Abstract
Soybean (Glycine max) accumulates several prenylated isoflavonoid phytoalexins, collectively referred to as glyceollins. Glyceollins (I, II, III, IV and V) possess modified pterocarpan skeletons with C5 moieties from dimethylallyl diphosphate, and they are commonly produced from (6aS, 11aS)-3,9,6a-trihydroxypterocarpan [(−)-glycinol]. The metabolic fate of (−)-glycinol is determined by the enzymatic introduction of a dimethylallyl group into C-4 or C-2, which is reportedly catalyzed by regiospecific prenyltransferases (PTs). 4-Dimethylallyl (−)-glycinol and 2-dimethylallyl (−)-glycinol are precursors of glyceollin I and other glyceollins, respectively. Although multiple genes encoding (−)-glycinol biosynthetic enzymes have been identified, those involved in the later steps of glyceollin formation mostly remain unidentified, except for (−)-glycinol 4-dimethylallyltransferase (G4DT), which is involved in glyceollin I biosynthesis. In this study, we identified four genes that encode isoflavonoid PTs, including (−)-glycinol 2-dimethylallyltransferase (G2DT), using homology-based in silico screening and biochemical characterization in yeast expression systems. Transcript analyses illustrated that changes in G2DT gene expression were correlated with the induction of glyceollins II, III, IV and V in elicitor-treated soybean cells and leaves, suggesting its involvement in glyceollin biosynthesis. Moreover, the genomic signatures of these PT genes revealed that G4DT and G2DT are paralogs derived from whole-genome duplications of the soybean genome, whereas other PT genes [isoflavone dimethylallyltransferase 1 (IDT1) and IDT2] were derived via local gene duplication on soybean chromosome 11.
Keywords: Gene duplication, Isoflavonoid, Phytoalexin, Prenyltransferase, Soybean
All nucleotide sequences reported in this paper have been submitted to the DDBJ/EMBL/GenBank database under accession numbers LC140926 (PT3), LC140927 (C4DT), LC140928 (IDT1), LC140929 (IDT2) and LC140930 (G2DT)
Introduction
Flavonoids comprise a widespread class of plant metabolites, and those with one or more dimethylallyl (C5) or geranyl (C10) groups present on their aromatic rings are known as prenylated flavonoids. The structures of prenylated flavonoids vary regarding the numbers and lengths of prenyl groups and modifications such as cyclization and hydroxylation. Hence, prenylated flavonoids are highly diverse, and they include approximately 1,000 compounds that are increasingly examined for their antioxidant, antitumor, antibacterial, antiviral and estrogenic activities (Yazaki et al. 2009). Although some of the properties are common to flavonoids with no prenyl groups, it is generally accepted that prenyl moieties increase lipophilicity and membrane permeability, and tend to potentiate the bioactivities of flavonoids (Botta et al. 2005, Yazaki et al. 2009, Yang et al. 2015). Prenylation of flavonoids is catalyzed by prenyltransferases (PTs), which transfer prenyl moieties from allylic prenyl diphosphate to flavonoid skeletons. The flavonoid-specific PT naringenin 8-dimethylallyltransferase (SfN8DT) was initially identified in the leguminous plant Sophora flavescens (Sasaki et al. 2008), and several plant PTs that act on flavonoids and other aromatic compounds have been molecularly and biochemically characterized (Akashi et al. 2009, Sasaki et al. 2011, Shen et al. 2012, Chen et al. 2013, Karamat et al. 2014, Li et al. 2014, Munakata et al. 2014, Wang et al. 2014, Li et al. 2015, Munakata et al. 2016). A recent report on hop PTs demonstrated that a complex of two PTs, as a metabolon, catalyzes three steps of sequential aromatic prenylation in β-bitter acid biosynthesis (Li et al. 2015). However, there is a wide gap between the number of identified PTs and that of known prenylated flavonoids and aromatic compounds. The fact that these characterized PTs have stringent substrate specificities indicates the presence of additional unidentified PTs.
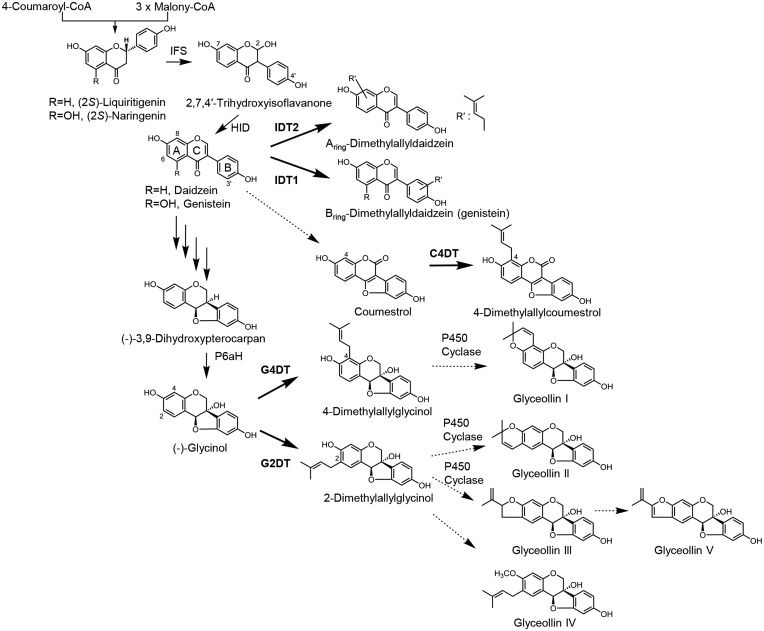
Isoflavonoids comprise a subclass of flavonoids, in which phenyl rings (B-rings) are attached to C-3 instead of C-2 in the flavonoid skeleton. Isoflavonoids are further classified as isoflavones, pterocarpans, coumestans or isoflavans, all of which are predominantly unique to leguminous plants (Aoki et al. 2000). More than half of these isoflavonoids are prenylated, and many prenylated isoflavonoids are considered to be inducible antimicrobial phytoalexins. Glyceollins, a series of prenylated pterocarpans, are well known to be the phytoalexins of soybean (Glycine max) (Tahara and Ibrahim 1995). During glyceollin biosynthesis, the dimethylallyl moiety is introduced at either C-4 or C-2 of the pterocarpan precursor (−)-glycinol [(6aS, 11aS)-3,9,6a-trihydroxypterocarpan; Fig. 1]. Although most glycinol biosynthetic enzymes and their genes have been characterized, little is known about the enzymes responsible for the succeeding prenylation and cyclization reactions, which lead to the formation of the characteristic structure of glyceollins. A homology-based approach has led to the isolation of a cDNA encoding dimethylallyl diphosphate (DMAPP):(−)-glycinol 4-dimethylallyltransferase (G4DT), which yields 4-dimethylallylglycinol, the direct precursor of glyceollin I (Akashi et al. 2009), but not 2-dimethylallylglycinol, suggesting stringent regiospecificity of G4DT for the C-4 position of glycinol. These data suggest that another PT is involved in the biosynthesis of glyceollins II–V (Fig. 1), which should be referred to as DMAPP:(−)-glycinol 2-dimethylallyltransferase (G2DT). Recent reports also illustrated that elicitor-treated soybeans accumulate glyceollins and several prenylated isoflavonoids, including 4-dimethylallylcoumestrol, 3′-dimethylallyldaidzein, 3′-dimethylallylgenistein and 8-dimethylallyldaidzein (Cheng et al. 2011, Simons et al. 2011) (Fig. 1), further indicating the presence of several isoflavonoid PTs with varying substrate specificities and regiospecificities in soybean.
Fig. 1.
Biosynthetic pathways of prenylated isoflavonoids in soybean. Bold arrows show reactions catalyzed by the PTs that were characterized in this study. As the candidates of IDT1 and IDT2 products, 3′-dimethylallyldaidzein, 3′-dimethylallylgenistein and 8-dimethylallyldaidzein were identified in soybean (Cheng et al. 2011), but the genuine products of IDT1 and IDT2 were not determined. The other PT products have been reported. See text. Dashed arrows represent putative steps. C4DT, coumestrol 4-dimethylallyltransferase; G2DT, (−)-glycinol 2-dimethylallyltransferase; G4DT, (−)-glycinol 4-dimethylallyltransferase; IDT1, isoflavone dimethylallyltransferase 1; IDT2, isoflavone dimethylallyltransferase 2; IFS, 2-hydroxyisoflavanone synthase; HID, 2-hydroxyisoflavanone dehydratase; P6aH, pterocarpan 6a-hydroxylase.
The structural diversity of plant specialized metabolites is generally associated with a functional diversification of enzymes caused by gene duplication and nucleotide substitutions, in some cases leading to changes in substrate specificity, regiospecificity and expression patterns (Ober 2005). In our previous studies, the tandem clusters of paralogous genes encoding biosynthetic enzymes in flavonoid and triterpenoid pathways, which suggests their origin from local gene duplication (LGD), were observed in the chromosomes of a model legume Lotus japonicus (e.g. Shimada et al. 2003, Sawai et al. 2006, Shimada et al. 2007). Hence, comprehensive identification of isoflavonoid PT genes in soybean, the whole-genome information of which is also available, and their mapping in chromosomes may reveal the molecular basis of the structural diversity of prenylated isoflavonoids in terms of molecular evolution.
In the present study, we used genome sequence information and expressd sequence tags (ESTs) from soybeans to characterize PTs that are involved in isoflavonoid metabolism. Candidate PTs of the isoflavonoid pathway were selected using homology-based in silico screening, and their catalytic functions were confirmed in recombinant yeast microsomes. In these investigations, we identified four novel PTs, one of which is G2DT, and revealed the characteristic arrangements of PT genes in the soybean genome. The present data indicate that soybean PT genes were diversified via LGD and whole-genome duplication (WGD) followed by nucleotide substitution.
Results
Identification of candidate genes for isoflavonoid PTs using in silico screening
Previous studies revealed that (iso)flavonoid-specific PTs exhibit relatively high sequence homology with homogentisate phytyltransferase (HPT), which is encoded by AtVTE2‐1 [National Center for Biotechnology Information (NCBI) accession No. AY089963)] of Arabidopsis thaliana (Sasaki et al. 2008, Akashi et al. 2009). Based on this notion, we isolated 11 candidate PTs from soybean by in silico screening of homologous sequences in the soybean genomic database (Phytozome v11.0) using AtVTE2‐1 as the query (Table 1). Among the 11 candidate genes, Glyma.13G097800 (DQ231059), Glyma.10G295300 (AB434690) and Glyma.01G134600 (GR848991) corresponded to PT1, PT2 and PT3, respectively, which were previously reported by Akashi et al. (2009). PT1 had 78% sequence identity with AtVTE2‐1, PT2 was found to encode G4DT, whereas PT3 remained uncharacterized due to limited sequence information at that time (Akashi et al. 2009). EST database searches were also performed for transcripts of the candidate genes, but no ESTs for the four candidates Glyma.03G033100, Glyma.10G070100, Glyma.10G070300 and Glyma.11G210500 were found at NCBI. In addition, their transcripts were not detected by PCR using the cDNA templates prepared from yeast extract-treated cultured soybean cells, which were used for cDNA cloning. Thus, in the present study, we performed functional analyses of the candidate genes Glyma.10G070200, Glyma.11G210300, Glyma.11G210400 and Glyma.20G245100 (designated PT4, PT5, PT6 and PT7, respectively), and PT3 (Table 1).
Table 1.
Predicted genes with high sequence identity to AtVTE2‐1 from the soybean genome database
| Sequence ID of the Phytozome database (v11.0) | Identity of the nucleotide and deduced amino acid sequence (in parentheses) to AtVTE2‐1 (%) | Full-length coding sequence information available using EST data | Function of the encoded protein |
|---|---|---|---|
| Glyma.13G097800 (PT1) | 66 (78) | Yes | HPTa |
| Glyma.03G033100 | 54 (55) | No | – |
| Glyma.10G070100 | 58 (54) | No | – |
| Glyma.10G070300 | 57 (49) | No | – |
| Glyma.11G210500 | 60 (56) | No | – |
| Glyma.10G295300 (PT2) | 59 (49) | Yes | G4DTb |
| Glyma.01G134600 (PT3) | 58 (50) | Yes | – |
| Glyma.10G070200 (PT4) | 58 (49) | Yes | C4DTc |
| Glyma.11G210300 (PT5) | 60 (55) | Yes | IDT1c |
| Glyma.11G210400 (PT6) | 61 (56) | Yes | IDT2c |
| Glyma.20G245100 (PT7) | 58 (47) | Yes | G2DTc |
a Predicted according to high identity with AtVTE2‐1 (Akashi et al. 2009).
b Experimentally characterized previously (Akashi et al. 2009).
c Experimentally characterized in this study; see text.
PT3, PT4, PT5, PT6 and PT7 were predicted to encode polypeptides of 393, 412, 402, 410 and 408 amino acids in length, respectively, and they had 47–56% identity with AtVTE2‐1 (Table 1). Amino acid sequences were aligned using Clustal Omega (Supplementary Fig. S1), and putative N-terminal transit peptides of 26, 44, 35, 43, and 43 amino acids in length and 8–9 transmembrane regions, respectively, were identified using transmembrane hidden Markov model (TMHMM) and WoLF PSORT programs. Predicted amino acid sequences also contained two motifs that are highly conserved among divalent cation-dependent PTs (Liang et al. 2002; Supplementary Fig. S1).
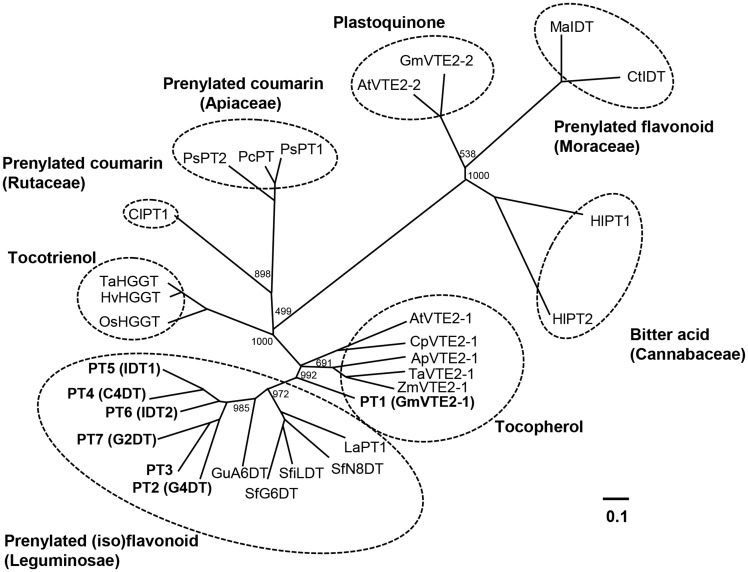
On further examination, the phylogenetic relationships among PT1–PT7, related PTs and PTs that act on aromatic compounds including flavonoids and isoflavonoids were investigated using the Neighbor–Joining method (Fig. 2). In the phylogenetic tree, PT3–PT7 are included in a monophyletic clade together with previously reported isoflavonoid PTs from Leguminosae, such as SfG6DT, LaPT and G4DT, suggesting their involvement in the biosynthesis of prenylated (iso)flavonoids. In this clade, PT3–PT7 and G4DT are intimately related to each other compared with those from other leguminous plants, namely Glycyrrhiza uralensis (GuA6DT), S. flavescens (SfG6DT, SfiLDT and SfN8DT) and Lupinus albus (LaPT1). In contrast, PTs responsible for prenylated (iso)flavonoid biosynthesis in Moraceae are located at a distant branch from those of Leguminosae. The PTs of Apiaceae and Cannabaceae, which are involved in the biosyntheses of prenylated coumarins and bitter acids, respectively, also form separate clades. In contrast to PTs in these specialized metabolisms, HPT (encoded by the orthologs of AtVTE2-1), homogentisate geranylgeranyltransferase (HGGT) and homogentisate prenyltransferase (encoded by the orthologs of AtVTE2-2), which are responsible for the biosyntheses of tocopherols, tocotrienols and plastoquinone, respectively, form monophyletic clades, each of which contains PTs of the same function from various plant species (Fig. 2).
Fig. 2.
Phylogenetic relationships among the soybean PTs and related PTs that act on the aromatic compounds of higher plants. Amino acid sequences were analyzed using the ClustalW program (DDBJ), and the Neighbor–Joining tree was produced from the results of 1,000 bootstrap replicates. Numbers denote bootstrap values (maximum 1,000). Homogentisate phytyltransferase for tocopherol biosynthesis, homogentisate geranylgeranyltransferase for tocotrienol biosynthesis, and homogentisate prenyltransferase for plastoquinone biosynthesis are shown as VTE2-1, HGGT and VTE2-2, respectively. Other PTs are involved in the biosynthesis of prenylated plant specialized metabolites including (iso)flavonoids. Species abbreviations: Ap, Allium porrum; At, Arabidopsis thaliana; Cl, Citrus limon; Cp, Cuphea pulcherrima; Ct, Cudrania tricuspidata; Gm, Glycine max; Gu, Glycyrrhiza uralensis; Hl, Humulus lupulus; Hv, Hordeum vulgare; La, Lupinus albus; Ma, Morus alba; Os, Oryza sativa; Pc, Petroselinum crispum; Ps, Pastinaca sativa; Sf, Sophora flavescens; Ta, Triticum aestivum; Zm, Zea mays. Accession numbers for the proteins used in the phylogenetic tree are as follows: ApVTE2-1 (DQ231057), AtVTE2‐1 (AY089963), AtVTE2‐2 (DQ231060), CIPT1 (AB813876), CpVTE2-1 (DQ231058), CtIDT (KM262660), G4DT (AB434690), GmVTE2-1 (DQ231059), GmVTE2-2 (DQ231061), GuA6DT (KJ123716), HIPT1 (AB543053), HIPT2 (KM222442), HvHGGT (AY222860), LaPT1 (JN228254), MaIDT (KM262659), OsHGGT (AY222862), PcPT (AB825956), PsPT1 (KM017083), PsPT2 (KM017084), SfG6DT (AB604224), SfiLDT (AB604223), SfN8DT (AB325579), TaHGGT (AY222861), TaVTE2-1 (DQ231056) and ZmVTE2-1 (DQ231055).
Cloning and functional analysis of cDNAs for PT3–PT7
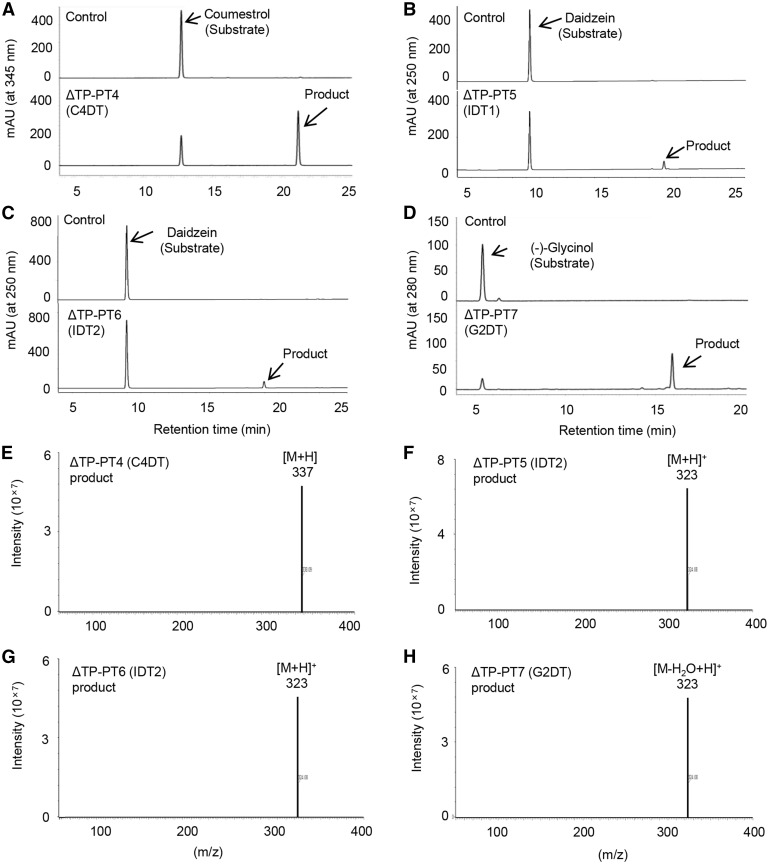
Coding sequences (CDSs) were amplified by PCR using cDNA templates from elicitor-treated cultured soybean cells, although the CDS of PT6 was artificially synthesized due to unsuccessful PCR amplification. Because some plastid-localized PTs partially or completely failed to be functionally expressed in yeast with the transit peptide (Akashi et al. 2009, Shen et al. 2012, Munakata et al. 2014, Li et al. 2015), the CDSs of the N-terminal-truncated forms without putative transit peptides were also PCR-amplified and introduced into yeast expression vectors. Subsequently, 10 constructs, namely the native CDSs of PT3, PT4, PT5, PT6 and PT7 and their truncated forms ΔTP-PT3, ΔTP-PT4, ΔTP-PT5, ΔTP-PT6 and ΔTP-PT7, respectively, were expressed in yeast. Recombinant yeast microsomes were then incubated with DMAPP, and the (iso)flavonoid substrates (2RS)-naringenin, (2S)-liquiritigenin, daidzein, genistein, coumestrol and (−)-glycinol, in the presence of Mg2+, and the reaction mixtures were analyzed using HPLC. Recombinant microsomes expressing ΔTP-PT4 and ΔTP-PT7 displayed dimethylallyltransferase activity toward coumestrol and (−)-glycinol, respectively, and produced single products (Fig. 3A, D). Conversely, microsomes expressing ΔTP-PT5 and ΔTP-PT6 produced single products when incubated with daidzein (Fig. 3B, C). Prenylation products were then confirmed using ultra-performance liquid chromatography (UPLC)–triple quadrupole mass spectrometry (TQMS) analysis in the electrospray ionization (ESI)-positive ion mode. A mass-to-charge ratio (m/z) of 337 was determined for the reaction product obtained from ΔTP-PT4-expressing microsomes and was matched to that of protonated ions of coumestrol with a dimethylallyl group (Fig. 3E). Furthermore, the m/z values of the reaction products from ΔTP-PT5- and ΔTP-PT6- (323 and 323) expressing microsomes corresponded to those of protonated ions of dimethylallyl-conjugated daidzein (Fig. 3F, G). The reaction product from ΔTP-PT7-expressing microsomes gave an m/z of 323, which was in good agreement with that of dehydrated and protonated ions of (−)-glycinol with a dimethylallyl group (Fig. 3H). The native (untruncated) CDS constructs of PT4, PT5, PT6 and PT7 in recombinant microsomes yielded the same products as those from their truncated forms. Microsomes expressing ΔTP-PT3 or native PT3 exhibited no dimethylallyltransferase activity toward any of the tested substrates.
Fig. 3.
Analysis of reaction products of N-terminal-truncated soybean PTs. Enzymatic reaction products of C4DT (A), IDT1 (B), IDT2 (C) and G2DT (D) were analyzed using HPLC. Assay mixtures contained DMAPP, microsome fractions of yeast expressing the truncated form of PT, and one of the following prenyl-accepting substrates: coumestrol (A), daidzein (B, C) or (−)-glycinol (D). Microsomes from yeast cells that were transformed with the empty (no insert cDNA) expression vector were used as negative controls. MS spectra of the reaction products obtained using C4DT (E), IDT1 (F), IDT2 (G) and G2DT (H) were analyzed in the ESI-positive mode.
The enzymatic product from microsomes expressing ΔTP-PT4 was purified and analyzed using 1H-nuclear magnetic resonance (1H-NMR) to investigate further the chemical structure, and the data obtained were compared with those from the authentic sample of the substrate coumestrol. The dimethylallyl moiety of the ΔTP-PT4 product was confirmed by characteristic signals at 1.64 (3H, s, H-5′) and 1.81 (3H, s, H-4′) for two methyl groups, 3.47 (2H, d, J = 6.9 Hz, H-1′) for one methylene and 5.22 (1H, m, H-2′) for one methine. The C-4 position, to which the dimethylallyl moiety is attached, was defined by the disappearance of the coumestrol H-4 signal and by a change from the coumestrol double-doublet H-2 signal to a doublet signal of the product. These data indicate that the enzymatic product of PT4-expressing microsomes is 4-dimethylallylcoumestrol. Accordingly, the enzyme encoded by PT4 was designated as DMAPP:coumestrol 4-dimethylallyltransferase (C4DT).
In further 1H-NMR analyses, the chemical structure of the purified enzymatic product from the microsomes expressing ΔTP-PT7 was determined, and the signals for the dimethylallyl moiety were identified as follows: 1.72 (6H, s, H-4′, H-5′) for two methyl groups, 3.28 (2H, d, J = 6.9 Hz, H-1′) for one methylene and 5.35 (1H, m, H-2′) for one methine. In addition, prenylation at the C-2 position was indicated by the disappearance of the glycinol H-2 signal and by the replacement of doublet H-1 and H-4 signals from glycinol to singlet signals in the product (Akashi et al. 2009). Taken together, these data reveal that the product from PT7-expressing microsomes is 2-dimethylallylglycinol, which is a direct precursor of glyceollins II and III. Accordingly, the enzyme encoded by PT7 was defined as DMAPP:(6aS, 11aS)-3,9,6a-trihydroxypterocarpan 2-dimethylallyltransferase [(−)-glycinol 2-dimethylallyltransferase; G2DT].
Microsomes expressing ΔTP-PT5 and ΔTP-PT6 exhibited dimethylallyltransferase activities against daidzein and yielded products with distinct HPLC retention times of 18.7 and 18.9 min, respectively (Fig. 3B, C). Hence, the enzymes encoded by PT5 and PT6 were designated DMAPP:isoflavone dimethylallyltransferase 1 (IDT1) and IDT2, respectively. However, weak enzymatic activity in microsomes expressing ΔTP-PT5 and ΔTP-PT6 did not yield products sufficient for determining chemical structures via NMR analyses. Hence, the position of the dimethylallyl group was estimated using tandem mass spectrometry (MS/MS) signals from a fragment ion at m/z 137, which was generated from the A-ring of daidzein following a retro Diels–Alder reaction (Maul et al. 2008). The presence of this signal indicated that the A-ring of the reaction product obtained by IDT1 was not enzymatically modified, suggesting prenylation of the B-ring (Supplementary Fig. S2A). In contrast, this signal was absent in experiments with IDT2, suggesting prenylation of the A-ring (Supplementary Fig. S2B).
Biochemical characterizations of C4DT, IDT1, IDT2 and G2DT
The substrate specificities of four N-terminal-truncated soybean PTs were tested using (2RS)-naringenin, (2S)-liquiritigenin, daidzein, genistein, coumestrol and (−)-glycinol. 2,7,4′-Trihydroxyisoflavanone, which is the direct daidzein precursor (Fig. 1), was also used for the assays of IDT1 and IDT2. In these experiments, C4DT and G2DT only exhibited activity toward coumestrol and (−)-glycinol, respectively, whereas IDT1 and IDT2 exhibited comparable activity toward daidzein and genistein but not toward 2,7,4′-trihydroxyisoflavanone (Table 2). In further experiments, C4DT, IDT1, IDT2 or G2DT was incubated without divalent cations and in the presence of 5 mM EDTA, and no products were detected, demonstrating the enzymatic requirement for divalent cations for their catalysis. Moreover, all four enzymes preferred Mg2+ to Mn2+ (Supplementary Fig. S3). Lineweaver–Burk plots of G2DT revealed apparent Km values for (−)-glycinol and DMAPP of 183 and 121 µM, respectively.
Table 2.
Substrate specificities of recombinant PTs for prenyl acceptors
| Prenyl acceptors | Relative activities (pmol mg microsomal protein−1 s−1) | |||
|---|---|---|---|---|
| C4DT | IDT1 | IDT2 | G2DT | |
| (2RS)-Naringenin | ND | ND | ND | ND |
| (2S)-Liquiritigenin | ND | ND | ND | ND |
| Genistein | ND | 6.9 ± 0.1 | 1.1 ± 0.1 | ND |
| Daidzein | ND | 8.7 ± 0.7 | 1.1 ± 0.1 | ND |
| Coumestrol | 1,800 ± 60 | ND | ND | ND |
| (−)-Glycinol | ND | ND | ND | 15.1 ± 1.0 |
| 2,7,4′-Trihydroxyisoflavanone | – | ND | ND | – |
Relative activities of C4DT, IDT1, IDT2 and G2DT toward various flavonoids and isoflavonoids.
Values are presented as mean ± SD of three biological samples.
ND, not detected; –, not tested.
Elicitation responses and expression analyses in cultured cells and isolated leaves of soybean
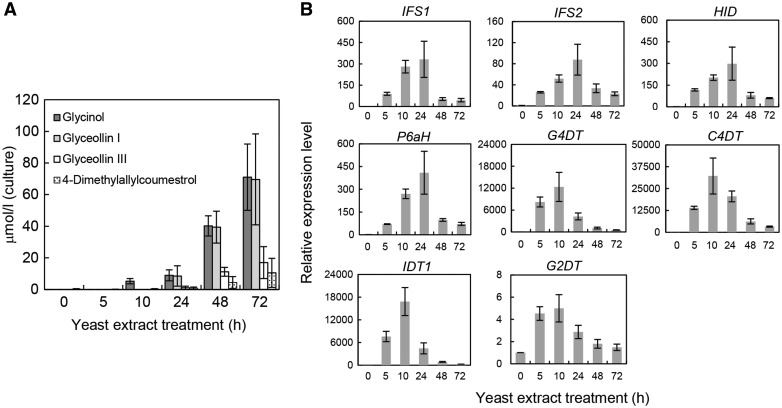
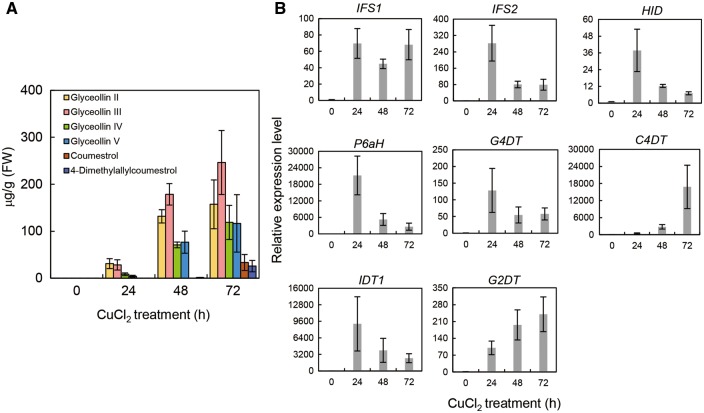
Prenylated isoflavonoid contents and the corresponding transcript levels of PT genes were analyzed using soybean cells in suspension cultures following treatments with yeast extract [0.3% (w/v) medium]. Yeast extract treatments led to increased accumulation of glyceollins I and III, reflecting enhancement of the enzymatic activities of G4DT and G2DT (Akashi et al. 2009). Moreover, the C4DT product 4-dimethylallylcoumetrol was also detected with the two glyceollin isomers (Fig. 4A), and the glyceollin precursor glycinol was increasingly present 10–72 h after treatments with yeast extract. In this system, glyceollin I was the major glyceollin isomer, and it was increasingly present 24–72 h after yeast extract treatment; conversely, the glyceollin III level was approximately a quarter of those of glycinol and glyceollin I. 4-Dimethylallylcoumetrol content was slightly increased following elicitor treatment (Fig. 4A). In contrast, none of the intermediates of glyceollin I and III, 4-dimethylallylglycinol and 2-dimethylallylglycinol, other glyceollin isomers or putative products of IDT1 and IDT2 (dimethylallyldaidzein or dimethylallylgenistein) was detected. Real-time PCR analyses demonstrated that elicitor treatment caused transient up-regulation of C4DT, IDT1, G2DT and G4DT, with co-ordinated transient increases in the expression of the glycinol biosynthetic genes 2-hydroxyisoflavanone synthase 1 (IFS1), IFS2, 2-hydroxyisoflavanone dehydratase (HID) and pterocarpan 6a-hydroxylase (P6aH) (Fig. 4B). The expression of G2DT was induced by elicitor treatment, but it reached a maximum transcript level of no more than 5-fold higher than the steady-state expression level.
Fig. 4.
Time course of prenylated isoflavonoid accumulation (A) and relative transcript levels of biosynthetic genes (B) in yeast extract-treated soybean suspension cultures. 4-Dimethylallylcoumestrol levels were determined as coumestrol equivalents. Transcript levels were expressed relative to that of the housekeeping gene SKIP16 and were normalized to those of non-treated cells (at 0 h). Values are presented as the mean ± SD of triplicate biological samples. Accession numbers are as follows: SKIP16, CD397253; IFS1, AF195798; IFS2, AF022462; HID, AB154415; P6aH, D83968; and G4DT, AB434690.
Aiming at an alternative phytoalexin induction system in which 2-dimethylallylglycinol-derived glyceollins (II–V) are principally induced, isolated soybean leaves were treated with 1 mM cupric chloride (CuCl2), and glyceollin production was determined according to previous reports on leguminous phytoalexin induction (Dewick 1977, Keen et al. 1986, Yuk et al. 2011). These experiments confirmed the accumulation of glyceollins II–V, again implying increased G2DT activity (Supplementary Text S1). In particular, the levels of glyceollins II–V were increased following CuCl2 treatment, reaching 150–250 µg g−1 FW at 72 h. Conversely, glyceollin I and dimethylallylisoflavones (dimethylallyldaidzein and dimethylallylgenistein) were not detected. Coumestrol and 4-dimethylallylcoumestrol were also induced by CuCl2 treatment (Fig. 5A). Subsequent real-time PCR experiments demonstrated that the expression of IFS1, IFS2, HID, P6aH, G4DT, C4DT, IDT1 and G2DT was increased by CuCl2 treatment (Fig. 5B), as observed in cultured cells treated with yeast extract. In particular, G2DT transcripts increased by 100-fold. The transcripts of the four genes Glyma.03G033100, Glyma.10G070100, Glyma.10G070300 and Glyma.11G210500 were also undetected in the CuCl2-treated isolated leaves, as observed in the yeast extract-treated cultured cells.
Fig. 5.
Time course of prenylated isoflavonoid accumulation (A) and relative transcript levels of biosynthetic genes (B) in soybean leaves following treatment with CuCl2. Glyceollins II, IV and V were determined as glyceollin I equivalents. Other conditions were the same as those described in Fig. 4.
Arrangements of PT genes in soybean chromosomes
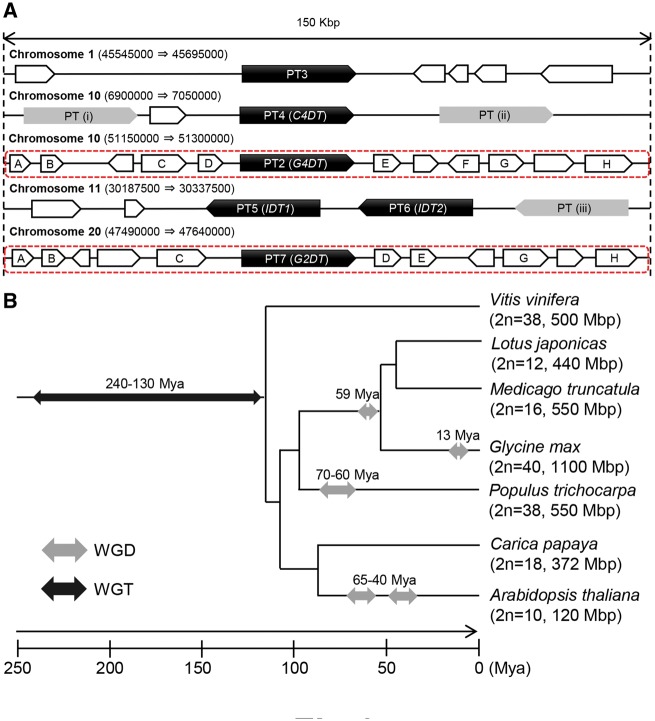
To investigate the evolutionary processes of soybean PT genes responsible for the presence of numerous prenylated isoflavonoids, we investigated the locations of PT2–PT7 and the annotations of adjacent genes using the soybean genome database. Fig. 6A shows a schematic of gene arrangements within approximately 75 kb upstream and downstream regions of each PT gene. The isoflavone PT genes IDT1 and IDT2 are located within 40 kb of each other on chromosome 11, and Glyma.11G210500 is located close to this tandem cluster. C4DT is also accompanied by Glyma.10G070100 and Glyma.10G070300. G4DT and G2DT, which encode enzymes that act on (−)-glycinol, are located in syntenic regions on chromosomes 10 and 20, respectively (Fig. 6A).
Fig. 6.
Chromosomal arrangements of PT genes and evolutionary processes of the soybean genome. (A) Gene arrangements in 75 kb upstream and downstream regions adjacent to PT genes (PT2–PT7) in soybean chromosomes. Numbers in parentheses show the positions of the 150 kb regions in each chromosome. PT (i), PT (ii) and PT (iii) denote the uncharacterized genes Glyma.10G070100, Glyma.10G070300 and Glyma.11G210500, respectively (Table 1). The flanking genes are annotated as follows: A, TBC RabGap/TBC domain-containing protein; B, PAP/fibrillin; C, callose synthase; D, zinc finger protein; E, isopropylmalate synthase; F, membrane protein; G, protein kinase; and H, transporter. The other genes are not functionally annotated. (B) Phylogenetic relationships among species of rosids with complete genome sequences. The phylogenetic tree was abstracted from Fawcett et al. (2009). Double arrows show WGD and whole-genome triplication (WGT) events (Severin et al. 2011). Glycine max experienced a recent WGD event 13 million years ago (Mya).
Discussion
More than half of the isoflavonoids found in leguminous plants have additional cyclic or acyclic prenyl moieties that originate from dimethylallyl or geranyl groups, and these prenyl modifications often enhance biological activities (Tahara and Ibrahim 1995). The identification of PTs that accept various isoflavonoid substrates is critical to understanding of the structural variations of prenylated isoflavonoids in leguminous plants. In addition, genome arrangements of PT genes might be partly linked to the evolutionary diversification of prenylated isoflavonoids in soybean. Soybean is a good resource for understanding isoflavonoid PT genes, as it produces a vast variety of prenylated isoflavonoids represented by glyceollins, and its detailed whole-genome information is available for gene mining. Characterization of the enzymes responsible for phytoalexin production may facilitate breeding of leguminous crops with enhanced resistance to pathogens.
In the present study, we performed molecular and functional characterization of soybean isoflavonoid PTs using a homology-based approach and identified the four PTs C4DT, IDT1, IDT2 and G2DT. Their deduced transmembrane domains and divalent cation requirements (Supplementary Figs. S1, S3) were almost consistent with those of previously reported (iso)flavonoid PTs (Sasaki et al. 2008, Akashi et al. 2009, Sasaki et al. 2011, Shen et al. 2012, Chen et al. 2013, Li et al. 2014, Wang et al. 2014). Moreover, the putative N-terminal transit peptide sequences of the soybean PTs implied their plastid localizations and their functions in prenylation reactions occurring in the plastid or its related subcellular compartment, as described in previous works on SfN8DT and G4DT (Sasaki et al. 2008, Akashi et al. 2009), although their subcellular localizations were not experimentally verified. Among the PT candidates obtained by in silico screening, we did not analyze functionally the four genes, Glyma.03G033100, Glyma.10G070100, Glyma.10G070300 and Glyma.11G210500 because their transcripts were not observed in elicitor-treated cultured cells and the isolated leaves of soybean, as well as in the EST database at NCBI. Their spatio-temporal expression may be strictly regulated.
The roles of soybean PTs in the biosynthesis of prenylated isoflavonoid
Our data illustrated that C4DT catalyzes the production of 4-dimethylallylcoumestrol from coumestrol (Fig. 3A). To our knowledge, this is the first identified plant PT that acts on coumestans including coumestrol as substrates. 4-Dimethylallylcoumestrol was induced in yeast extract-treated suspension cultures and in isolated leaves treated with CuCl2. In agreement with this, transcription of C4DT was up-regulated prior to the increase in 4-dimethylallylcoumestrol levels in both of these systems (Figs. 4A, B, 5A, B). A previous study revealed that the disease resistance-inducing herbicide lactofen induces 4-dimethylallylcoumestrol accumulation in the ‘minimal-wound snapped cotyledon assay’ (Cheng et al. 2011). Dimethylallylcoumestrol accumulation coupled with the expression patterns of C4DT on CuCl2 treatment suggests its involvement in defensive responses to biotic or abiotic stresses (Figs. 4, 5).
IDT1 and IDT2 were found to catalyze dimethylallylation of isoflavones (daidzein and genistein), presumably producing B- and A-ring prenylated isoflavones, respectively (Fig. 3B, C). In the previously reported ‘minimal-wound snapped cotyledon assay’, production of the B- and A-ring prenylated isoflavones 3′-dimethylallyldaidzein (3′-dimethylallylgenistein) and 8-dimethylallyldaidzein, respectively, which are possible products of IDT1 and IDT2, respectively, was induced by lactofen and by a glucan from the cell walls of the pathogenic bacterium Phytophthora sojae (Cheng et al. 2011). In the present study, IDT1 transcript levels increased by approximately 10,000-fold after elicitor treatment of cultured cells and isolated leaves, implying that it is involved in defense responses (Figs. 4B, 5B). However, 3′-dimethylallyldaidzein, 3′-dimethylallylgenistein, 8-dimethylallyldaidzein and other prenylated isoflavones were not detected in these induced tissues, and IDT2 transcripts were not detected in cultured cells or isolated leaves. Hence, these data do not support the roles of IDT1 or IDT2 in the biosynthesis of 3′-dimethylallyldaidzein (3′-dimethylallylgenistein) or 8-dimethylallyldaidzein. Further investigation will be required to elucidate their roles in soybean isoflavonoid biosynthesis.
G2DT was found to catalyze the conversion of (−)-glycinol into 2-dimethylallylglycinol, which is a precursor of glyceollins II, III, IV and V (Fig. 3D). The regiospecificity of this prenylation reaction was unequivocally confirmed in comparisons of 1H-NMR spectra for glycinol and the G2DT products. The apparent Km values of G2DT for (−)-glycinol (183 µM) and DMAPP (121 µM) were comparable with those of G4DT (68 and 150 µM, respectively), whereas the affinity of G4DT for the prenyl acceptor was slightly higher (Akashi et al. 2009). To test the role of G2DT in plant tissues, we employed two induction systems of glyceollins: suspension-cultured cells, which were previously used for the analysis of G4DT (Akashi et al. 2009), and isolated leaves, in which glyceollins II and III (Keen et al. 1986) and IV and V (Yuk et al. 2011) were strongly induced by elicitation (Pseudomonas syringae pv. pisi and iodoacetate) and wounding, respectively. Following yeast extract treatment of the suspension-cultured cells, up-regulation of G2DT transcripts by approximately 5-fold and slight accumulation of glyceollin III were observed (Fig. 4A, B). In additional experiments using CuCl2 in isolated soybean leaves, glyceollins II–V were markedly induced (Fig. 5A), although our elicitation procedures differed from those of previous studies. Moreover, G2DT transcription was increased by >100-fold prior to the accumulation of glyceollin isomers (Fig. 5B). Taken together, the pathway via 2-dimethylallylglycinol was induced more intensively in CuCl2-treated isolated leaves than in yeast extract-treated cultured cells, and the relative transcript level of G2DT was consistent with the accumulation levels of 2-dimethylallylglycinol-derived glyceollins (II–V) in the induction systems. These results support the notion that G2DT is involved in glyceollin II–V biosynthesis. In contrast, glyceollin I was not detected in elicited leaves despite the considerable 100-fold increase in G4DT transcript levels (Fig. 5B). This discrepancy suggests the conversion of glyceollin I to an unknown end-product or inactivation by (post)translational regulation of G4DT, warranting further investigation of metabolite profiling and G4DT protein expression.
In glyceollin biosynthesis, the subsequent cyclization reactions are reportedly catalyzed by cytochrome P450 monooxidases (P450s), but none of them has been molecularly characterized (Welle and Grisebach 1988, Ayabe and Akashi 2006). The molecular clones for G4DT and G2DT will be used to prepare the substrates for the P450 cyclases, facilitating their molecular identification (Fig. 1). Because G4DT (and probably also G2DT) is plastid localized, the identification of the P450s involved in the next step of G4DT and G2DT will help to reveal the intriguing subcellular compartment in which glyceollins are produced.
Molecular evolution of isoflavonoid PTs in leguminous plants
Phylogenetic analysis of the PTs responsible for the prenylation of aromatic compounds revealed that clades are formed in two distinct manners (Fig. 2). One includes the PTs involved in the biosyntheses of tocopherols, tocotrienols and plastoquinone, which are essential for the basic activities of plants at the cellular level and thus can be considered to reflect ‘primary’ metabolism. In this case, a clade consists of the PTs that possess the same catalytic function but originate from various plant species. In contrast, PTs involved in plant specialized metabolism, such as the biosyntheses of prenylated (iso)flavonoids, coumarins and bitter acids, form their clades in the other manner, in which close phylogenetic relationships are observed among the PTs that originate from the same plant species but not among those that possess the same or similar function. The latter pattern was only clarified after multiple (iso)flavonoid PTs were identified from two species, S. flavescens and soybean. These phylogenetic relationships suggest that primary metabolism PTs were established prior to speciation in the angiosperm and that PTs in plant specialized metabolism were diversified after the establishment of each plant lineage. PTs that act on isoflavones, such as IDT1, IDT2, LaPT, SfG6DT, MaIDT and CtIDT, have thus far been identified from several plant lineages, and they are dispersedly located in the phylogenetic tree, suggesting their convergent origins. Non-leguminous MaIDT and CtIDT, which are located in the distant branch, are likely to have originated from the PTs responsible for plastoquinone biosynthesis, whereas the other isoflavone PTs from leguminous plants probably originated from those for tocopherols (Fig. 2).
The functional diversification process of soybean isoflavonoid PTs suggested by the genome organization
The genomic distribution of the six PT genes investigated in our studies and their adjacent genes in soybean chromosomes led to two notable findings (Fig. 6A). First, IDT1 and IDT2, which encode PTs with similar specificity for isoflavone substrates but distinct regiospecificities of prenylation, were located in tandem on the same chromosome. Furthermore, other PT genes were found adjacent to the IDT1–IDT2 cluster and C4DT, although their expression was not detected in this study. The two genes (IDT1 and IDT2) were reportedly produced by an LGD after the recent WGD (Wang et al. 2015). Taken together, clusters of PT genes including putative isoflavonoid PT genes indicate that they emerged via LGD events in soybean. Accordingly, subsequent nucleotide substitution mutations in the duplicate genes probably changed the properties of encoded PTs. In the genome of a model legume Lotus japonicus, tandem clusters of genes that encoded enzymes of similar but distinct catalytic functions were observed, including those encoding the two oxidosqualene cyclases, cycloartenol synthase and lupeol synthase (Sawai et al. 2006), Type I and II chalcone isomerases (Shimada et al. 2003), and pterocarpan reductases with various stereospecificities (Akashi et al. 2006, Shimada et al. 2007). The PT gene clusters on chromosomes 10 and 11 of soybean provide evolutionary examples of biosynthetic genes in specialized metabolism via LGD followed by nucleotide substitution.
Secondly, we observed a syntenic relationship between the approximately 150 kb flanking region of G4DT on chromosome 10 and that of G2DT on chromosome 20 (Fig. 6A). Recent genomic investigations of angiosperm indicated frequent WGDs caused by polyploidization and suggested that repeated WGDs were responsible for speciation between angiosperm lineages (Fawcett et al. 2009, Van de Peer et al. 2009, Severin et al. 2011). Soybean has a relatively large number of chromosomes (2n = 40) compared with other leguminous plants and has a complicated genome organization, in which >75% of genes are present in multiple copies. This striking genomic feature of soybean is attributed to WGDs approximately 13 and 59 million years ago (Schmutz et al. 2010; Fig. 6B). The synteny observed in the flanking regions of G4DT and G2DT demonstrates that the two genes are located in homoeologous regions deriving from the WGD that reportedly occurred 13 million years ago (Severin et al. 2011, Wang et al. 2015), and they have been functionally specialized from a common ancestral PT gene. These findings indicate that diversification of genes in plant specialized metabolism can occur after WGDs as well as LGDs. The previously reported conserved gene duplicates in homoeologous genomic regions in soybean included PT3 and Glyma.03G033100, as well as G4DT and G2DT (Severin et al. 2011). Their functions remain unknown at present, but their closely related functions may be clarified in a future study.
Materials and Methods
Chemicals
DMAPP was purchased from Cayman Chemical, and (2RS)-naringenin and coumestrol were purchased from Tokyo Chemical Industry Co., Ltd. and Sigma-Aldrich, respectively. Daidzein and genistein were purchased from LC Laboratories, and (2S)-liquiritigenin was obtained from laboratory stock. (−)-Glycinol and glyceollins I and III were prepared as described previously (Akashi et al. 2009). 2,7,4′-Trihydroxyisoflavanone was prepared using Escherichia coli expressing the cDNA for CYP93C2 (Uchida et al. 2015).
Plant materials
Maintenance of soybean callus and treatment of suspension cultures with yeast extract were performed as described previously (Akashi et al. 2009). Soybean seeds (cv. Fuki) were purchased from Takii Seed Co., Ltd., germinated on an artificial soil and grown in a greenhouse at 25°C under a 16 h light/8 h dark photoperiod. Leaves were then isolated from 5-week-old plants and soaked in 1 mM CuCl2 solution.
Analytical methods
HPLC analyses were performed with an LC-2000 series HPLC system (JASCO) equipped with a multiwavelength detector (MD-2010, JASCO) using a TSK-Gel ODS-80™ column (4.6 × 150 mm; Tosoh) and a flow rate of 1.0 ml min−1 at 40°C. Gradient elution was performed using water (A) and methanol (B) or acetonitrile (C) with the following gradient programs: program 1 for analysis of prenylated isoflavonoids in suspension cultures (0–30 min, 40–100% B); program 2 for analysis of prenylated isoflavonoids in soybean leaves (0–20 min, 40–80% C); and program 3 for enzyme activity assays of PTs (0–30 min, 20–80% C). Eluates were monitored at 260 nm for isoflavones, 280 nm for (−)-glycinol and glyceollin isomers, and 345 nm for coumestrol and 4-dimethylallylcoumestrol.
UPLC-TQMS analyses were performed using a Quattro premier XE (Waters) instrument. UPLC separation was performed using an ACQUITY UPLC BEH C18 column (2.1 × 100 mm, Waters) with a flow rate of 0.38 ml min−1 at 40°C. Gradient elution was performed with water (D) and methanol (E) or acetonitrile (F) in the presence of 0.1% (v/v) formic acid as follows: program 1 for analyses of prenylated isoflavonoids in suspension cultures (0–2 min, 5–30%; 2–10 min, 30–70%; and 10–12 min, 70–95% E); and program 2 for analyses of prenylated isoflavonoids in soybean leaves and the enzyme activity assays of PTs (0–2 min, 5–30%; 2–10 min, 30–70%; and 10–12 min, 70–95% F). MS spectra were measured using Quattro Preminer ver 4.1 software (Waters) under the following conditions: ESI-positive mode, source temperature of 120°C, desolvation gas with a flow rate of 800 l h−1 at 400°C, cone gas with a flow rate of 50 l h−1, cone voltage of 40 V and capillary voltage of 3 kV. The MS/MS spectra of IDT1 and IDT2 products were acquired using a collision energy ramp of 10–40 eV.
In silico screening of candidate genes for putative isoflavonoid PTs
Putative genes encoding isoflavonoid PTs were retrieved from the soybean database at Phytozome v11.0 (https://phytozome.jgi.doe.gov/pz/portal.html#) using the amino acid sequence of A. thaliana HPT (AtVTE2‐1, AY089963) as the query, and corresponding transcripts were searched in the EST database at NCBI. The correspondence of the sequence ID in Wm82.a2.v1 is shown in Supplementary Table S1. Protein motifs were predicted using TMHMM (http://www.cbs.dtu.dk/services/TMHMM/) and Wolf PSORT programs (http://www.genscript.com/psort/wolf_psort.html). Predicted genes that were located close to isoflavonoid PT genes on soybean chromosomes were also examined using the soybean database at Phytozome v11.0.
Cloning of cDNAs for putative isoflavonoid PTs
Total RNA was prepared from the elicitor-treated soybean cells using an SV total RNA isolation system (Promega), and cDNA templates for PCR amplification of CDSs (excluding PT6) were synthesized using a SuperScript III First-Strand Synthesis System (Invitrogen). Because the PCR product of PT6 was not obtained using the cDNA samples, a template for PT6 was synthesized using GeneArt technology (Invitrogen). Subsequently, full-length and N-terminal-truncated CDSs were amplified using KOD-Plus polymerase (Toyobo). The primers used for cDNA cloning are listed in Supplementary Table S2. The PCR products of PT4, PT5 and PT6 were subcloned into the entry vector pCR8/GW/TOPO or pENTR/D-TOPO Gateway Technology and introduced into the yeast expression vector pYES-DEST52 using a Gateway LR Clonase II Enzyme Mix (Invitrogen). The PCR products of PT3 and PT7 were directly cloned into the yeast expression vector pYES2.1/TOPO (Invitrogen).
PT assays
Saccharomyces cerevisiae BJ2168 cells were transformed with expression vectors, and recombinant protein expression and microsome preparations were performed as described previously (Akashi et al. 1998). The protein contents of microsomes were determined using the Bradford method (Bradford 1976), and PT assays were performed as described previously (Akashi et al. 2009) with some modifications. Briefly, to a total reaction volume of 200 µl, substrates and microsomes were added at the indicated concentrations and incubated for the indicated times. The specific activities of C4DT, IDT1, IDT2 and G2DT were determined after incubating 400 µM prenyl accepter, 400 µM DMAPP and recombinant microsomes containing 15–100 µg of protein at 30°C for 15–60 min. Kinetic studies of G2DT were performed in the presence of 5, 10, 20, 40, 80, 160 or 400 µM (−)-glycinol and a fixed concentration of DMAPP (400 µM) or with a fixed concentration of (−)-glycinol (400 µM) and varying concentrations (10, 20, 40, 80, 160 and 400 µM) of DMAPP after incubation with microsomes containing 50 µg of protein at 30°C for 15 min. Apparent Km values were calculated using Lineweaver–Burk plots.
Identification of C4DT and G2DT reaction products
To identify the C4DT product, 800 µg of coumestrol, 800 µg of DMAPP and recombinant microsomes expressing the truncated form of PT4 (∼13 mg of protein) were incubated at 30°C overnight. Subsequently, the reaction product was purified from an ethyl acetate extract of the reaction mixture using silica gel thin-layer chromatography (TLC) on a plate (Kieselgel F254; Merck) with chloroform : methanol (9 : 1, v/v) as the eluting solvent. NMR spectra were recorded on a JMN ECA-500 system (JEOL). 4-Dimethylallylcoumestrol, 1H-NMR (DMSO-d6) δ: 1.64 (3H, s, H-5′), 1.81 (3H, s, H-4′), 3.47 (2H, d, J = 6.9 Hz, H-1’), 5.22 (1H, m, H-2′), 6.93 (1H, d, J = 8.6 Hz, H-2), 6.94 (1 H, dd, J = 2.3, 8.6 Hz, H-8), 7.15 (1 H, d, J = 2.3 Hz, H-10), 7.68 (1 H, d, J = 8.6 Hz, H-7), 7.69 (1 H, d, J = 8.6 Hz, H-1). To validate the signal assignments of 4-dimethylallylcoumestrol, authentic coumestrol was analyzed using NMR. Coumestrol, 1H-NMR (DMSO-d6) δ: 6.91 (1 H, d, J = 2.3 Hz, H-4), 6.94 (1 H, dd, J = 2.3, 8.6 Hz, H-2), 6.95 (1H, dd, J = 2.3, 8.6 Hz, H-8), 7.17 (1H, d, J = 2.3 Hz, H-10), 7.70 (1H, d, J = 8.6 Hz, H-7), 7.86 (1H, d, J = 8.6 Hz, H-1); 13C-NMR (DMSO-d6); δ: 98.7 (C-10), 102.0 (C-6a), 103.0 (C-4), 104.1 (C-11b), 113.8 (C-2), 114.0 (C-8), 114.6 (C-6b), 120.6 (C-7), 122.7 (C-1), 154.7 (C-4a), 156.0 (C-9), 157.0 (C-10a), 157.6 (C-6), 159.5 (C-11a), 161.3 (C-3). To identify the G2DT product, 400 µg of (−)-glycinol, 400 µg of DMAPP and recombinant microsomes expressing the truncated form of PT7 (∼7 mg of protein) were incubated at 30°C overnight. The reaction product was then purified from ethyl acetate extract of the reaction mixture using silica gel TLC with hexane : ethyl acetate (3 : 7, v/v) as the eluting solvent. 2-Dimethylallylglycinol, 1H-NMR (acetone-d6) δ: 1.72 (6H, s, H-4′, H-5′), 3.28 (2H, d, J = 6.9 Hz, H-1′), 3.97 (1H, d, J = 11.5 Hz, H-6), 4.09 (1H, d, J = 11.5 Hz, H-6), 5.23 (1H, s, H-11a), 5.35 (1H, m, H-2′), 6.25 (1H, d, J = 2.3 Hz, H-10), 6.35 (1H, s, H-4), 6.42 (1H, dd, J = 2.3, 8.0 Hz, H-8), 7.16 (1H, s, H-1), 7.19 (1H, d, J = 8.0 Hz, H-7).
Prenylated isoflavonoid analyses in elicitor-treated soybean tissues
Aliquots (10 ml) of elicited suspension cultures (250 ml) were periodically collected and extracted with ethyl acetate using a Polytron homogenizer as described previously (Akashi et al. 2009). Elicitor-treated leaves were periodically collected and ground into a fine powder in liquid nitrogen using a mortar and pestle and then extracted with 20 vols. of methanol overnight. Extracts were evaporated to dryness, dissolved in methanol and analyzed using HPLC and UPLC-TQMS. Coumestrol and prenylated isoflavonoids were identified by comparing retention times, UV-VIS spectra and MS spectra with those of authentic standards.
Real-time PCR of genes involved in the biosynthesis of prenylated isoflavonoids
Real-time PCR was performed using SYBR Green PCR Master Mix and a Fast Real-Time PCR system (Applied Biosystems) according to the manufacturer’s protocol. Data were analyzed using the comparative Ct method with the housekeeping gene SKP1/ASK-interacting protein 16 (SKIP16) as an internal standard (Hu et al. 2009). The primers for real-time PCR are listed in Supplementary Table S2.
Phylogenetic analysis
Amino acid sequences of selected PTs with specificity for aromatic substrates including (iso)flavonoids were analyzed using the ClustalW program (Thompson et al. 1994) of the DNA Data Bank of Japan. The phylogenetic tree was constructed using the Neighbor–Joining method from the results of 1,000 bootstrap replicates and was presented as a non-root tree using TreeView (Page 1996).
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan [Grant-in-Aid for Scientific Research (C) (No. 21510232)] and the Nakato Scholarship Foundation (Japan) [a Grant-in-Aid to K.Y.].
Acknowledgements
We wish to thank Tsuyoshi Nakagawa (Nihon University) for technical assistance and Dr. Kai Uchida for critical reading of the manuscript and many helpful suggestions.
Disclosures
The authors have no conflicts of interest to declare.
Glossary
Abbreviations
- C4DT
coumestrol 4-dimethylallyltransferase
- CDS
coding sequence
- DMAPP
dimethylallyl diphosphate
- ESI
electrospray ionization
- EST
expressed sequence tag
- G2DT
(−)-glycinol 2-dimethylallyltransferase
- G4DT
(−)-glycinol 4-dimethylallyltransferase
- HGGT
homogentisate geranylgeranyltransferase
- HID
2-hydroxyisoflavanone dehydratase
- HPT
homogentisate phytyltransferase
- IDT
isoflavone dimethylallyltransferase
- IFS
2-hydroxyisoflavanone synthase
- LGD
local gene duplication
- m/z
mass to-charge ratio
- MS/MS
tandem mass spectrometry
- NCBI
the National Center for Biotechnology Information, NMR, nuclear magnetic resonance
- P450
cytochrome P450 monooxygenase
- P6aH
pterocarpan 6a-hydroxylase
- PT
prenyltransferase
- TLC
thin-layer chromatography
- TQMS
triple quadrupole mass spectrometry
- TMHMM
transmembrane hidden Markov model
- UPLC
ultra-performance liquid chromatography
- WGD
whole-genome duplication
References
- Akashi T., Aoki T., Ayabe S. (1998) Identification of a cytochrome P450 cDNA encoding (2S)-flavanone 2-hydroxylase of licorice (Glycyrrhiza echinata L.; Fabaceae), which represents licodione synthase and flavone synthase II. FEBS Lett. 431: 287–290. [DOI] [PubMed] [Google Scholar]
- Akashi T., Koshimizu S., Aoki T., Ayabe S. (2006) Identification of cDNAs encoding pterocarpan reductase involved in isoflavan phytoalexin biosynthesis in Lotus japonicus by EST mining. FEBS Lett. 580: 5666–5670. [DOI] [PubMed] [Google Scholar]
- Akashi T., Sasaki K., Aoki T., Ayabe S., Yazaki K. (2009) Molecular cloning and characterization of a cDNA for pterocarpan 4-dimethylallyltransferase catalyzing the key prenylation step in the biosynthesis of glyceollin, a soybean phytoalexin. Plant Physiol. 149: 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Akashi T., Ayabe S. (2000) Flavonoids of leguminous plants: structure, biological activity, and biosynthesis. J. Plant Res. 113: 475–488. [Google Scholar]
- Ayabe S., Akashi T. (2006) Cytochrome P450s in flavonoid metabolism. Phytochem. Rev. 5: 271–282. [Google Scholar]
- Botta B., Vitali A., Menendez P., Misiti D., Delle Monache G. (2005) Prenylated flavonoids: pharmacology and biotechnology. Curr. Med. Chem. 12: 717–739. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Chen R., Liu X., Zou J., Yin Y., Ou B., Li J., et al. (2013) Regio- and stereospecific prenylation of flavonoids by Sophora flavescens prenyltransferase. Adv. Synth. Catal. 355: 1817–1828. [Google Scholar]
- Cheng J., Yuan C., Graham T.L. (2011) Potential defense-related prenylated isoflavones in lactofen-induced soybean. Phytochemistry 72: 875–881. [DOI] [PubMed] [Google Scholar]
- Dewick P.M. (1977) Biosynthesis of pterocarpan phytoalexins in Trifolium pratense. Phytochemistry 16: 93–97. [Google Scholar]
- Fawcett J.A., Maere S., Van de Peer Y. (2009) Plants with double genomes might have had a better chance to survive the Cretaceous–Tertiary extinction event. Proc. Natl. Acad. Sci USA 106: 5737–5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., Fan C., Li H., Zhang Q., Fu Y.F. (2009) Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT–PCR. BMC Mol. Biol. 10: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamat F., Olry A., Munakata R., Koeduka T., Sugiyama A., Paris C., et al. (2014) A coumarin-specific prenyltransferase catalyzes the crucial biosynthetic reaction for furanocoumarin formation in parsley. Plant J. 77: 627–638. [DOI] [PubMed] [Google Scholar]
- Keen N.T., Lyne R.L., Hymowitz T. (1986) Phytoalexin production as a chemosystematic parameter within the genus Glycine. Biochem. Syst. Ecol. 14: 481–486. [Google Scholar]
- Li J., Chen R., Wang R., Liu X., Xie D., Zou J., et al. (2014) GuA6DT, a regiospecific prenyltransferase from Glycyrrhiza uralensis, catalyzes the 6-prenylation of flavones. Chembiochem 15: 1673–1681. [DOI] [PubMed] [Google Scholar]
- Li H., Ban Z., Qin H., Ma L., King A.J., Wang G. (2015) A heteromeric membrane-bound prenyltransferase complex from Humulus lupulus catalyzes three sequential aromatic prenylations in the bitter acid pathway. Plant Physiol. 167: 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P.H., Ko T.P., Wang A.H.J. (2002) Structure, mechanism and function of prenyltransferases. Eur. J. Biochem. 269: 3339–3354. [DOI] [PubMed] [Google Scholar]
- Maul R., Schebb N.H., Kulling S.E. (2008) Application of LC and GC hyphenated with mass spectrometry as tool for characterization of unknown derivatives of isoflavonoids. Anal. Bioanal. Chem. 391: 239–250. [DOI] [PubMed] [Google Scholar]
- Munakata R., Inoue T., Koeduka T., Karamat F., Olry A., Sugiyama A., et al. (2014) Molecular cloning and characterization of a GDP-specific aromatic prenyltransferase from Citrus limon. Plant Physiol. 166: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata R., Olry A., Karamat F., Courdavault V., Sugiyama A., Date Y., et al. (2016) Molecular evolution of parsnip (Pastinaca sativa) membrane-bound prenyltransferases for linear and/or angular furanocoumarin biosynthesis. New Phytol. 211: 332–344. [DOI] [PubMed] [Google Scholar]
- Ober D. (2005) Seeing double: gene duplication and diversification in plant secondary metabolism. Trends Plant Sci. 10: 444–449. [DOI] [PubMed] [Google Scholar]
- Page R.D. (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12: 357–358. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Mito K., Ohara K., Yamamoto H., Yazaki K. (2008) Cloning and characterization of naringenin 8-prenyltransferase, a flavonoid-specific prenyltransferase of Sophora flavescens. Plant Physiol. 146: 1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Tsurumaru Y., Yamamoto H., Yazaki K. (2011) Molecular characterization of a membrane-bound prenyltransferase specific for isoflavone from Sophora flavescens. J. Biol. Chem. 286: 24125–24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai S., Shindo T., Sato S., Kaneko T., Tabata S., Ayabe S., et al. (2006) Functional and structural analysis of genes encoding oxidosqualene cyclases of Lotus japonicus. Plant Sci. 170: 247–257. [Google Scholar]
- Schmutz J., Cannon S.B., Schlueter J., Ma J., Mitros T., Nelson W., et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183. [DOI] [PubMed] [Google Scholar]
- Severin A.J., Cannon S.B., Graham M.M., Grant D., Shoemaker R.C. (2011) Changes in twelve homoeologous genomic regions in soybean following three rounds of polyploidy. Plant Cell 23: 3129–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G., Huhman D., Lei Z., Snyder J., Sumner L.W., Dixon R.A. (2012) Characterization of an isoflavonoid-specific prenyltransferase from Lupinus albus. Plant Physiol. 159: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada N., Aoki T., Sato S., Nakamura Y., Tabata S., Ayabe S. (2003) A cluster of genes encodes the two types of chalcone isomerase involved in the biosynthesis of general flavonoids and legume-specific 5-deoxy(iso)flavonoids in Lotus japonicus. Plant Physiol. 131: 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada N., Sato S., Akashi T., Nakamura Y., Tabata S., Ayabe S., et al. (2007) Genome-wide analyses of the structural gene families involved in the legume-specific 5-deoxyisoflavonoid biosynthesis of Lotus japonicus. DNA Res. 14: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R., Vincken J.P., Bohin M.C., Kuijpers T.F., Verbruggen M.A., Gruppen H. (2011) Identification of prenylated pterocarpans and other isoflavonoids in Rhizopus spp. elicited soya bean seedlings by electrospray ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 25: 55–65. [DOI] [PubMed] [Google Scholar]
- Tahara S., Ibrahim R.K. (1995) Prenylated isoflavonoids—an update. Phytochemistry 38: 1073–1094. [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K., Akashi T., Aoki T. (2015) Functional expression of cytochrome P450 in Escherichia coli: an approach to functional analysis of uncharacterized enzymes for flavonoid biosynthesis. Plant Biotechnol. 32: 205–213. [Google Scholar]
- Van de Peer Y., Fawcett J.A., Proost S., Sterck L., Vandepoele K. (2009) The flowering world: a tale of duplications. Trends Plant Sci. 14: 680–688. [DOI] [PubMed] [Google Scholar]
- Wang R., Chen R., Li J., Liu X., Xie K., Chen D., et al. (2014) Molecular characterization and phylogenetic analysis of two novel regio-specific flavonoid prenyltransferases from Morus alba and Cudrania tricuspidata. J. Biol. Chem. 289: 35815–35825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chu S., Zhu Y., Cheng H., Yu D. (2015) Positive selection drives neofunctionalization of the UbiA prenyltransferase gene family. Plant Mol. Biol. 87: 383–394. [DOI] [PubMed] [Google Scholar]
- Welle R., Grisebach H. (1988) Induction of phytoalexin synthesis in soybean: enzymatic cyclization of prenylated pterocarpans to glyceollin isomers. Arch. Biochem. Biophys. 263: 191–198. [DOI] [PubMed] [Google Scholar]
- Yang X., Jiang Y., Yang J., He J., Sun J., Chen F., et al. (2015) Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci. Technol. 44: 93–104. [Google Scholar]
- Yazaki K., Sasaki K., Tsurumaru Y. (2009) Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 70: 1739–1745. [DOI] [PubMed] [Google Scholar]
- Yuk H.J., Curtis-Long M.J., Ryu H.W., Jang K.C., Seo W.D., Kim J.Y., et al. (2011) Pterocarpan profiles for soybean leaves at different growth stages and investigation of their glycosidase inhibitions. J. Agric. Food Chem. 59: 12683–12690. [DOI] [PubMed] [Google Scholar]