Abstract
The rates of anthropogenic climate change substantially exceed those at which forest ecosystems – dominated by immobile, long-lived organisms – are able to adapt. The resulting maladaptation of forests has potentially detrimental effects on ecosystem functioning. Furthermore, as many forest-dwelling species are highly dependent on the prevailing tree species, a delayed response of the latter to a changing climate can contribute to an extinction debt and mask climate-induced biodiversity loss. However, climate change will likely also intensify forest disturbances. Here, we tested the hypothesis that disturbances foster the reorganization of ecosystems and catalyze the adaptation of forest composition to climate change. Our specific objectives were (i) to quantify the rate of autonomous forest adaptation to climate change, (ii) examine the role of disturbance in the adaptation process, and (iii) investigate spatial differences in climate-induced species turnover in an unmanaged mountain forest landscape (Kalkalpen National Park, Austria). Simulations with a process-based forest landscape model were performed for 36 unique combinations of climate and disturbance scenarios over 1000 years. We found that climate change strongly favored European beech and oak species (currently prevailing in mid- to low-elevation areas), with novel species associations emerging on the landscape. Yet, it took between 357 and 706 years before the landscape attained a dynamic equilibrium with the climate system. Disturbances generally catalyzed adaptation and decreased the time needed to attain equilibrium by up to 211 years. However, while increasing disturbance frequency and severity accelerated adaptation, increasing disturbance size had the opposite effect. Spatial analyses suggest that particularly the lowest and highest elevation areas will be hotspots of future species change. We conclude that the growing maladaptation of forests to climate and the long lead times of autonomous adaptation need to be considered more explicitly in the ongoing efforts to safeguard biodiversity and ecosystem services provisioning.
Keywords: climate change adaptation, forest dynamics, forest ecosystem management, Kalkalpen National Park, natural disturbance, novel ecosystems, species turnover, succession
Introduction
For long-lived organisms such as trees, the rapid progress of anthropogenic climate change (Collins et al., 2013) means that they will experience a distinctly different environment toward the end of their life compared to the conditions under which they have established, resulting in disequilibrium between the vegetation composition and the environment. Such a growing maladaptation of the prevailing vegetation to climate is likely to negatively affect the provisioning of a wide range of ecosystem services to society (Temperli et al., 2012; Lavorel et al., 2015). Furthermore, the occurrence of forest-dwelling species is strongly linked to the prevalence of specific tree species (Bergman et al., 2012; Thom et al., 2016). Increasingly, maladapted forests may thus commit ecosystems to an extinction debt (i.e., a delayed extinction of species due to a protracted response of the ecosystem) (Kitzes & Harte, 2015) and mask the rate and severity of the ongoing biodiversity loss (Thuiller et al., 2005) due to a delayed response of tree species to a changing climate. Consequently, rapid climate change induces high uncertainty into the management of forest ecosystems for the provisioning of ecosystem services and the conservation of biodiversity (Millar et al., 2007; Seidl & Lexer, 2013), as experiences made under relatively constant climatic conditions (with climate and vegetation in equilibrium) are increasingly rendered inapplicable.
Theory suggests that disturbance catalyzes change in ecosystems (Gunderson & Holling, 2001), and can thus reduce the disequilibrium between the prevailing species composition and changing environmental conditions. This notion applies to both natural disturbances in unmanaged systems and silvicultural interventions in managed systems, given that management allows adaptation processes such as natural regeneration to ensue after a disturbance. We here refer to a reduction of the disequilibrium between vegetation composition and climate through natural processes as ‘autonomous adaptation’ (in short referred to as adaptation in the remainder of the text). Processes through which disturbance fosters adaptation include the modification of competition among species, increased resource availability, and a reset of system-level connectedness after disturbance (i.e., a shift from primarily system-internal control mechanisms such as the competition for light to mainly external controls from, for instance, climate and the availability of seeds) (see e.g., Pickett et al., 1989; Pulsford et al., 2016). As a result of these processes, disturbances can initiate ecosystem reorganization by providing opportunities for new species to invade a site, or giving already present but suppressed species the chance to attain dominance.
Studies that have investigated disturbance–climate relationships generally suggest an intensification of natural disturbance activity in the future as a result of climate change (Dale et al., 2001; Seidl et al., 2014a). Hitherto, these disturbance changes have been mainly discussed as a potential threat to ecological resilience (Johnstone et al., 2016; Seidl et al., 2016). However, based on theoretical understanding of ecosystem dynamics (Gunderson & Holling, 2001) such changes can also be hypothesized to facilitate the adaptation of forests to climatic changes, as more disturbance results in a larger share of landscapes being in the state of reorganization that follows after disturbance (see also Serra-Diaz et al., 2015). The increasing level of natural disturbance observed in many ecosystems around the globe currently could thus also be interpreted as a mechanism through which ecosystems reduce a growing maladaptation to a rapidly changing climate. The role of disturbance in shaping future ecosystem composition and reducing the climate–vegetation disequilibrium has as of yet been widely overlooked in the discussion of changing disturbance regimes under climate change.
Yet, changes in key ecosystem processes such as disturbances can also lead to profoundly altered ecosystem dynamics in both natural and managed forests. This has the potential to result in ecological novelty (i.e., dissimilarity of a system relative to a reference baseline Radeloff et al., 2015) and the emergence of no-analog combinations of species (i.e., species communities that did not exist under past climatic conditions Williams & Jackson, 2007). Whether such novel trajectories of ecosystems are compatible with goals of conservation and ecosystem services provisioning remains unclear to date.
Understanding the potential trajectories of forest ecosystems under climate change is thus of paramount importance for ecosystem management. A general proposition frequently found in the ecosystem management literature is that species will shift to higher latitudes and elevations due to global warming (Lenoir et al., 2008; Chen et al., 2011). Such trajectories are confirmed by many studies using empirically calibrated species distribution models (e.g., Hanewinkel et al., 2013; Zimmermann et al., 2013). Species distribution models are powerful tools representing the fundamental niche of a species, and allowing the potential future distribution of a species’ niche to be mapped in relation to projected climatic changes. However, they do assume climate–vegetation equilibrium of the current vegetation, and do not consider relevant processes such as migration and competition among species (Elith & Leathwick, 2009). As a result, important aspects of adaptation such as time lags in the turnover of the current species composition to a changing future climate are disregarded (see e.g., Bertrand et al., 2011; Meier et al., 2012). Furthermore, as the effects of changes in species interactions in response to a changing climate are not considered, shifts in the realized niche of species and the potential rise of novel species communities remain scarcely investigated (but see e.g., Thuiller et al., 2015).
Here, we studied the interactions between vegetation, disturbance, and climate in a complex, unmanaged mountain forest landscape. Our objectives were to (i) assess the time lags in the response of tree species composition and association to changing climatic conditions, (ii) examine the role of disturbance as possible facilitator of this adaptation process, and (iii) study how species turnover in response to different climate and disturbance regimes differs in space. We hypothesized long time lags in the autonomous adaption of the tree species composition (i.e., a trajectory toward the species that are most competitive under the given environmental conditions, without a consideration of management interventions) to climate change (Aitken et al., 2008; Meier et al., 2012). Furthermore, we expected the adaptation processes to be catalyzed by intensifying disturbance activity (e.g., through opening niches for the establishment of locally novel species, eventually resulting in a change in the tree species competition), consequently reducing vegetation-climate disequilibrium. Specifically, we hypothesized a stronger influence of disturbance frequency and size (i.e., creating more and bigger opportunities for reorganization) than disturbance severity (see also Gunderson & Holling, 2001; Turner, 2010). Finally, we hypothesized that changing climate and disturbance regimes create local novelty in forest ecosystems, that is, future tree species compositions and associations that are currently not present at a site, as both processes alter local environmental conditions, the availability of resources, and the competitive relations between species (see also Seastedt et al., 2008). In particular, we expected novelty to be most distinctive at low elevations where no-analog environmental conditions will emerge under climate change (see also Williams & Jackson, 2007).
Materials and methods
Study area
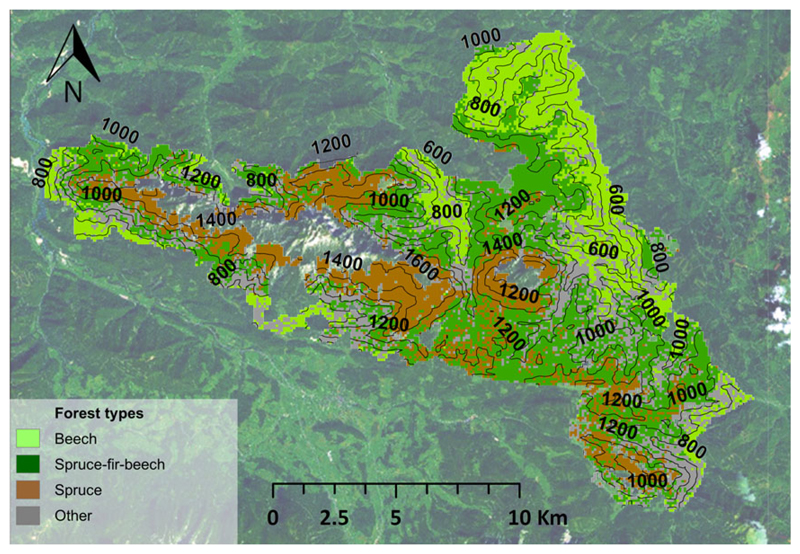
We investigated Kalkalpen National Park (KANP), a forest landscape located at N47.47°, E14.22° in the northern front range of the Austrian Alps. We chose the landscape scale as the focal scale for our analyses, as it allows both large scale processes such as species migration and fine scale processes such as competition between individual trees to be addressed in an integrative manner. In addition to climate and natural disturbance – the processes of interest in this study – forest management is a prominent driver of tree species change (Naudts et al., 2016). We here controlled for the influence of forest management by focusing our analysis on a national park landscape. KANP is the largest forest wilderness in Austria (total area of 20 856 ha) and covers an elevation range from 385 to 1963 m asl. Under current climatic conditions, the landscape encompasses three of the most important forest types of Central Europe, that is, European beech [Fagus sylvatica (L.)] forests, Norway spruce [Picea abies (L.) Karst.] forests, and mixed forests of Norway spruce, silver fir [Abies alba (Mill.)], and European beech (Fig. 1). Since its establishment in 1997, active forest management has ceased in the core zone of KANP.
Fig. 1.
Simulated potential natural vegetation under historical climate at Kalkalpen National Park, showing the three most important forest types. Contour lines indicate elevation above sea level in meters.
Simulation model
We used the individual-based forest landscape and disturbance model iLand to simulate trajectories of tree species change under a range of different climate and disturbance scenarios at KANP. iLand is a high-resolution, process-based model operating on the grain of individual trees, simulating spatially explicit forest landscape dynamics (Seidl et al., 2012a). Processes in iLand are embedded in a hierarchical framework, accounting for interactions between tree (e.g., growth, mortality, competition for resources), stand (availability of water, nutrients), and landscape levels (disturbance, seed dispersal). The resource availability of each individual tree is derived based on a light use efficiency approach (Landsberg & Waring, 1997) combined with ecological field theory (Walker et al., 1989). Resource utilization explicitly considers the effects of temperature, radiation, soil water and nutrient availability as well as vapor pressure deficit at daily time steps, and atmospheric carbon dioxide (CO2) concentration on an annual basis (Seidl et al., 2012a). Individual tree mortality is determined by carbon starvation. Regeneration depends on the local availability of seeds (determined by species-specific seed dispersal kernels around mature trees) as well as on favorable environmental conditions (e.g., light availability, temperature) (Seidl et al., 2012a,b). iLand simulates ecosystem carbon stocks and fluxes and incorporates an agent-based forest management module (Rammer & Seidl, 2015). A wide range of disturbance regimes can be simulated by employing process-based disturbance modules. Currently, iLand includes modules for wildfire (Seidl et al., 2014b), wind (Seidl et al., 2014c), and bark beetle disturbance (Seidl & Rammer, 2016). To study disturbance impacts in detail users can also specify alternative disturbance regimes by specifying distributions for disturbance frequency, size, and severity. The model was successfully parameterized, tested, and applied in ecosystems in Central (Seidl et al., 2012a; Silva Pedro et al., 2015, 2016; Thom et al., 2016) and Northern Europe (Seidl et al., 2014c) as well as in the northwestern United States (Seidl et al., 2012a,b, 2014b). For the current study system, simulated productivity, climate sensitivity, and potential natural vegetation composition were previously evaluated successfully against independent data (Thom et al., 2016).
Initialization and drivers of landscape development
We compiled data for climate, soil, and current vegetation at KANP to initialize and simulate future trajectories of landscape development. Simulations under baseline climate were conducted via resampling years from the period 1950 to 2010. We used three different combinations of global and regional circulation models under A1B forcing as our main projections of transiently changing climatic conditions until the end of the 21th century: CNRM-RM4.5 (Radu et al., 2008) driven by the general circulation models (GCM) ARPEGE, and MPI-REMO (Jacob, 2001) as well as ICTP-RegCM3 (Pal et al., 2007) driven by the GCM ECHAM5. A1B assumes rapid growth of economies and global population, while technological development and policies aim to achieve a balance across fossil and nonfossil energy sources. It is thus often considered to be the ‘business-as-usual’ pathway within the SRES scenario family (IPCC, 2000). To evaluate the sensitivities of our findings to a broader range of climate change scenarios, we furthermore studied the effect of the climate change signal from the least and most severe representative concentration pathway (RCP) scenarios (RCP2.6 and RCP8.5, respectively) provided for Austria (Alder & Hostetler, 2013) based on ECHAM5 – RegCM3. We then tested whether these projections under RCP forcing significantly differed from our findings under A1B forcing regarding the time required to reach dynamic equilibrium (see Fig. S1 and Table S4 in the Supporting Information for details).
From 2100 onwards, we assumed a stabilization of the climate and CO2 at the level of 2080–2099, with years being resampled randomly with replacement. This arbitrary cessation of climate change was assumed here to gauge the time required for the ecosystem to catch up with climate and marks a clear starting point for assessing time lags of vegetation response to a changed climate (see also Solomon et al., 2009). The average climate assumed for the years beyond 2100 in the three A1B projections represents a temperature change of between +3.1 and +3.3 °C, and a precipitation change of between –89 and +141 mm relative to the baseline period. All climate data were bias-corrected by means of quantile mapping (Déqué, 2007) against gridded weather station data at 1 × 1 km resolution (Haiden et al., 2011) and statistically downscaled to a 100 × 100 m grid using daily weather gradients within the study area.
We employed the same 100 × 100 m grid to initialize soil conditions in the simulations. Based on measurements of soil depth and soil type on a regular inventory grid across the KANP (N = 710), Kobler (2004) developed statistical models of soil properties using environmental drivers as predictors. We utilized these models to derive wall-to-wall estimates of soil conditions across the landscape. Additional soil information required for simulation (i.e., soil texture and plant-available nitrogen) was imputed based on a stratified sampling from data derived from the Austrian National Forest Inventory (N = 557) (Seidl et al., 2009).
Information on current tree vegetation was derived from a combination of different data sources. We used aerial photo-analysis and terrestrial inventory plot data (N = 1122) to derive tree species composition, diameter at breast height (dbh) distributions, and stem density at the level of stand polygons (median stand size: 1.4 ha). Stand age was determined from forest inventory and planning data, and airborne LiDAR (Light Detection and Ranging) was used to estimate canopy height as well as the stockable area within a stand. LiDAR data were further utilized to determine the initial tree locations in iLand. Altogether, we initialized more than 2 × 106 trees of 17 different species in stand polygons representing 15 540 ha, characterizing the state of tree vegetation in the year 1999. To accommodate recent disturbance events at KANP, we subsequently simulated tree growth from 1999 to 2013 and imposed the recent disturbance history as recorded in the disturbance monitoring system of KANP (see also Thom et al., 2016 for further details on landscape initialization). The year 2013 was subsequently used as the initial year for all scenario analyses. We assumed a small background probability of seeds from tree species in neighboring forests to enter the KANP landscape (see Table S2 for full list of tree species). The relative abundance of seeds from different tree species was determined from neighboring forest types, and dispersal into the study landscape was tree species specific following the same dispersal kernels as used in the dynamic simulation.
Simulation design
To capture the potentially extensive lead times of tree species adaptation, we simulated forest development at KANP over a period of 1000 years. For each of the four main climate projections described in the previous section, we investigated nine different disturbance scenarios, with one representing a no-disturbance control and the remaining eight imposing combinations of two levels of disturbance frequency, severity, and size (Table 1). Instead of dynamically simulating specific disturbance agents and their interaction with climate change (cf. Seidl & Rammer, 2016), we chose to employ generic disturbance scenarios and implement an orthogonal design in which disturbance frequency, severity, and size are independent of each other and of the imposed climate projections. Studying all factorial combinations of independently varying frequencies, severities, and sizes under all different climate projections allowed us to robustly address our third hypothesis, namely that disturbance frequency and size affect adaptation more strongly than disturbance severity.
Table 1.
Factorial design of disturbance scenarios
| Disturbance scenario R/S/M | Rotation period (years) | Severity (%) | Mean size (ha) | |
|---|---|---|---|---|
| 1 | ∞/0/0 | – | – | – |
| 2 | 250/50/5.3 | 250 | 50 | 5.3 |
| 3 | 125/50/5.3 | 125 | 50 | 5.3 |
| 4 | 250/100/5.3 | 250 | 100 | 5.3 |
| 5 | 125/100/5.3 | 125 | 100 | 5.3 |
| 6 | 250/50/53.4 | 250 | 50 | 53.4 |
| 7 | 125/50/53.4 | 125 | 50 | 53.4 |
| 8 | 250/100/53.4 | 250 | 100 | 53.4 |
| 9 | 125/100/53.4 | 125 | 100 | 53.4 |
The disturbance scenarios represent all possible combinations of two levels of disturbance frequency (here expressed as disturbance rotation period), severity, and mean disturbance size. Severity is characterized as trees with dbh >10 cm killed within the disturbed perimeter.
Our low-intensity disturbance variant (disturbance scenario 2, Table 1) assumed a disturbance rotation period of 250 years (see Thom et al., 2013), a mean disturbance size of 5.3 ha (corresponding to the current mean disturbance size as determined from the disturbance inventory at KANP), and a disturbance severity of 50% (i.e., 50% of trees with dbh >10 cm are killed within the disturbed perimeter; cf. Janda et al. (2016), who report average severities ranging from 29.0% to 74.9% of canopy removed in Central European mountain forests). This disturbance scenario thus roughly corresponds to the current disturbance regime at KANP, which is mainly dominated by wind and bark beetles (cf. Seidl & Rammer, 2016). In the disturbance scenario with the highest intensity (disturbance scenario 9), disturbance rotation period was halved to 125 years, severity doubled to 100%, and disturbance size increased tenfold to a mean size of 53.4 ha. This extreme scenario thus represents the possibility of drastically changed disturbance regimes under future climate conditions (see Seidl & Rammer 2016 for an analysis of the climate sensitivity of wind and bark beetle disturbances at KANP). The location of disturbances was implemented stochastically in each simulation run, with disturbance size determined by drawing from a negative exponential distribution with the respective mean size. Whether one or more disturbance events occurred in a simulated year was determined by a probability function based on disturbance rotation period and disturbance size. To account for stochasticity in our analyses, we conducted 10 replicated simulations per scenario. To scrutinize the robustness of our results in the face of stochasticity, we investigated the between-replicate coefficient of variation at the level of individual tree species (see Table S1 for details). In summary, our simulation design consisted of 4 climate projections × 9 disturbance scenarios × 10 replicates = 360 simulations of the 15 540 ha landscape over 1000 years (i.e., 5.59 billion hectare-years simulated). The factorial design allowed us to separate the effects of different climate forcings from the different constituents of the disturbance regime (rotation period, severity, size) with regard to their influence on forest composition.
Analyses
We tested for differences in the share of individual tree species among climate and disturbance scenarios at the end of the simulation period at a spatial grain of 100 m cells, using Wilcoxon rank-sum test. As an indicator for the speed of adaptation to changing climatic conditions, we used the elapsed time until the simulated landscape reached a dynamic equilibrium in species composition, that is, the point in time after which fluctuations in tree species composition were negligible (White & Jentsch, 2001). We expected all simulations to eventually reach a dynamic equilibrium, as our landscape was large relative to disturbance frequency and size (on average none of the disturbance scenarios affected more than 1% of landscape area per year) (Turner et al., 1993), and we assumed a stabilization of climate change beyond year 2100. We considered a simulation as being in dynamic equilibrium when the temporal variation in basal area of all species present on the landscape was within a range of ±2 m2 ha−1 of their respective simulation endpoint (defined as the average basal area per tree species in the last 200 years of the simulation). To assess the influence of this a priori set equilibrium definition on our findings, we conducted a sensitivity analysis, investigating the effect of different threshold values (±1.5 and ±2.5 m2 ha−1, respectively) on the estimated time until a dynamic equilibrium was reached.
Furthermore, we estimated migration speed in elevation as the mean annual change rate of a species’ leading edge, defined as the 90th percentile of the elevation distribution of a species on the landscape. To test changes in the elevation distribution of species, we derived species shares for each 100 m cell and approximated the altitudinal distribution of each species using either a Gaussian (if a clear optimum was present) or a 2nd order polynomial function. Moreover, we asked whether species do change individually or in associations, using Spearman’s rank correlation analysis to quantify the strength of association between species (and changes therein) at the same 100-m spatial grain.
To obtain a more mechanistic insight into the effect of disturbance on tree species adaptation, we utilized our set of orthogonal disturbance scenarios to test for differences between disturbance extent (i.e., percent area disturbed) and disturbance impact (i.e., percent basal area disturbed) on the emergence of a dynamic landscape equilibrium.
Finally, we analyzed the emerging tree species compositions under different climate and disturbance regimes with regard to their local novelty compared to baseline conditions. Specifically, we quantified species turnover triggered by climate change, that is, the deviation of tree species shares under climate change from baseline values at the end of the simulation period, and its distribution across the landscape. To that end, we summed the differences in tree species shares between scenarios across all species and divided by two to account for the fact that a 10% increase in one species ipso facto has to lead to a 10% decrease in another species. The theoretical maximum species turnover was thus 100% for each cell, where 100% indicated that the tree species composition under climate change contains no species of the composition simulated under baseline conditions (see also Radeloff et al., 2015).
Results
Temporal trajectories of forest succession
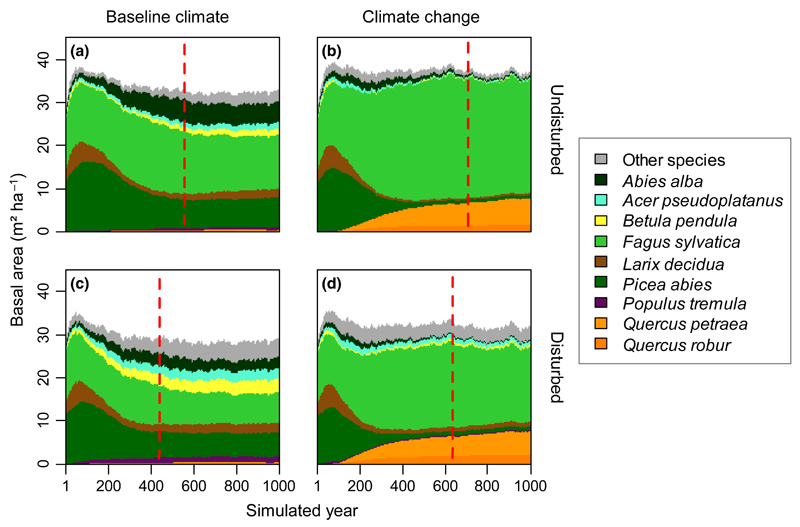
Forest management activities prior to 1997 and recent severe disturbances (most notably the storm Kyrill in 2007) have significantly affected KANP. Consequently, all scenarios resulted in a considerable increase in mean basal area during the first 50–100 years relative to the current forest conditions (Fig. 2). Although the most drastic tree species changes occurred during the first centuries of the simulation, we found that particularly European beech and oak species [Qercus petraea (Matt.) and Quercus robur (L.)] were slow to stabilize on the landscape. Our simulations indicated a distinct impact of climate change on forest succession (Fig. 2; Table S2). While the average time to reach dynamic equilibrium under baseline climate conditions was 558 years (Fig. 2a), a changing climate prolonged the time needed for the landscape to be in dynamic equilibrium with its environment by 148 years on average if disturbance was neglected (Fig. 2b). At the landscape scale, European beech benefited most strongly from changing climate conditions (P < 0.001) and increased its tree species share by +28.5 and +32.2 percentage points compared to baseline climate for scenarios with and without disturbances, respectively. Although negligible under baseline climate, the abundance of oaks strongly increased with warming (+20.0 and +22.9 percentage points for Q. petraea and Q. robur combined; P < 0.001). Norway spruce, one of the main tree species at KANP under baseline climate, strongly decreased within the first 300–400 years under climate change, and only held a minor share of the tree species composition at the end of the simulation period (−19.4 and −16.7 percentage points, respectively, P < 0.001). Conversely, silver fir was suppressed by past forest management and recovered during the first centuries of the simulation under baseline climate. However, similarly to Norway spruce, it declined substantially under climate change conditions (−13.5 and −8.1 percentage points, respectively, P < 0.001).
Fig. 2.
Trajectories of forest succession at KANP, summarized for four scenarios: (a) baseline climate without disturbance, (b) climate change without disturbance, (c) baseline climate with disturbance, and (d) climate change with disturbance. Values are averages over all scenarios and replicates in the respective categories. Climate change includes projections for all three A1B scenarios. Tree species with ≥5% basal area in at least one of the four scenarios are shown explicitly (see Table S2 for more information). The red-dashed line indicates the point in time when a dynamic equilibrium was reached on average.
The role of disturbance in forest adaptation to changing environmental conditions
In addition to climate, disturbance also strongly influenced the trajectories of forest succession at the landscape scale. Overall, disturbance strongly favored early-seral species (most notably silver birch (Betula pendula [Roth]), European larch (Larix decidua [Mill.]), sycamore maple (Acer pseudoplatanus [L.]), and aspen (Populus tremula [L.])) at the expense of late successional species (Fig. 2c, d; Table S2). While European beech was most positively affected by climate change among all tree species, it was also the species affected most severely by disturbances under both baseline and climate change conditions (a reduction of −13.4 and −17.1 percentage points under baseline and climate change conditions, respectively, P < 0.001). Also silver fir declined substantially by −5.8% under baseline climate conditions as a result of disturbance (P < 0.001). Disturbances reduced the time to reach dynamic equilibrium by on average 119 years (21.3%) under baseline climate conditions (Fig. 2c) and by 72 years (10.2%) under changed climatic conditions (Fig. 2d).
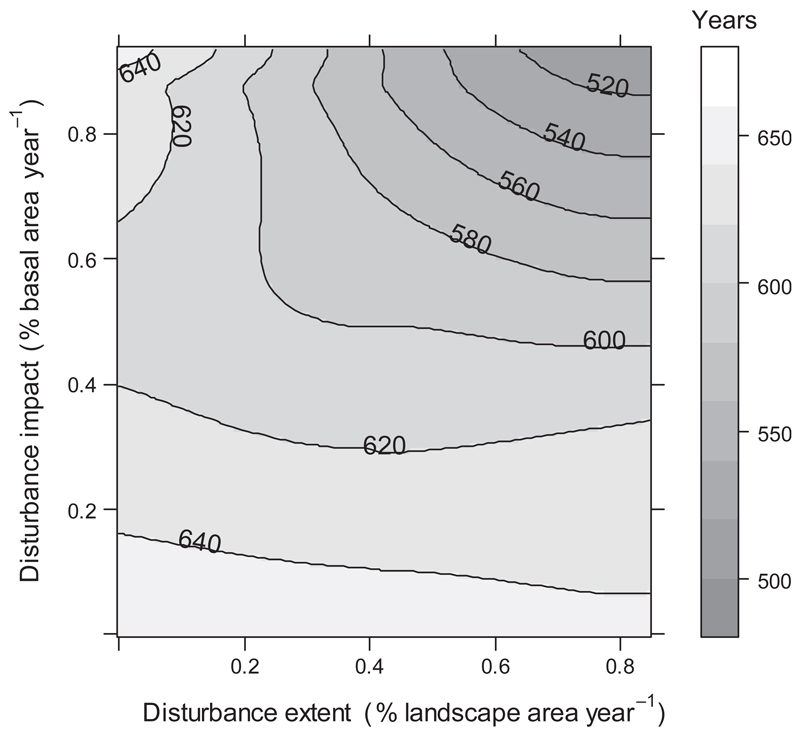
A subsequent in-depth analysis at the level of the individual constituents of the disturbance regime revealed that both disturbance extent and impact had an important influence on the adaptation of tree species to changing environmental conditions (Fig. 3). For instance, the adaptation speed was accelerated by more than 100 years compared to undisturbed conditions when disturbance affected 0.8% of basal area and 0.8% of landscape extent per year. Specifically, we found synergistic effects between disturbance extent and impact – if the disturbance impact on basal area was low, the spatial extent of disturbance had only a weak influence on the speed of adaptation. With increasing disturbance impact, however, the effect of an increasing spatial extent of disturbance also increased. Likewise, an increase in disturbance extent also amplified the effect of disturbance impact on the adaptation speed of the system.
Fig. 3.
Simulated time until the tree species composition at KANP is fully adapted to climate, as influenced by disturbance impact (in % of basal area removed per year) and disturbance extent (in % of the total area of KANP disturbed per year). Isolines indicate the time needed (in years) to reach a dynamic equilibrium in the tree species composition and were derived via a loess regression. For the current analysis, climate was assumed to change until the end of the 21st century and kept stable afterward, allowing vegetation to catch up.
While an increasing area affected by disturbance promoted forest succession, the speed of adaptation was found to be negatively related to mean disturbance size in most scenarios (Table 2). Overall, scenarios with a tenfold increase in mean disturbance size (to 53.4 ha) prolonged the time it took for the landscape to reach dynamic equilibrium by +24.8 years on average, compared to scenarios that assumed the historically observed mean disturbance size of 5.3 ha. However, when increased disturbance size also coincided with high severity and frequency adaptation was further slowed, and it took on average +71 years longer for the landscape to reach a dynamic equilibrium with climate (cf. scenarios 5 and 9 in Table 2). In contrast, both increased severity (−64.3 years compared to current severity) and increased frequency (−54.5 years compared to current frequency) generally accelerated forest adaptation. Tree species composition at KANP adapted most quickly to the new climatic conditions in disturbance scenario 5 (disturbance rotation period 125 years, severity 100% and mean size 5.3 ha). In this scenario, species adaptation was 201 years (baseline) and 211 years (climate change) faster than in scenarios excluding disturbances.
Table 2.
The time the tree species composition needs to adapt to changing environmental conditions
| Disturbance scenario R/S/Ms | Baseline climate |
Climate change |
|||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| 1 | ∞/0/0 | 558 | 0 | 706 | 111 |
| 2 | 250/50/5.3 | 497 | 15 | 659 | 50 |
| 3 | 125/50/5.3 | 450 | 10 | 655 | 51 |
| 4 | 250/100/5.3 | 434 | 8 | 647 | 43 |
| 5 | 125/100/5.3 | 357 | 12 | 495 | 166 |
| 6 | 250/50/53.4 | 495 | 20 | 670 | 78 |
| 7 | 125/50/53.4 | 465 | 22 | 659 | 78 |
| 8 | 250/100/53.4 | 434 | 33 | 675 | 90 |
| 9 | 125/100/53.4 | 384 | 27 | 610 | 110 |
Shown are the average years as well as standard deviations (SD) per disturbance scenario that are required until a dynamic equilibrium in the vegetation composition was reached on the landscape. Climate was assumed to change until the end of the 21st century and kept stable afterward, allowing vegetation to catch up.
A sensitivity analysis of the equilibrium definition used here showed that our results were generally robust to different threshold levels for defining stabilization in the species composition, but also revealed considerable sensitivity of the time needed for adaptation (Tables S3.1 and S3.2). Furthermore, assuming more extreme climatic change (scenario RCP8.5) prolonged the time needed to adaptation by 68 years, while more moderate future climate conditions accelerated it by 19 years on average across all disturbance scenarios (Table S4). These more extreme scenarios did, however, not differ significantly from the ensemble of climate change projections studied here in 17 of the 18 scenarios and did not alter the overall results regarding the disturbance effect on tree species adaptation.
Species shifts and novel species compositions
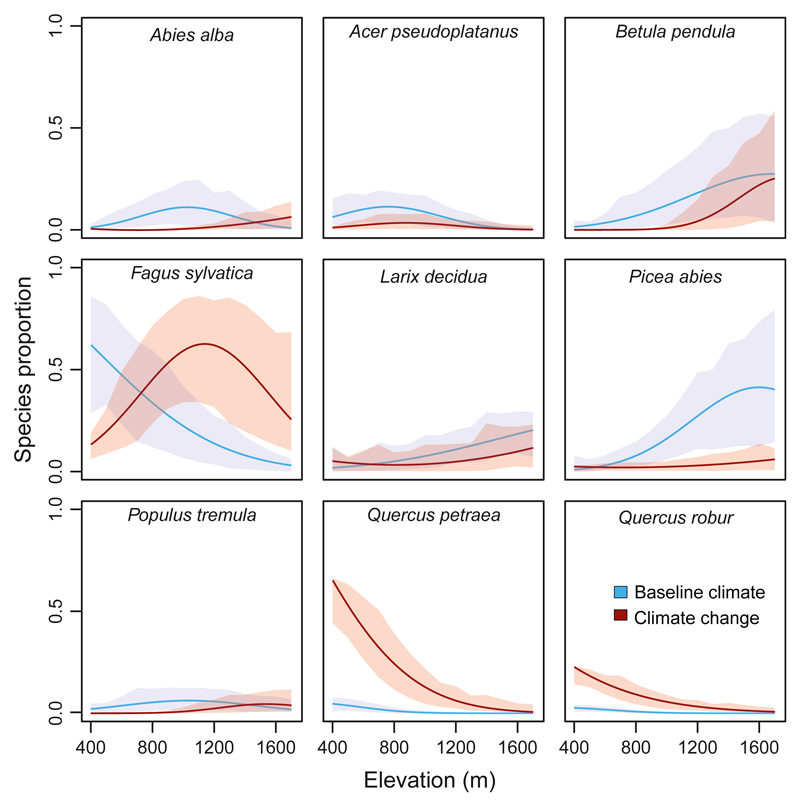
Not all tree species shifted upwards in elevation in response to warming temperatures. While most species, such as European beech (+0.26 m asl yr−1), oak species (+0.24 m and +0.27 m asl yr−1 for Q. petraea and Q. robur, respectively), and silver fir (+0.37 m asl yr−1), migrated upwards with climate change, the leading edges of the distribution of Norway spruce (−0.02 m asl yr−1) and European larch (+0.00 m asl yr−1) remained virtually constant compared to baseline climate. To further elucidate changes in species over elevation, we examined their distributions over elevation at the end of the simulation (Fig. 4). This analysis showed that oak species (mainly Q. petraea) invaded the elevation band that was formerly beech dominated (cf. also Fig. S2.4 with Figs S2.8 and S2.9). Norway spruce and European larch, on the other hand, which were prominent tree species at higher elevations under baseline climate, became scarce under changed climatic conditions even at their past elevation optima (Figs S2.5 and S2.6).
Fig. 4.
Distribution of the nine most common tree species over elevation at Kalkalpen National Park at the end of the 1000 year simulation period (a species proportion of 1 indicates a basal area share of 100%). Lines represent fitted distributions over simulated species occurrence. Shaded areas indicate the 5th to 95th percentile range across all replicates and disturbance scenarios.
Our analyses also indicated that climate change modified species associations as a result of changing competitive relationships among species (Tables 3 and 4). For instance, we found a substantial change in the association between European beech and oak species: While they were clearly positively related under baseline climate conditions (indicating co-occurrence in the warmer, low-elevation areas of the landscape), they were moderately negatively associated under changed climatic conditions (indicating growing niche separation, with oaks outcompeting beech in the exceedingly warm low-elevation parts of the landscape, and beech moving into higher elevation portions of the landscape). The relation between European beech and Norway spruce, on the other hand, became less negative under climate change, and the association of silver fir with European beech and Norway spruce remained positive. Correlations among early successional species such as silver birch, European larch, and European aspen were positive under both baseline climate and climate change scenarios.
Table 3.
Association between tree species under baseline climate conditions
| Species | Abies alba | Acer pseudoplatanus | Betula pendula | Fagus sylvatica | Larix decidua | Picea abies | Populus tremula | Quercus petraea | Quercus robur |
|---|---|---|---|---|---|---|---|---|---|
| A. alba | 1 | ||||||||
| A. pseudoplatanus | 0.17 | 1 | |||||||
| B. pendula | −0.385 | −0.495 | 1 | ||||||
| F. sylvatica | 0.446 | 0.477 | −0.865 | 1 | |||||
| L. decidua | −0.417 | −0.658 | 0.754 | −0.781 | 1 | ||||
| P. abies | 0.112 | −0.578 | 0.449 | −0.634 | 0.623 | 1 | |||
| P. tremula | −0.355 | 0.212 | 0.637 | −0.468 | 0.269 | −0.121 | 1 | ||
| Q. petraea | −0.063 | 0.44 | −0.524 | 0.656 | −0.456 | −0.687 | −0.097 | 1 | |
| Q. robur | −0.063 | 0.448 | −0.519 | 0.663 | −0.467 | −0.7 | −0.082 | 0.925 | 1 |
Shown is Spearman’s rank correlation coefficient, derived from all scenario combinations simulated.
Table 4.
Association between tree species under changed climatic conditions
| Species | Abies alba | Acer pseudoplatanus | Betula pendula | Fagus sylvatica | Larix decidua | Picea abies | Populus tremula | Quercus petraea | Quercus robur |
|---|---|---|---|---|---|---|---|---|---|
| A. alba | 1 | ||||||||
| A. pseudoplatanus | −0.313 | 1 | |||||||
| B. pendula | 0.809 | −0.175 | 1 | ||||||
| F. sylvatica | 0.454 | −0.305 | 0.154 | 1 | |||||
| L. decidua | 0.055 | 0.086 | 0.402 | −0.625 | 1 | ||||
| P. abies | 0.182 | 0.107 | 0.324 | −0.348 | 0.69 | 1 | |||
| P. tremula | 0.772 | −0.045 | 0.969 | 0.134 | 0.378 | 0.287 | 1 | ||
| Q. petraea | −0.856 | 0.178 | −0.876 | −0.358 | −0.2 | −0.219 | −0.854 | 1 | |
| Q. robur | −0.843 | 0.297 | −0.78 | −0.508 | −0.013 | −0.097 | −0.746 | 0.952 | 1 |
Shown is Spearman’s rank correlation coefficient, derived from all scenario combinations simulated.
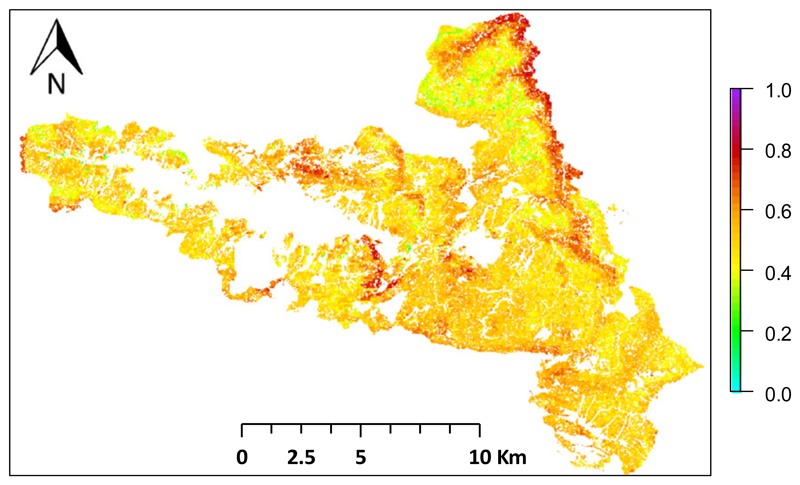
These changes in individual species ranges and species associations resulted in the emergence of local novelty in tree species composition. Comparing baseline climate under the current disturbance regime (scenario 2, Table 1) with climate change under intensified disturbance (scenario 9) revealed a species turnover of 51.8% (CI ± 16.5%), indicating that more than half of the basal area of every stand will on average be replaced by novel tree species under changing climate and disturbance regimes (Fig. 5). In all scenarios, species turnover ‘hotspots’ showed a distinct bimodal distribution over elevation, with most pronounced changes being simulated at the lowest and highest elevations. However, disturbances dampened this spatial pattern of novel tree species compositions on the landscape and resulted in species turnover being more evenly distributed across the landscape cf. (Fig. S3).
Fig. 5.
Species turnover as a result of changing climate and disturbance regimes. The map shows local species change (100 × 100 m grid) as a consequence of climate change and intensified disturbances (disturbance scenario 9) compared to baseline climate under historic disturbance (disturbance scenario 2). A value of 1 indicates a replacement of 100% of the trees relative to baseline climate.
Discussion
Drivers of forest adaptation
Climate change will strongly modify environmental conditions within the lifetime of a single generation of trees, and thus results in high uncertainty with regard to the spatiotemporal trajectories of forest ecosystems (see also Manusch et al., 2014). Here, we showed that the adaptation of forests to these changing conditions can lag several centuries behind climatic trajectories, but also that disturbances significantly decrease the temporal mismatch between the vegetation composition and the climate system. To our knowledge, we here presented the first comprehensive analysis on how changes in disturbance frequency, severity, and size could influence the growing disequilibrium between forest vegetation and the climate system. Our study highlights the importance of landscape-scale processes such as seed dispersal and disturbance to understand how forest ecosystems will change with progressing climate change. The process-based modeling approach employed here did not only track the fundamental niche of species, but also simulated species movement and establishment, as well as changing relative resource use efficiency between species. Consequently, we were able to not only account for direct (e.g., temperature), but also indirect (e.g., changing competition) impacts of climate change. A remaining limitation of our modeling approach is the omission of within-species variation. The considerable ecophysiological plasticity within species, for instance, could dampen vegetation changes in response to climate (Fady et al., 2016).
As expected, we found the current vegetation composition at NPKA to differ considerably from the potential natural vegetation derived under current climate conditions. As in many parts of Central Europe (e.g., Emmer et al., 1998; Knoke et al., 2005), historic management has strongly favored Norway spruce at the expense of European beech in our study area. Although active management has ceased at NPKA almost 20 years ago, the current tree species composition is still to a large degree the legacy of past land use. Hence forests at KANP are currently in disequilibrium with the prevailing climate conditions. Our simulations suggest that it takes several centuries before the vegetation composition is in a dynamic equilibrium with climate conditions at NPKA even in the absence of a changing climate. This comparably slow return to a natural tree species composition suggests that compositional changes in recently installed protected areas that have previously been altered by humans are currently more strongly driven by recovery from past land use than by climate change. It also underlines that in some areas targeted ecosystem restoration measures could be of value to accelerate the trajectory toward natural system states after intensive human alteration (e.g., Covington et al., 1997; Zerbe, 2002).
The expected climate change will have a strong impact on the natural vegetation composition in Central European forest landscapes and prolongs the time it takes for the current vegetation to adapt to its environment. Under past climate lower elevations were dominated by European beech, higher elevations by Norway spruce, and mid-elevation areas by a mixture of European beech, Norway spruce, and silver fir. This spatial pattern changed under climate change in our simulations, with European beech dominating large parts of the landscape and oak invading the lower reaches of KANP once a dynamic equilibrium between climate and vegetation was reached. These findings are in line with broad-scale studies using species distribution models (SDMs) (e.g., Hanewinkel et al., 2013; Zimmermann et al., 2013), but only emerged after century-long time lags in our spatiotemporally explicit analysis, confirming the hypothesis of a highly protracted tree species adaptation to changing climatic conditions. In this regard, it is interesting to note that recent paleoecological work even suggested that forest ecosystems may remain in disequilibrium with climate for several millennia (Herzschuh et al., 2016). Our analysis revealed mean annual change rates in elevation of the most common tree species from −0.02 to +0.37 m asl yr−1 under climate change. These change rates are below the majority of woody species’ change rates reported by Lenoir et al. (2008), who investigated elevational optimum shifts of plants in France from 1905 to 2005, but in contrast to our study, they also included ecosystems that have been continuously managed, while we excluded direct human interventions in our analysis. Differences in methodology for determining elevational change rates might also account for some of the divergence, while overall our estimates still fall within the large variation reported empirically (Lenoir et al., 2008). Furthermore, for the interpretation of our findings, it is important to note that elevational shifts in our analysis were also to some degree limited by the specific topographic conditions of our study landscape. Norway spruce and European larch, for instance, were already occupying the highest elevation areas of the landscape under baseline climate and were thus no more able to expand their territory upwards.
Our results generally support the expectation of a facilitating effect of disturbance on species adaptation. Because climate change is predicted to intensify future disturbance activity (Seidl et al., 2014a; Millar & Stephenson, 2015), it is likely that disturbances will reduce the climate – vegetation disequilibrium in forest ecosystems. In this regard, we here were able to disentangle the effects of changes in disturbance rotation period, severity, and size using a factorial simulation experiment. We found that increases in the different characteristics of the disturbance regime affected the compositional maladaptation to climate conditions in distinctly different ways: While increases in disturbance frequency and severity reduced the lag times of autonomous adaptation, increasing disturbance size prolonged the time it took for the landscape to reach a dynamic equilibrium. An increasing disturbance frequency implies an accelerated progression through the adaptive cycle of ecosystem dynamics, and a larger proportion of the landscape in the stages of reorganization and renewal (Gunderson & Holling, 2001). Larger sizes of high severity disturbance events, on the other hand, result in increased dispersal distances for species, which slows recovery and favors early-seral species over the slowly invading cohort of new late-seral species (Seidl et al., 2014b). This finding on the differential effects of changes in attributes of the disturbance regime suggests that the potential positive effect of greater disturbance activity on species adaptation will be contingent on the nature of disturbance change affecting the landscape. While an increase in disturbance frequency, as projected for many areas (Mouillot et al., 2002; Bentz et al., 2010) might aid adaptation, the emergence of ‘megadisturbances’ (Stephens et al., 2014; Millar & Stephenson, 2015) could further aggravate the maladaptation of vegetation to the emerging climate conditions.
A limitation of our study in this regard was the assumption of equal disturbance sensitivities between species. Wind and bark beetles are the most prominent disturbance agents in Central Europe, and a high share of Norway spruce also implicates a high predisposition of forests to those agents (Thom et al., 2013). This suggests that the abundance of particularly disturbance-sensitive species on the landscape might be over-estimated in our analysis. Future work should address this issue by investigating the effects of prominent disturbance agents on landscape trajectories explicitly and account for dynamic feedbacks between vegetation change and the disturbance regime (see e.g., Temperli et al., 2013; Seidl & Rammer, 2016).
Future forests and ecological novelty
In line with previous research, we found early-seral species to benefit from higher levels of disturbance, as they are better able to recolonize disturbed parts of the landscape (Swanson et al., 2011). This suggests that under the changed disturbance regimes expected for the future, early-seral species might play a more prominent role in forest dynamics than in the past. For the landscape investigated here our results suggests a homogenization of the tree species composition as a result of climate change, with European beech-dominated forest types strongly increasing their prevalence over other forest types. This effect is, however, partly offset by disturbances, which foster tree species diversity on the landscape (Silva Pedro et al., 2016). These trends are of particular relevance in the context of biodiversity conservation, as tree species diversity is an important predictor for the diversity of a wide variety of other species groups (see e.g., Díaz et al., 2005; Thom et al., 2016).
In this context, another important finding of our analysis is the rise of local novelty as a result of climate change. We found a high turnover of species for the large majority of locations within our study landscape (93.2% of the landscape had a species turn-over of >40%, Fig. 5). While on average species turn-over rates were similar in disturbed and undisturbed simulations, we found that neglecting disturbance leads to an overestimation of novelty in high- and low-elevation areas of KANP (Fig. S3). Furthermore, we found that previous associations of species changed considerably, as tree species responded individually to the emerging climate conditions (see also Hanson & Weltzin, 2000). An aspect we did not account for in the analysis of emerging novel ecosystem composition, however, was the potential for an invasion of alien tree species (see e.g., Radeloff et al., 2015). However, as we here focus on a national park where anthropogenic disturbances are minimized and no exotic tree species will be introduced through management, this omission might be of minor importance for our study system. Nonetheless, specifically the role of disturbances on invasion of alien species requires further attention. While anthropogenic disturbances are generally regarded as catalysts of the invasion of nonnative species (Chytrý et al., 2008; Pysek et al., 2010), only a limited number of studies have assessed the impact of natural disturbances in this regard (see e.g., Davies et al., 2009).
Forest management implications
The uncertainty in future trajectories of forest ecosystems resulting from the rapidly changing environmental conditions poses a key challenge for forest management and conservation (Millar et al., 2007). Our study suggests that disturbance is an important facilitator of the autonomous adaptation of forest ecosystems to changing environmental conditions. We thus suggest that disturbances should be seen as opportunity for forest adaptation in the context of management (see also Seidl et al., 2016). As the slow migration of trees does not allow them to track rapid climatic changes (see also McLachlan et al., 2005), active management should be considered to shorten the extensive lead times, in order to sustain biodiversity and the provisioning of ecosystem services in the future (Seidl et al., 2011). In this regard, our results suggest that particularly forests in low- and high-elevation zones are highly vulnerable to climate change-induced species change, and should thus be in the focus of monitoring and management. However, our findings also showed that changes might be considerably slower than suggested by species distribution models (e.g., Hanewinkel et al., 2013). In fact, our study indicated the absence of strong climate-induced changes in the tree species composition over the next 100 years compared to baseline climate. However, this slow response of forests must not be mistaken for insensitivity or robustness of forests to a changing climate, but rather indicates a growing disequilibrium between forests and climate, and commits forests to strong future alterations in species composition. The effects of this growing maladaptation on biodiversity and ecosystem services provisioning are currently not sufficiently understood, yet will likely be a major factor to consider in the ecosystem management of the coming decades.
Supplementary Material
Acknowledgements
This study was supported by the Austrian Science Fund FWF (grant P 25503-B16). R. Seidl acknowledges further support through a Marie Curie Career Integration Grant of the European Community (PCIG12-GA-2012 334104). The simulation results presented here have been generated at the Vienna Scientific Cluster (VSC). We thank three anonymous Reviewers for their helpful comments on an earlier version of the manuscript.
References
- Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Applications. 2008;1:95–111. doi: 10.1111/j.1752-4571.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JR, Hostetler SW. CMIP5 Global Climate Change Viewer. [accessed 8 July 2016];US Geological survey. 2013 doi: 10.5066/F72J68W0. Available at: http://regclim.coas.oregonstate.edu/gccv/index.html. [DOI] [Google Scholar]
- Bentz BJ, Régnière J, Fettig CJ, et al. Climate change and bark beetles of the western United States and Canada: direct and indirect effects. BioScience. 2010;60:602–613. [Google Scholar]
- Bergman K-O, Jansson N, Claesson K, Palmer MW, Milberg P. How much and at what scale? Multiscale analyses as decision support for conservation of saproxylic oak beetles. Forest Ecology and Management. 2012;265:133–141. [Google Scholar]
- Bertrand R, Lenoir J, Piedallu C, et al. Changes in plant community composition lag behind climate warming in lowland forests. Nature. 2011;479:517–520. doi: 10.1038/nature10548. [DOI] [PubMed] [Google Scholar]
- Chen I, Hill JK, Ohlemuller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- Chytrý M, Jarošík V, Pyšek P, Hájek O, Knollová I, Tichý L, Danihelka J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology. 2008;89:1541–1553. doi: 10.1890/07-0682.1. [DOI] [PubMed] [Google Scholar]
- Collins M, Knutti R, Arblaster J, et al. Long-term climate change: projections, commitments and irreversibility. In: Stocker TF, Qin D, Plattner G-K, et al., editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK and New York, NY, USA: 2013. pp. 1029–1136. [Google Scholar]
- Covington W, Fule P, Moore M, et al. Restoring ecosystem health in ponderosa pine forests of the southwest. Journal of Forestry. 1997;95:23–29. [Google Scholar]
- Dale VH, Joyce LA, Mcnulty S, et al. Climate Change and Forest Disturbances. BioScience. 2001;51:723. [Google Scholar]
- Davies KW, Svejcar TJ, Bates JD. Interaction of historical and nonhistorical disturbances maintains native plant communities. Ecological Applications. 2009;19:1536–1545. doi: 10.1890/09-0111.1. [DOI] [PubMed] [Google Scholar]
- Déqué M. Frequency of precipitation and temperature extremes over France in an anthropogenic scenario: model results and statistical correction according to observed values. Global and Planetary Change. 2007;57:16–26. [Google Scholar]
- Díaz I, Armesto J, Reid S, Sieving K, Willson M. Linking forest structure and composition: avian diversity in successional forests of Chiloé Island, Chile. Biological Conservation. 2005;123:91–101. [Google Scholar]
- Elith J, Leathwick JR. Species distribution models: ecological explanation and prediction across space and time. Annual Review of Ecology, Evolution, and Systematics. 2009;40:677–697. [Google Scholar]
- Emmer IM, Fanta J, Kobus AT, Kooijman A, Sevink J. Reversing borealization as a means to restore biodiversity in Central-European mountain forests – an example from the Krkonoše Mountains, Czech Republic. Biodiversity and Conservation. 1998;7:229–247. [Google Scholar]
- Fady B, Aravanopoulos FA, Alizoti P, et al. Evolution-based approach needed for the conservation and silviculture of peripheral forest tree populations. Forest Ecology and Management. 2016;375:66–75. [Google Scholar]
- Gunderson LH, Holling CS. Panarchy: Understanding Transformations in Human and Natural Systems. Island Press; Washington, Covelo, London: 2001. [Google Scholar]
- Haiden T, Kann A, Wittmann C, Pistotnik G, Bica B, Gruber C. The integrated nowcasting through comprehensive analysis (INCA) system and its validation over the Eastern Alpine region. Weather and Forecasting. 2011;26:166–183. [Google Scholar]
- Hanewinkel M, Cullmann DA, Schelhaas M-J, Nabuurs G-J, Zimmermann NE. Climate change may cause severe loss in the economic value of European forest land. Nature Climate Change. 2013;3:203–207. [Google Scholar]
- Hanson PJ, Weltzin JF. Drought disturbance from climate change: response of United States forests. Science of the Total Environment. 2000;262:205–220. doi: 10.1016/s0048-9697(00)00523-4. [DOI] [PubMed] [Google Scholar]
- Herzschuh U, Birks HJB, Laepple T, Andreev A, Melles M, Birgham-Grette J. Glacial legacies on interglacial vegetation at the Pliocene-Pleistocene transition in NE Asia. Nature Communications. 2016;7:11967. doi: 10.1038/ncomms11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. Special report on emission scenarios. In: Nakicenovic N, Swart R, editors. Contribution of Working Group III of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: 2000. pp. 1–570. [Google Scholar]
- Jacob D. A note to the simulation of the annual and inter-annual variability of the water budget over the Baltic Sea drainage basin. Meteorology and Atmospheric Physics. 2001;77:61–73. [Google Scholar]
- Janda P, Trotsiuk V, Mikolas M, et al. The historical disturbance regime of mountain Norway spruce forests in the Western Carpathians and its influence on current forest structure and composition. Forest Ecology and Management. 2016 doi: 10.1016/j.foreco.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone JF, Allen CD, Franklin JF, et al. Changing disturbance regimes, ecological memory, and forest resilience. Frontiers in Ecology and the Environment. 2016;14:369–378. [Google Scholar]
- Kitzes J, Harte J. Predicting extinction debt from community patterns. Ecology. 2015;96:2127–2136. doi: 10.1890/14-1594.1. [DOI] [PubMed] [Google Scholar]
- Knoke T, Stimm B, Ammer C, Moog M. Mixed forests reconsidered: a forest economics contribution on an ecological concept. Forest Ecology and Management. 2005;213:102–116. [Google Scholar]
- Kobler J. Risikokarten als Planungsgrundlage für Flächenbewirtschaftung und Tourismuslenkung im Nationalpark Kalkalpen Oberösterreich. Faculty of Earth Sciences, Geography and Astronomy, University of Vienna; Vienna: 2004. pp. 1–304. [Google Scholar]
- Landsberg JJ, Waring RH. A generalised model of forest productivity using simplified concepts of radiation-use efficiency carbon balance and partitioning. Forest Ecology and Management. 1997;95:209–228. [Google Scholar]
- Lavorel S, Colloff MJ, Mcintyre S, et al. Ecological mechanisms underpinning climate adaptation services. Global Change Biology. 2015;21:12–31. doi: 10.1111/gcb.12689. [DOI] [PubMed] [Google Scholar]
- Lenoir J, Gegout JC, Marquet PA, de Ruffray P, Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- Manusch C, Bugmann H, Wolf A. The impact of climate change and its uncertainty on carbon storage in Switzerland. Regional Environmental Change. 2014;14:1437–1450. [Google Scholar]
- McLachlan JS, Clark JS, Manos PS. Molecular indicators of tree migration capacity under rapid climate change. Ecology. 2005;86:2088–2098. [Google Scholar]
- Meier ES, Lischke H, Schmatz DR, Zimmermann NE. Climate, competition and connectivity affect future migration and ranges of European trees. Global Ecology and Biogeography. 2012;21:164–178. [Google Scholar]
- Millar CI, Stephenson NL. Temperate forest health in an era of emerging megadisturbance. Science. 2015;349:823–826. doi: 10.1126/science.aaa9933. [DOI] [PubMed] [Google Scholar]
- Millar CI, Stephenson NL, Stephens SL. Climate change and forests of the future: managing in the face of uncertainty. Ecological Applications. 2007;17:2145–2151. doi: 10.1890/06-1715.1. [DOI] [PubMed] [Google Scholar]
- Mouillot F, Rambal S, Joffre R. Simulating climate change impacts on fire frequency and vegetation dynamics in a Mediterranean ecosystem. Global Change Biology. 2002;8:423–437. [Google Scholar]
- Naudts K, Chen Y, McGrath MJ, Ryder J, Valade A, Otto J, Luyssaert S. Europe’s forest management did not mitigate climate warming. Science. 2016;351:597–600. doi: 10.1126/science.aad7270. [DOI] [PubMed] [Google Scholar]
- Pal JS, Giorgi F, Bi X, et al. Regional climate modeling for the developing world: the ICTP RegCM3 and RegCNET. Bulletin of the American Meteorological Society. 2007;88:1395–1409. [Google Scholar]
- Pickett STA, Kolasa J, Armesto JJ, Collins SL. The ecological concept of disturbance and its expression at various hierarchical levels. Oikos. 1989;54:129–136. [Google Scholar]
- Pulsford SA, Lindenmayer DB, Driscoll DA. A succession of theories: purging redundancy from disturbance theory. Biological Reviews. 2016;91:148–167. doi: 10.1111/brv.12163. [DOI] [PubMed] [Google Scholar]
- Pysek P, Jarosik V, Hulme PE, et al. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proceedings of the National Academy of Sciences. 2010;107:12157–12162. doi: 10.1073/pnas.1002314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeloff VC, Williams JW, Bateman BL, et al. The rise of novelty in ecosystems. Ecological Applications. 2015;25:2051–2068. doi: 10.1890/14-1781.1. [DOI] [PubMed] [Google Scholar]
- Radu R, Déqué M, Somot S. Spectral nudging in a spectral regional climate model. Tellus A. 2008;60:898–910. [Google Scholar]
- Rammer W, Seidl R. Coupling human and natural systems: simulating adaptive management agents in dynamically changing forest landscapes. Global Environmental Change. 2015;35:475–485. [Google Scholar]
- Seastedt TR, Hobbs RJ, Suding KN. Management of novel ecosystems: are novel approaches required? Frontiers in Ecology and the Environment. 2008;6:547–553. [Google Scholar]
- Seidl R, Lexer MJ. Forest management under climatic and social uncertainty: trade-offs between reducing climate change impacts and fostering adaptive capacity. Journal of Environmental Management. 2013;114:461–469. doi: 10.1016/j.jenvman.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Seidl R, Rammer W. Climate change amplifies the interactions between wind and bark beetle disturbance in forest landscapes. Landscape Ecology. 2016 doi: 10.1007/s10980-016-0396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Rammer W, Lexer MJ. Schätzung von Bodenmerkmalen und Modellparametern für die Waldökosystemsimulation auf Basis einer Großrauminventur. Allgemeine Forst- und Jagdzeitung. 2009;180:35–44. [Google Scholar]
- Seidl R, Rammer W, Lexer MJ. Adaptation options to reduce climate change vulnerability of sustainable forest management in the Austrian Alps. Canadian Journal of Forest Research. 2011;41:694–706. [Google Scholar]
- Seidl R, Rammer W, Scheller RM, Spies TA. An individual-based process model to simulate landscape-scale forest ecosystem dynamics. Ecological Modelling. 2012a;231:87–100. [Google Scholar]
- Seidl R, Spies TA, Rammer W, Steel EA, Pabst RJ, Olsen K. Multi-scale drivers of spatial variation in old-growth forest carbon density disentangled with Lidar and an individual-based landscape model. Ecosystems. 2012b;15:1321–1335. [Google Scholar]
- Seidl R, Schelhaas M-J, Rammer W, Verkerk PJ. Increasing forest disturbances in Europe and their impact on carbon storage. Nature Climate Change. 2014a;4:806–810. doi: 10.1038/nclimate2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Rammer W, Spies TA. Disturbance legacies increase the resilience of forest ecosystem structure, composition, and functioning. Ecological Applications. 2014b;24:2063–2077. doi: 10.1890/14-0255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Rammer W, Blennow K. Simulating wind disturbance impacts on forest landscapes: tree-level heterogeneity matters. Environmental Modelling & Software. 2014c;51:1–11. [Google Scholar]
- Seidl R, Spies TA, Peterson DL, Stephens SL, Hicke JA. Searching for resilience: addressing the impacts of changing disturbance regimes on forest ecosystem services. Journal of Applied Ecology. 2016;53:120–129. doi: 10.1111/1365-2664.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Diaz JM, Scheller RM, Syphard AD, Franklin J. Disturbance and climate microrefugia mediate tree range shifts during climate change. Landscape Ecology. 2015;30:1039–1053. [Google Scholar]
- Silva Pedro M, Rammer W, Seidl R. Tree species diversity mitigates disturbance impacts on the forest carbon cycle. Oecologia. 2015;177:619–630. doi: 10.1007/s00442-014-3150-0. [DOI] [PubMed] [Google Scholar]
- Silva Pedro M, Rammer W, Seidl R. A disturbance-induced increase in tree species diversity facilitates forest productivity. Landscape Ecology. 2016;31:989–1004. [Google Scholar]
- Solomon S, Plattner G-K, Knutti R, Friedlingstein P. Irreversible climate change to carbon dioxide emissions. Proceedings of the National Academy of Sciences. 2009;106:1704–1709. doi: 10.1073/pnas.0812721106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SL, Burrows N, Buyantuyev A, et al. Temperate and boreal forest mega-fires: characteristics and challenges. Frontiers in Ecology and the Environment. 2014;12:115–122. [Google Scholar]
- Swanson ME, Franklin JF, Beschta RL, et al. The forgotten stage of forest succession: early-successional ecosystems on forest sites. Frontiers in Ecology and the Environment. 2011;9:117–125. [Google Scholar]
- Temperli C, Bugmann H, Elkin C. Adaptive management for competing forest goods and services under climate change. Ecological Applications. 2012;22:2065–2077. doi: 10.1890/12-0210.1. [DOI] [PubMed] [Google Scholar]
- Temperli C, Bugmann H, Elkin C. Cross-scale interactions among bark beetles, climate change, and wind disturbances: a landscape modeling approach. Ecological Monographs. 2013;83:383–402. [Google Scholar]
- Thom D, Seidl R, Steyrer G, Krehan H, Formayer H. Slow and fast drivers of the natural disturbance regime in Central European forest ecosystems. Forest Ecology and Management. 2013;307:293–302. [Google Scholar]
- Thom D, Rammer W, Dirnböck T, et al. The impacts of climate change and disturbance on spatio-temporal trajectories of biodiversity in a temperate forest landscape. Journal of Applied Ecology. 2016 doi: 10.1111/1365-2664.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W, Lavorel S, Araujo MB, Sykes MT, Prentice IC. Climate change threats to plant diversity in Europe. Proceedings of the National Academy of Sciences. 2005;102:8245–8250. doi: 10.1073/pnas.0409902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W, Pollock LJ, Gueguen M, Münkemüller T. From species distributions to meta-communities. Ecology Letters. 2015;18:1321–1328. doi: 10.1111/ele.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MG. Disturbance and landscape dynamics in a changing world. Ecology. 2010;91:2833–2849. doi: 10.1890/10-0097.1. [DOI] [PubMed] [Google Scholar]
- Turner MG, Romme W, Gardner RH, O’Neill R, Kratz T. A revised concept of landscape equilibrium: disturbance and stability on scaled landscapes. Landscape Ecology. 1993;8:213–227. [Google Scholar]
- Walker J, Sharpe PJH, Penridge LK, Wu H. Ecological field theory: the concept and field tests. Vegetatio. 1989;83:81–95. [Google Scholar]
- White PS, Jentsch A. The search for generality in studies of disturbance and ecosystem dynamics. Progress in Botany. 2001;62:399–450. [Google Scholar]
- Williams JW, Jackson ST. Novel climates, no-analog communities, and ecological surprises. Frontiers in Ecology and the Environment. 2007;5:475–482. [Google Scholar]
- Zerbe S. Restoration of natural broad-leaved woodland in Central Europe on sites with coniferous forest plantations. Forest Ecology and Management. 2002;167:27–42. [Google Scholar]
- Zimmermann NE, Jandl R, Hanewinkel M, et al. Potential future ranges of tree species in the Alps. In: Cerbu GA, Hanewinkel M, Gerosa G, Jandl R, editors. Management Strategies to Adapt Alpine Space Forests to Climate Change Risks. InTech; New York, NY: 2013. pp. 37–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.