon-free regimens for HCV are much better tolerated and side effects are unlikely to be a significant limitation to the implementation of HCV PEP. Thus, while there is minimal perceived risk of HCV PEP to the individual, there is also not a clear benefit as early HCV infection can be eradicated with FDA-approved, highly effective DAA regimens. Furthermore, the implementation of HCV PEP carries significant financial implications.
There is no available cost-effectiveness analysis for HCV PEP, although given the high cost of DAA (on average 54 600–94 500 US dollars [USD] per 12-week course) and the large number of patients needed to treat to abort 1 early infection, it is it unlikely that an intervention that prevents such a rare event would provide adequate value for money to be considered cost-effective by commonly cited US willingness-to-pay thresholds. This is all the truer in the setting of highly efficacious combination DAA therapies for established infection. In the setting of chronic HCV, infection cure rates exceed 95%, an outcome that clearly differentiates HCV from the other occupational blood-borne pathogens. Although HIV PEP has been reported to be cost-effective in the occupational exposure setting, these models correctly assume that the failure to prevent incurable chronic HIV infection will necessitate lifelong antiretroviral therapy [69].
The low incidence rate of HCV transmission in the setting of an occupational exposure also creates limitations in feasibility of conducting a clinical trial to determine efficacy and safety, which would be necessary before HCV PEP could be recommended and implemented in the healthcare setting. For sample size calculations in clinical trials, there is a standard assumption of a desired power (usually 80%–90%) to detect a significant difference at a prespecified level of significance, usually 5% [70]. Due to the low incidence rate of HCV transmission (estimated 1.9%) in the setting of an occupational exposure, the sample size of a clinical trial to assess the efficacy would have to be large enough to detect a relatively small difference between groups, even if it is highly efficacious.
For clinical trials with extremely low incidence rates, the common assumptions used for sample size calculations are not feasible. For example, assuming an incidence rate of 1.9% for the control arm and the ability to show an incidence of approximately 1% in the intervention arm, the fixed sample size analysis (power 90%, significance level 5%) suggests a sample size of up to 6532 (3266 per group) subjects [70]. Assuming 18 200 USD per 4 weeks of DAA PEP for the intervention arm, the cost of drug alone would be 59.4 million USD—a cost unlikely to be offset by the early prevention of a maximum of 62 cases of acute HCV. On the other hand, the cost for the delivery of highly effective, all-oral DAA regimens to persons who are acutely infected is anticipated to be approximately 63 000 USD for an 8-week course (ledipasvir/sofosbuvir) or 54 600 USD for a 12-week course (elbasvir/grazoprevir) or approximately 3.39 million USD to treat the maximum of 62 persons with acute HCV infection following exposure. In fact, recent studies of acute infection suggest that high rates of eradication (83%–100%) with abbreviated treatment length, including 6 weeks, may be possible depending on how early the patient is in the acute course of infection [71, 72]. Importantly, both strategies—PEP and early treatment of HCV infection—are expected to result in the absence of chronic infection in the vast majority of exposed persons.
Chow et al have proposed a different method for sample size calculations in the setting of extremely low incidence rates of outcome of interest based on precision analysis [70]. Using the same theoretical clinical trial as described above, assuming an incidence rate of 1.9% for the control arm and the ability to show a 50% relative reduction to an incidence rate of 1% in the intervention arm, the precision sample size analysis suggests that a sample size of 1100 subjects per group (N = 2200) would be needed to reach statistical significance. The power for correctly detecting a difference of 1.0% would be 53.37%. While this decreases the drug-related costs to 20 million USD, the cost is still >5-fold higher than treating the few patients who develop active infection.
COST ANALYSIS
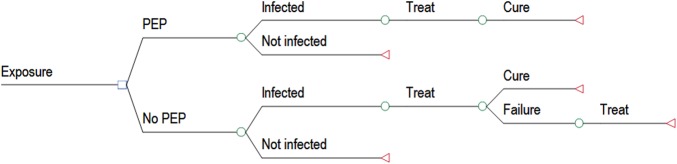
To explore the costs associated with PEP in the healthcare setting, we performed a simple decision analysis to examine the relative costs of PEP after a needlestick exposure to HCV-positive bodily fluids. Two strategies were compared (Figure 3): (1) PEP with DAA daily for 4 weeks, vs (2) No PEP; treat only patients who develop active infection HCV.
Figure 3.
Diagram of Decision Analysis of Post-exposure Prophylaxis (PEP) for Hepatitis C virus infection as compared to no PEP. Open squares and circles show decision points in the analysis and open triangles show end points in the analysis.
We assumed a baseline rate of postexposure HCV infection of 1.9% and further assumed that PEP was 100% effective at preventing infection. We assumed that everyone who developed active infection was treated and that treatment was 98% effective, with no deaths from therapy and no chronic infections (treatment failure). The base-case assumed therapy for PEP consisted of a combination DAA therapy with elbasvir/grazoprevir given for 4 weeks, which is currently the least costly available therapy. For patients who became infected, we assumed treatment for acute infection with ledipasvir/sofosbuvir for 8 weeks. Patients for whom acute therapy failed were given NS5A-sparing therapy of simeprevir plus sofosbuvir plus ribavirin for 24 weeks. While on any therapy, we assumed that patients were seen by a physician with HCV viral RNA testing at baseline, week 4, end of therapy (EOT), and EOT plus 12 weeks, and comprehensive metabolic panel performed at baseline, week 4, and EOT. Cost estimates for these interventions are shown in Table 3.
Table 3.
Cost Estimates for Decision Analysis
| Item | Cost | Source |
|---|---|---|
| Elbasvir/grazoprevir (1 wk) | $4550 | AWP [73] |
| Ledipasvir/sofosbuvir (1 wk) | $7875 | AWP [73] |
| Ribavirin (1 wk) | $71a | AWP [73] |
| Simeprevir (1 wk) | $5530 | WAC [74] |
| Sofosbuvir (1 wk) | $7000 | WAC [74] |
| HCV RNA | $79 | CMS [75] |
| Complete metabolic panel | $19 | CMS [75] |
| Office visit | $79 | CMS [76] |
| Cost of PEPb | $18 200 | |
| Cost of acute treatmentc | $63 000 |
Abbreviations: AWP, average wholesale price; CMS, Center for Medicare and Medicaid Services; HCV, hepatitis C virus; PEP, postexposure prophylaxis; RNA, ribonucleic acid; WAC, wholesale acquisition cost.
a Based on generic cost.
b Cost of postexposure prophylaxis with elbasvir/grazoprevir for 4 weeks.
c Cost of treatment of acute infection with ledipasvir/sofosbuvir for 8 weeks.
The results of our model showed that treating 100 exposed patients with PEP would cost 1 857 272 USD vs 132 870 USD in the no PEP strategy. In sensitivity analysis, we considered a range of costs for treatment of acute HCV, but even at the highest end of the range (94 500 USD for 12 weeks of therapy), the PEP strategy was still more expensive by a factor of 9. Likewise, we considered a range of probabilities for infection after exposure, but even at a rate of 10%, the PEP strategy was still significantly more expensive. To achieve cost savings for PEP, the cost of medications would need to drop to 1329 USD per week. However, this assumes that the cost of treatment for acute therapy does not change; in a 2-way sensitivity analysis where the costs of therapy decrease for both PEP and acute treatment, PEP remains the more expensive option. We examined the cost of a shorter PEP regimen and found that any regimen longer than 2 days would still be more expensive than the no-PEP option.
We would also acknowledge the less tangible issues surrounding an occupational exposure that carries the risk of a blood-borne pathogen infection. There is a clear psychological impact on not only the HCW but also their family and in particular their sexual partners. Furthermore, the development of an acute blood-borne infection can carry particular significance for individuals who engage in work that potentially places others at risk for acute infection (eg, surgeons). We did not account for worry and anxiety in the model, in part because it is difficult to project the differences in these emotions between the PEP and no PEP groups and it was beyond the scope of this article; however, it is unlikely to change the outcome of the model. Here we focused on the HCW because we have the most reliable data on the risk per exposure. However, PEP and pre-exposure prevention of HCV infection are even more important for groups with a higher risk of transmission, including people with intravenous drug use and some HIV-infected men who have sex with men. Additional work is needed to address the role of DAA in HCV prevention in these groups.
CONCLUSIONS
Occupational transmission of HCV is uncommon, yet of the 3 most prevalent healthcare-related blood-borne pathogens, it remains the only infection without available PEP and/or preexposure vaccine. There are many arguments for why PEP in HCV should not be recommended: (1) Risk of transmission in HCW is very low; (2) for the rare HCWs who develop acute infection, the eradication rate with highly efficacious and safe DAA combination therapies is near 100%; and (3) there is unlikely to be a scenario by which PEP is cost-effective compared with early HCV treatment, with the exception of a 2-day course of PEP. Based on acute HCV infection models using intravenously infected chimpanzees, there is little plausibility that 2 days of DAA therapy would block the first phase of viral replication. Thus, any studies of or recommendations for PEP would have to acknowledge that this intervention is not cost-effective. In addition, the clinical application of these results would need to consider differences in efficacy across genotypes and use a pan-genotypic regimen when feasible. The lack of understanding of the appropriate length of therapy for PEP and the lack of feasibility of conducting an adequately powered clinical trial to assess efficacy further solidify this argument. Instead, appropriate follow-up and postexposure testing, reassurance, and early treatment of acquired HCV infection with potent DAA combination therapies should be recommended.
Supplementary Data
Supplementary materials are available at http://academic.oup.com/cid. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (K23-AI096913-03 to S. N. and K01-AI083782 to D. P. H.) and the National Institute on Drug Abuse (K24 DA034621-01 to M. S. S. and R37DA013806 to D. L. T.).
Potential conflicts of interest. S. N. has received research funding from Vertex Pharmaceuticals, Merck, Gilead Sciences, Janssen Pharmaceuticals, AbbVie, Bristol-Meyers Squibb (BMS), and Tacere, and has served as a consultant and scientific advisor for Vertex Pharmaceuticals, Merck, Gilead Sciences, Janssen Pharmaceuticals, AbbVie, and BMS. M. S. S. has received research funding (paid to Johns Hopkins University) from AbbVie, BMS, Gilead Sciences, Janssen Pharmaceuticals, and Merck, and has served as a consultant and scientific advisor to AbbVie, Cocrystal, Gilead Sciences, Janssen Pharmaceuticals, Merck, and Trek. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Porta C, Handelman E, McGovern P. Needlestick injuries among health care workers: a literature review. AAOHN J 1999; 47:237–44. [PubMed] [Google Scholar]
- 2.Panlilio AL, Orelien JG, Srivastava PU, Jagger J, Cohn RD, Cardo DM; NaSH Surveillance Group; EPINet Data Sharing Network. Estimate of the annual number of percutaneous injuries among hospital-based healthcare workers in the United States, 1997–1998. Infect Control Hosp Epidemiol 2004; 25:556–62. [DOI] [PubMed] [Google Scholar]
- 3.Deuffic-Burban S, Delarocque-Astagneau E, Abiteboul D, Bouvet E, Yazdanpanah Y. Blood-borne viruses in health care workers: prevention and management. J Clin Virol 2011; 52:4–10. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. Recommendations for prevention of HIV transmission in health-care settings. MMWR Morb Mortal Wkly Rep 1987; 36(suppl 2):1S–18S. [PubMed] [Google Scholar]
- 5.Garner JS; Hospital Infection Control Practices Advisory Committee. Guideline for isolation precautions in hospitals. Infect Control Hosp Epidemiol 1996; 17:53–80. [DOI] [PubMed] [Google Scholar]
- 6.Beekmann SE, Henderson DK. Health care workers and hepatitis: risk for infection and management of exposures. Infect Dis Clin Pract 1992; 1:424–8. [Google Scholar]
- 7.Hernandez ME, Bruguera M, Puyuelo T et al. Risk of needle-stick injuries in the transmission of hepatitis C virus in hospital personnel. J Hepatol 1992; 16:56–8. [DOI] [PubMed] [Google Scholar]
- 8.Mitsui T, Iwano K, Masuko K et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology 1992; 16:1109–14. [PubMed] [Google Scholar]
- 9.Sodeyama T, Kiyosawa K, Urushihara A et al. Detection of hepatitis C virus markers and hepatitis C virus genomic-RNA after needlestick accidents. Arch Intern Med 1993; 153:1565–72. [PubMed] [Google Scholar]
- 10.Lanphear BP, Linnemann CC, Gannon CG et al. Hepatitis C virus infection in healthcare workers: risk of exposure and infection. Infect Control Hosp Epidemiol 1994; 15:745–50. [DOI] [PubMed] [Google Scholar]
- 11.Puro V, Petrosillo N, Ippolito G; Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Risk of hepatitis C seroconversion after occupational exposures in health care workers. Am J Infect Control 1995; 23:273–7. [DOI] [PubMed] [Google Scholar]
- 12.Arai Y, Noda K, Enomoto N et al. A prospective study of hepatitis C virus infection after needlestick accidents. Liver 1996; 16:331–4. [DOI] [PubMed] [Google Scholar]
- 13.Takagi H, Uehara M, Kakizaki S et al. Accidental transmission of HCV and treatment with interferon. J Gastroenterol Hepatol 1998; 13:238–43. [DOI] [PubMed] [Google Scholar]
- 14.Hasan F, Askar H, Al Khaliki J et al. Lack of transmission of hepatitis C virus following needlestick accidents. Hepatogastroenterology 1999; 46:1678–81. [PubMed] [Google Scholar]
- 15.Baldo V, Floreani A, Dal Vecchio L et al. Occupational risk of blood-borne viruses in healthcare workers: a 5-year surveillance program. Infect Control Hosp Epidemiol 2002; 23:325–7. [DOI] [PubMed] [Google Scholar]
- 16.Chung H, Kudo M, Kumada T et al. Risk of HCV transmission after needlestick injury, and the efficacy of short-duration interferon administration to prevent HCV transmission to medical personnel. J Gastroenterol 2003; 38:877–9. [DOI] [PubMed] [Google Scholar]
- 17.De Carli G, Puro V, Ippolito G; Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Risk of hepatitis C virus transmission following percutaneous exposure in health care workers. Infection 2003; 31:22–7. [PubMed] [Google Scholar]
- 18.Tomkins SE, Elford J, Nichols T et al. Occupational transmission of hepatitis C in healthcare workers and factors associated with seroconversion: UK surveillance data. J Viral Hepat 2012; 19:199–204. [DOI] [PubMed] [Google Scholar]
- 19.Henderson DK. Managing occupational risks for hepatitis C transmission in the health care setting. Clin Microbiol Rev 2003; 16:546–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson DK. HIV in the healthcare setting. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases. 7th ed New York, NY: Elsevier Churchill Livingstone, 2009; 3753–70. [Google Scholar]
- 21.Seeff LB, Wright EC, Zimmerman HJ et al. Type B hepatitis after needle-stick exposure: prevention with hepatitis B immune globulin: final report of the Veterans Administration Cooperative Study. Ann Intern Med 1978; 88:285–93. [DOI] [PubMed] [Google Scholar]
- 22.Yazdanpanah Y, De Carli G, Migueres B et al. Risk factors for hepatitis C virus transmission to health care workers after occupational exposure: a European case-control study. Clin Infect Dis 2005; 41:1423–30. [DOI] [PubMed] [Google Scholar]
- 23.Ippolito G, Puro V, Petrosillo N, De Carli G, Micheloni G, Magliano E. Simultaneous infection with HIV and hepatitis C virus following occupational conjunctival blood exposure. JAMA 1998; 280:28. [DOI] [PubMed] [Google Scholar]
- 24.Sulkowski M, Ray SC, Thomas DL. Needlestick transmission of hepatitis C. JAMA 2002; 287:2406–13. [DOI] [PubMed] [Google Scholar]
- 25.Rey D, Fritsch S, Schmitt C, Meyer P, Lang JM, Stoll-Keller F. Quantitation of hepatitis C virus RNA in saliva and serum of patients coinfected with HCV and human immunodeficiency virus. J Med Virol 2001; 63:117–9. [PubMed] [Google Scholar]
- 26.Farias A, Re V, Mengarelli S et al. Detection of hepatitis C virus (HCV) in body fluids from HCV monoinfected and HCV/HIV coinfected patients. Hepatogastroenterology 2010; 57:300–4. [PubMed] [Google Scholar]
- 27.Savasi V, Parrilla B, Ratti M, Oneta M, Clerici M, Ferrazzi E. Hepatitis C virus RNA detection in different semen fractions of HCV/HIV-1 co-infected men by nested PCR. Eur J Obstet Gynecol Reprod Biol 2010; 151:52–5. [DOI] [PubMed] [Google Scholar]
- 28.Ohto H, Terazawa S, Nobuhiko S et al. Transmission of hepatitis C virus from mothers to infants. N Engl J Med 1994; 330:744–50. [DOI] [PubMed] [Google Scholar]
- 29.Lin HH, Kao JH, Hsu HY et al. Possible role of high-titer maternal viremia in perinatal transmission of hepatitis C virus. J Infect Dis 1994; 169:638–41. [DOI] [PubMed] [Google Scholar]
- 30.Thomas DL, Villano SA, Riester KA et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1–infected mothers. J Infect Dis 1998; 177:1480–8. [DOI] [PubMed] [Google Scholar]
- 31.Bukh J, Meuleman P, Tellier R et al. Challenge pools of hepatitis C virus genotypes 1-6 prototype strains: replication fitness and pathogenicity in chimpanzees and human liver-chimeric mouse models. J Infect Dis 2010; 201:1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Morb Mortal Wkly Rep 2001; 50(RR-11):1–52. [PubMed] [Google Scholar]
- 33.Cox AL, Netski DM, Mosbruger T et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis 2005; 40:951–8. [DOI] [PubMed] [Google Scholar]
- 34.Page-Shafer K, Pappalardo BL, Tobler LH et al. Testing strategy to identify cases of acute hepatitis C virus (HCV) infection and to project HCV incidence rates. J Clin Microbiol 2008; 46:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orland JR, Wright TL, Cooper S. Acute hepatitis C. Hepatology 2001; 33:321–7. [DOI] [PubMed] [Google Scholar]
- 36.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 2006; 13:34–41. [DOI] [PubMed] [Google Scholar]
- 37.Thomas DL, Astemborski J, Rai RM et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000; 284:450–6. [DOI] [PubMed] [Google Scholar]
- 38.Soriano V, Mocroft A, Rockstroh J et al. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J Infect Dis 2008; 198:1337–44. [DOI] [PubMed] [Google Scholar]
- 39.Thomas DL, Thio CL, Martin MP et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009; 8:798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerlach JT, Diepolder HM, Zachoval R et al. Acute hepatitis C: high rate of both spontaneous and treatment induced viral clearance. Gastroenterology 2003; 125:80–8. [DOI] [PubMed] [Google Scholar]
- 41.Mosley JW, Operskalski EA, Tobler LH et al. The course of hepatitis C viremia in transfusion recipients prior to availability of antiviral therapy. J Viral Hepat 2008; 15:120–8. [DOI] [PubMed] [Google Scholar]
- 42.Vogel M, Dominguez S, Bhagani S et al. Treatment of acute HCV infection in HIV-positive patients: experience from a multicenter European cohort. Antivir Ther 2010; 15:267–79. [DOI] [PubMed] [Google Scholar]
- 43.Larghi A, Zuin M, Crosignani A et al. Outcome of an outbreak of acute hepatitis C among healthy volunteers participating in pharmacokinetics studies. Hepatology 2002; 36:993–1000. [DOI] [PubMed] [Google Scholar]
- 44.Spada E, Mele A, Berton A et al. Multispecific T cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut 2004; 53:1673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology 1999; 29:908–14. [DOI] [PubMed] [Google Scholar]
- 46.Grebely J, Page K, Sacks-Davis R et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014; 59:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest 2009; 119:1745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thimme R, Bukh J, Spangenberg HC et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A 2002; 99:15661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diepolder HM, Zachoval R, Hoffmann RM et al. Possible mechanism involving T lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 1995; 346:1006–7. [DOI] [PubMed] [Google Scholar]
- 50.Missale G, Bertoni R, Lamonaca V et al. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest 1996; 98:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heller T, Werner JM, Rahman F et al. Occupational exposure to hepatitis C virus: early T-cell responses in the absence of seroconversion in a longitudinal cohort study. J Infect Dis 2013; 208:1020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wedemeyer H, He XS, Nascimbeni M et al. Impaired effector function of hepatitis C virus–specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol 2002; 169:3447–58. [DOI] [PubMed] [Google Scholar]
- 53.Spira AI, Marx PA, Patterson BK et al. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med 1996; 183:215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai C-C, Follis KE, Sabo A et al. Prevention of SIV infection in macaques by (R)-9-(2phosphonylmethoxypropyl) adenine. Science 1995; 270:1197–9. [DOI] [PubMed] [Google Scholar]
- 55.Scheel TKH, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med 2013; 19:837–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai C-C, Emau P, Follis KE et al. Effectiveness of postinoculation (R)-9-(2phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol 1998; 72:4265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Stoddard MB, Wang S et al. Elucidation of hepatitis c virus transmission and early diversification by single genome sequencing. PLoS Pathogen 2012; 8:e1002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poordad F, McCone J, Bacon BR et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacobson IM, McHutchison JG, Dusheiko G et al. Telaprevir for previously untreated chronic hepatitis C infection. N Engl J Med 2011; 364:2405–16. [DOI] [PubMed] [Google Scholar]
- 60.Jacobson I, Dore G, Foster GR et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naïve patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomized, double-blind, placebo-controlled trial. Lancet 2014; 384:403–13. [DOI] [PubMed] [Google Scholar]
- 61.Lawitz E, Mangia A, Wyles D et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–87. [DOI] [PubMed] [Google Scholar]
- 62.Wyles DL, Ruane PJ, Sulkowski MS et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373:714–25. [DOI] [PubMed] [Google Scholar]
- 63.Afdhal N, Zeuzem S, Kwo P et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1483–93. [DOI] [PubMed] [Google Scholar]
- 64.Feld JJ, Kowdley KV, Coakley E et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370:1594–603. [DOI] [PubMed] [Google Scholar]
- 65.Zeuzem S, Ghalib R, Reddy KR et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med 2015; 163:1–13. [DOI] [PubMed] [Google Scholar]
- 66.Feld JJ, Jacobson IM, Hezode C et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015; 373:2599–607. [DOI] [PubMed] [Google Scholar]
- 67.Jiang M, Mani N, Lin C et al. In vitro phenotypic characterization of hepatitis C virus NS3 protease variants observed in clinical studies of telaprevir. Antimicrob Agents Chemother 2013; 57:6236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kohli A, Kattakuzhy S, Sidharthan S et al. Four-week direct-acting antiviral regimens in noncirrhotic patients with hepatitis C virus genotype 1 infection: an open-label, nonrandomized trial. Ann Intern Med 2015; 163:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pinkerton SD, Holtgrave DR, Pinkerton HJ. Cost-effectiveness of chemoprophylaxis after occupational exposure to HIV. Arch Intern Med 1997; 157:1972–80. [PubMed] [Google Scholar]
- 70.Chow SC, Chiu ST. Sample size and data monitoring for clinical trials with extremely low incidence rates. Ther Innov Regul Sci 2013; 47:438–46. [DOI] [PubMed] [Google Scholar]
- 71.Rockstroh J, Bhagani S, Hyland RH et al. Ledipasvir/sofosbuvir for 6 weeks in HIV-infected patients with acute HCV infection. In: Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, 22–25 February 2016 Abstract 154LB. [Google Scholar]
- 72.Deterding K, Spinner C, Schott E et al. Six weeks of sofosbuvir/ledipasvir (SOF/LDV) are sufficient to treat acute hepatitis C virus genotype 1 monoinfection: The HepNet Acute HCV IV Study. In: European Association for the Study of Liver Disease, Barcelona, Spain, 14–17 April 2016. [Google Scholar]
- 73.Red Book. Ann Arbor, MI: Truven Health Analytics, 2014. [Google Scholar]
- 74.Red Book. Ann Arbor, MI: Truven Health Analytics, 2010. [Google Scholar]
- 75.Center for Medicare and Medicaid Services. Clinical laboratory fee schedule. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html Accessed 17 December 2013.
- 76.Center for Medicare and Medicaid Services. Physician fee schedule. Available at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSlookup/index.html Accessed 17 December 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.