Abstract
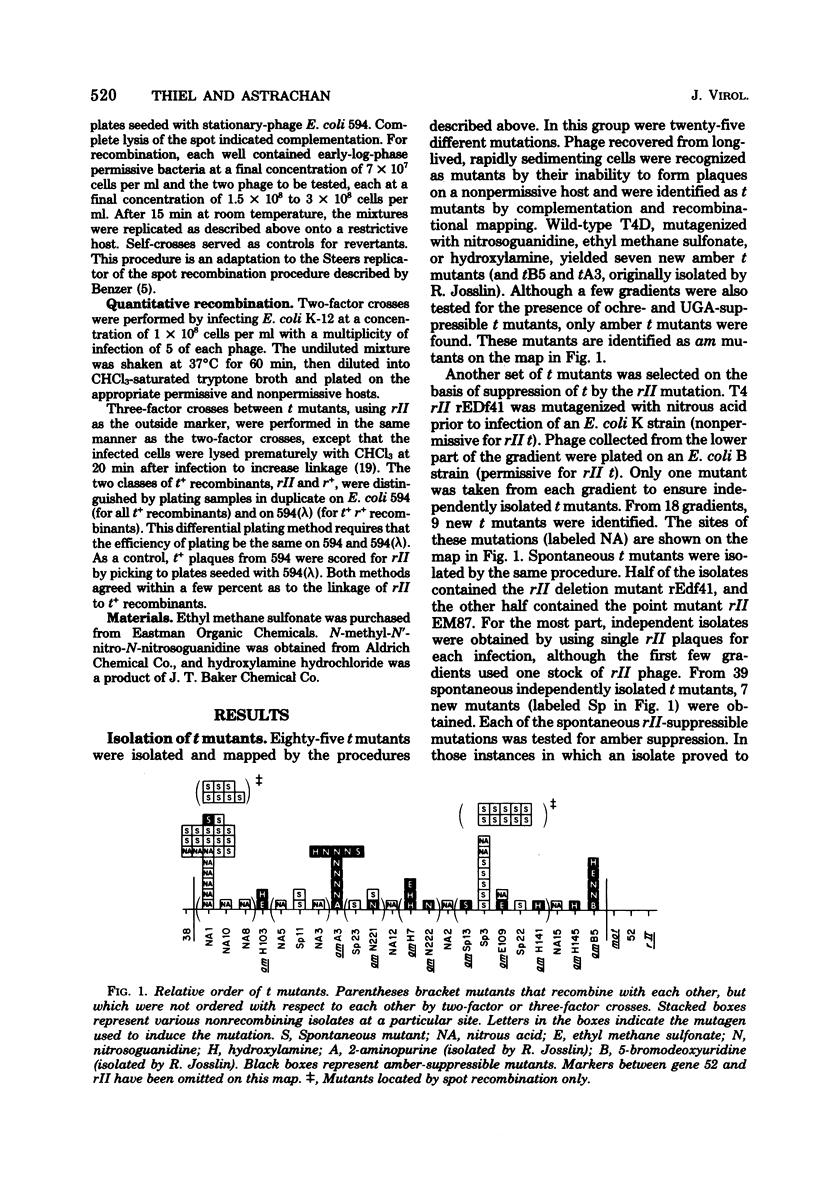
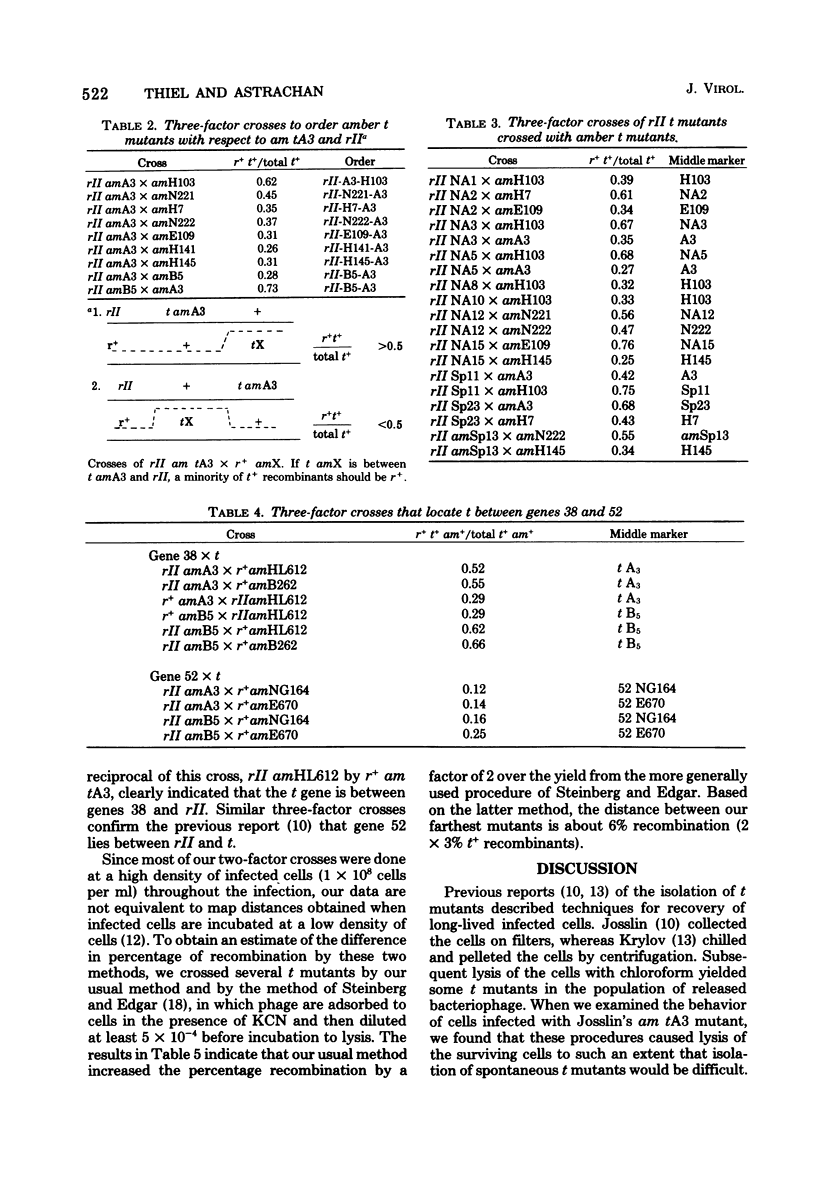
A procedure for selective isolation of T4 t mutants is described. At 120 min after infection of Escherichia coli cells with a low multiplicity of T4 bacteriophage, the mixture was sedimented through a linear sucrose gradient, and infected cells that remained intact were collected as the fastest sedimenting fraction. Ten to 50% of the phage released by chloroform treatment of this fraction were t mutants. Collection of a high proportion of t mutants depended on efficient elimination of cells that would survive because of superinfection lysis inhibition. This was accomplished by early addition of anti-T4 serum and heat-killed cells to inactivate progeny wild-type phage released at the normal burst time. Of 85 t mutants that were isolated and mapped, 23 new mutations were found, 14 of which are suppressible by an rII mutation and 9 of which are suppressible by rII or amber suppressors. Two hot-spot sites for spontaneous mutations were found; 14 mutants at one site, represented by a frameshift mutation, and 12 mutants at a second site were obtained from 39 spontaneous mutants independently isolated from different parental plaques. On our map of the t gene, the distance between the farthest t mutations is 6% recombination. A nonreverting triple t mutant, constructed to contain a frameshift mutation between two amber mutations, exhibited the same t mutant phenotype observed with revertible t mutants.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz W. C., Berger H. Selective allele loss in mixed infections with T4 bacteriophage. Genetics. 1973 Jan;73(1):1–11. doi: 10.1093/genetics/73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. ON THE TOPOGRAPHY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1961 Mar;47(3):403–415. doi: 10.1073/pnas.47.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. ON THE TOPOLOGY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1607–1620. doi: 10.1073/pnas.45.11.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W. The genetic control of spontaneous and induced mutation rates in bacteriophage T4. Genetics. 1973 Apr;73(Suppl):45–64. [PubMed] [Google Scholar]
- Emrich J. Lysis of T4-infected bacteria in the absence of lysozyme. Virology. 1968 May;35(1):158–165. doi: 10.1016/0042-6822(68)90315-2. [DOI] [PubMed] [Google Scholar]
- FOLSOME C. E. Specificity of induction of T4rII mutants by ultraviolet irradiation of extracellular phages. Genetics. 1962 May;47:611–622. doi: 10.1093/genetics/47.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josslin R. The lysis mechanism of phage T4: mutants affecting lysis. Virology. 1970 Mar;40(3):719–726. doi: 10.1016/0042-6822(70)90216-3. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Hamlett N. V., Berger H. Polynucleotide ligase in bacteriophage T4D recombination. Genetics. 1972 Oct;72(2):187–203. doi: 10.1093/genetics/72.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai F., Streisinger G., Miller B. The mechanism of lysis in phage T4-infected cells. Virology. 1967 Nov;33(3):398–404. doi: 10.1016/0042-6822(67)90115-8. [DOI] [PubMed] [Google Scholar]
- Rutberg B. A class of hybrids between bacteriophages T4B and T4D. Virology. 1969 Feb;37(2):243–251. doi: 10.1016/0042-6822(69)90204-9. [DOI] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREISINGER G., MUKAI F., DREYER W. J., MILLER B., HORIUCHI S. Mutations affecting the lysozyme of phage T4. Cold Spring Harb Symp Quant Biol. 1961;26:25–30. doi: 10.1101/sqb.1961.026.01.007. [DOI] [PubMed] [Google Scholar]
- Streisinger G, Bruce V. Linkage of Genetic Markers in Phages T2 and T4. Genetics. 1960 Sep;45(9):1289–1296. doi: 10.1093/genetics/45.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]


