Abstract
The medial collateral ligament is the most commonly injured ligament of the knee, with injury generally sustained in the athletic population as a result of valgus contact with or without tibial external rotation. The capacity of the medial collateral ligament to heal has been demonstrated in both laboratory and clinical studies; however, complete ruptures heal less consistently and may result in persistent instability. When operative intervention is deemed necessary, anatomical medial knee reconstruction is recommended. Post-operative rehabilitation focuses on early motion and the return of normal neuromuscular firing patterns with progression based on attainment of specific phase criteria and goals. The purpose of this clinical commentary is to discuss the determinants of phase progression and the importance of objectively assessing readiness for advancement that is consistent with post-operative healing. Additional tests and validated measures to assess readiness for sport are also presented.
Level of Evidence
5
Keywords: Medial knee, medial collateral ligament, reconstruction, rehabilitation, return to sport, periodization
BACKGROUND AND PURPOSE
The medial collateral ligament (MCL) is the most commonly injured ligament of the knee.1-3 The vast majority of significant MCL injuries occur secondary to a medially directed valgus force that is sustained just proximal or distal to the lateral knee joint.4 MCL injuries are more common in athletic cohorts compared to non-athletic cohorts, with the most injurious sports being collision and contact in nature. A greater risk of MCL injury is present in male athletes participating in intercollegiate as compared to intramural sports.5 A recent epidemiologic study of 346 MCL injuries in soccer players reported the average duration of time lost was 23.2 days, with higher grade injuries associated with greater lost time.6
Both laboratory and clinical studies have elucidated the healing capacity of the MCL complex; the MCL has the unique capability to heal with conservative measures in most cases.7,8 Non-operative treatment is often effective for grade I and II injuries, and can be effective in grade III injuries. The good healing potential of the MCL is secondary to the growth rate and functioning of its stem cells, as well as the expression of growth factors key to ligament healing.9-12
Both individual patient and biologic factors influence management of grade III tears. Pertinent patient factors to consider include timing of injury, level of activity, and presence of symptomatic instability. Injury location affects ligament healing; Frank et al13 reported slower healing and the development of abnormal morphology with insertional injuries as compared to midsubstance tears in a rabbit model. Wilson et al14 reported complete injuries where the MCL is avulsed from the tibial insertion failed to heal reliably in the athletic population.
A keen understanding of knee anatomy, involved structures and potential for healing are necessary to make informed management decisions regarding the surgeon's recommendation for surgical versus non-operative management of isolated grade III MCL injuries. If operative management is indicated, anatomic repair or reconstruction is recommended to improve overall patient function and to restore valgus stability.15,16 Anatomic medial knee reconstruction restores near native stability to the knee and, therefore, provides near normal ligament load distribution.17 Post-operatively, strict adherence to rehabilitation protocols is essential to enable optimal healing. A safe and effective rehabilitation protocol is informed by the existing literature, incorporating current rehabilitation principles, the science of soft tissue healing, and adaptations based on an individual's readiness to progress through progressive phases. The purpose of this clinical commentary is to discuss the determinants of phase progression and the importance of objectively assessing readiness for advancement that is consistent with post-operative healing. Additional tests and validated measures to assess readiness for sport are also presented.
ANATOMY
The primary structures of the medial aspect of the knee are the proximal and distal divisions of the superficial medial collateral ligament, the posterior oblique ligament, and the deep medial collateral ligament (Figure 1). The superficial MCL is the largest structure of the medial aspect of the knee, and is comprised of one femoral and two tibial attachments.18,19 The central arm of the posterior oblique ligament is a fibrous extension off the distal aspect of the semimembranosus, which reinforces the posteromedial aspect of the joint capsule. The deep MCL comprises the thickened joint capsule deep to the superficial MCL, and is divided into meniscofemoral and meniscotibial components. The MCL is the primary stabilizer to resist valgus loading and has been shown to contribute 78% to the restraining force on the medial side of the knee.20,21 Resistance to valgus instability is secondarily provided by the ACL.22
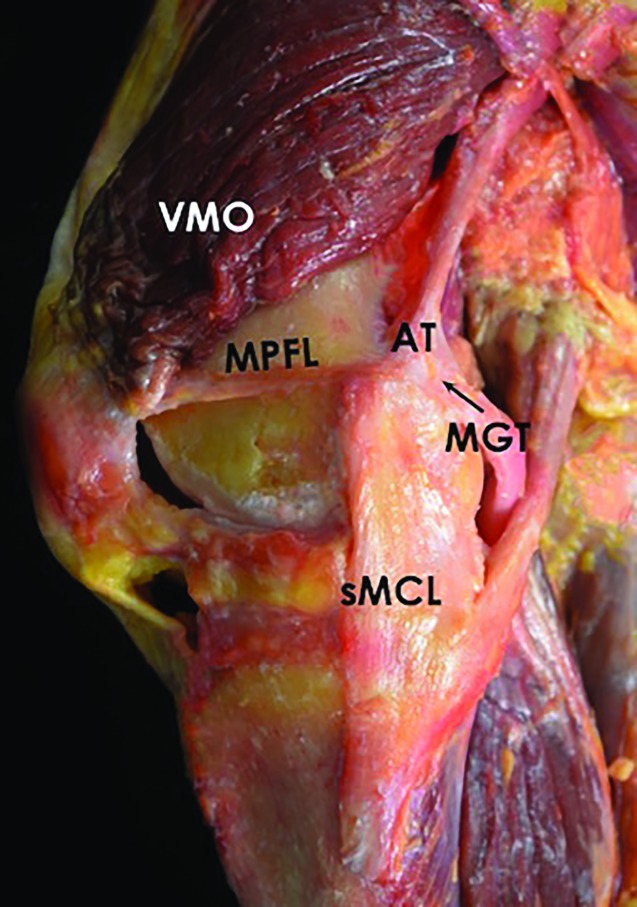
Figure 1.
Photograph of a knee, demonstrating the VMO, MPFL, AT, MGT, and sMCL. VMO, vastus medialis oblique; MPFL, medial patellofemoral ligament; AT, adductor tubercle; MGT, medial gastrocnemius tendon; sMCL, superficial medial collateral ligament.
The MCL and the posterior oblique ligament also contribute to resisting abnormal external tibial rotation.20,21 A controlled laboratory study investigating the structural properties of the medial knee ligaments found the superficial MCL had the highest load to failure and stiffness, followed by the posterior oblique ligament (POL) and deep MCL.23 The distribution of strain changes with flexion angle; Gardiner et al,24 in his cadaveric study measuring strain on the MCL during valgus loading, found the highest strain to occur at full extension on the posterior side of the MCL near the femoral insertion.
DIAGNOSIS
Isolated injury to the medial knee ligament complex most commonly occurs due to a laterally directed blow just proximal or distal to the knee joint with the foot planted. Depending on the amount of knee flexion present at the moment of injury, some tibial rotation or translation may occur. Coupled forces, such as valgus stress and external rotation, may result in damage to both the MCL and posterior oblique ligament.25 After an MCL injury, patients often describe feeling instability with side-to-side activities, particularly with cutting or pivoting maneuvers. Physical examination, including visual inspection, palpation and the application of valgus load in both full knee extension and 20 to 30 degrees of knee flexion, is the initial step of the diagnostic process. The degree of medial joint opening relative to the unaffected knee allows the examiner to assess injury to the MCL. The valgus stress test at zero degrees, assesses for a combined medial knee and cruciate injury; asymmetric opening in full extension indicates a combined injury to the MCL and posterior oblique ligament, and possibly the cruciate ligaments.26 Stability in full extension denotes no substantial damage to the posterior oblique ligament.27 Additionally, assessment of end-point integrity is utilized to determine the presence of an incomplete versus complete rupture.28 A valgus stress at 20-30 degrees of knee flexion is the primary test to evaluate for medial-sided ligament knee injury.
IMAGING
Correlation of valgus stress radiographs with medial knee ligament injuries has been performed,29 and deemed to provide objective and reproducible measurements of medial compartment gapping. In one study, when a clinician applied valgus load to a knee with a simulated isolated grade-III superficial MCL injury, medial gapping increased by 1.7 and 3.2 mm at 0 and 20 degrees of flexion, respectively, compared to that of an intact knee. A complete medial knee injury, including the superficial and deep MCL and posterior oblique ligament, increased gapping by 6.5 and 9.8 mm at 0 and 20 degrees, respectively, under the clinician-applied valgus load. The authors reported intraobserver repeatability and interobserver reproducibility with intraclass correlation coefficients of 0.99 and 0.98 in the measurement of medial gapping. In addition to the diagnostic process, valgus stress radiographs are useful for pre-operative planning and post-operative follow-up of patients.29 Magnetic resonance imaging (MRI) may be useful to determine both the location and the magnitude of ligamentous damage, as well as other injuries to the knee.
GRADING
Isolated medial knee injuries have been classified by the amount of subjective laxity detected at 30 degrees of knee flexion with application of valgus load; however, it is required that the patient is able to relax during testing in order to accurately assess ligamentous integrity. Grades 1, 2, and 3 MCL injury correspond to perceived gapping of the medial joint line of 3 to 5 mm, 6 to 10 mm, and >10 mm, respectively.2,28 However, as noted above, these reported measurements for the grades of medial joint gapping are subjective amounts of the medial joint line gapping and do not correlate to that objectively measured on stress radiographs. The American Medical Association Standard Nomenclature of Athletic Injuries30 has developed a widely utilized scale to grade medial knee injuries. An isolated grade I, first-degree tear presents with localized tenderness without laxity. An isolated grade II, second-degree tear presents with localized tenderness and partially torn MCL and POL fibers. An isolated grade III, third-degree tear presents denotes complete disruption of all three structures and no end point to valgus laxity testing.
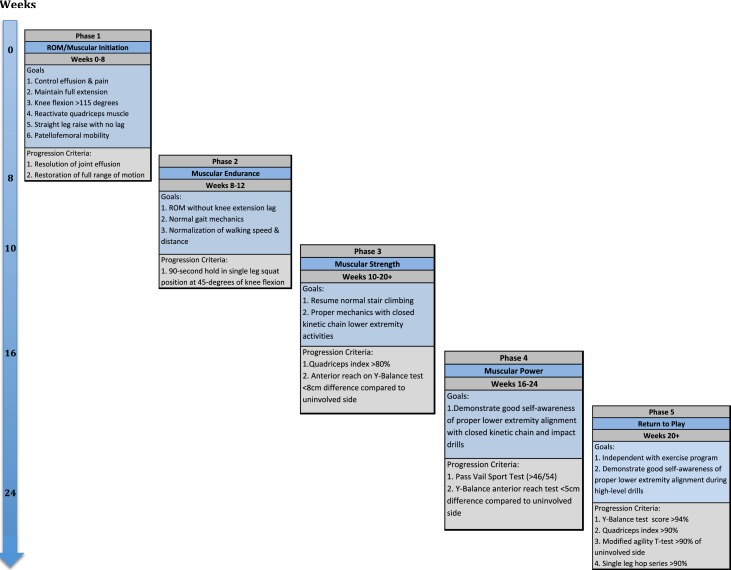
POST-OPERATIVE REHABILITATION & RETURN TO PLAY AFTER MCL RECONSTRUCTIONS
The post-operative rehabilitation protocol for grade III MCL injuries employed at the authors’ institution emphasizes early motion and restoration of neuromuscular firing patterns (see Appendix 1). Early motion, control of knee joint swelling and pain, and activation of the quadriceps during the initial recovery period after an anatomic knee ligament reconstruction is critical for overall recovery.31,32 After the initial period of non-weight bearing for 6 weeks in a hinged brace locked in extension when not working on knee motion in therapy, emphasis is placed on restoration of a proper gait pattern, functional exercise progression and the implementation of neuromuscular and proprioceptive-based exercises.31-33 The presence of concurrent cruciate ligament reconstructions or osteotomies may require significant modifications to the rehabilitation protocol in order to facilitate adequate soft tissue and/or bony healing.
Therapeutic Modalities
Proposed benefits of cryotherapy include the promotion of local vasoconstriction to control edema, as well as the reduction of pain.34-36 Most recommendations for the use of cold therapy are based on anecdotal experience, with limited scientific evidence to support the efficacy of specific cold modalities. Hubbard et al37 conducted a systematic review investigating the impact of cryotherapy on soft tissue injury and found cryotherapy to be effective in decreasing pain in the acute setting. Malanga et al36 conducted a review of multiple cryotherapies in the setting of low back pain and found cryotherapy to be effective for short-term reduction in pain.
Active compression cold therapy devices, such as the Game Ready® system (Game Ready®, Concord, Georgia, U.S.A.) are commonly employed post-operatively to reduce pain and swelling. In a prospective randomized study38 examining continuous long-term application of an active compression cold therapy system after anterior cruciate ligament reconstruction and the authors found significantly increased range of motion and functional knee scores when compared to cold therapy alone. Stockle and colleagues39 compared the impact of intermittent impulse compression (an air pad under the foot inflated every 20 seconds), standard cool packs, and continuous cryotherapy (ice water circulating between the ice box and the cold pad, with ice water was changed once per day) on edema in the treatment of acute foot and ankle trauma. The authors reported reduction in swelling by 74% with intermittent compression versus 70% with continuous cryotherapy and 45% with cool packs after four days of treatment. In the authors’ treatment protocol, patients utilize a Game Ready® on low compression for 30 minutes followed by 60 minutes off to allow for skin temperature recovery, with this cycle repeated a minimum of four times per day.
Bracing
Post-operatively, the brace is set at full extension for six weeks with the exception of during passive range of motion with the physical therapist until appropriate quadriceps control is achieved. In this initial phase, an emphasis is placed on achievement of full extension and 90 degrees of knee flexion by the end of the first two weeks. After two weeks, knee motion is increased as tolerated. Loss of pre-injury range of motion has been found to be one of the most frequently reported complications following knee ligament reconstruction surgery.40 After the six-week protection period, and in conjunction with the patient being able to clinically demonstrate active quadriceps control during terminal extension, in addition to passive knee flexion greater than 90 degrees, a transition to a return to sport brace is made. A timeframe for discontinuing all brace use is patient dependent and variable, with the decision being informed by consideration of environmental risk factors, athletic/work demands and physiological healing times.
Weight Bearing Progression
A period of non-weight bearing is recommended immediately following surgery.2,14,41 After six weeks of protected weight bearing; a program that focuses on increasing the patient's weight bearing tolerance is implemented. This period of progressive weight bearing is designed to incrementally increase loading volume to reduce the risk of a rehabilitation setback through overloading the joint and reconstructed/repaired structures.16 Options to increase patient tolerance to weight bearing include resisted biking, walking in a pool, or walking on an anti-gravity treadmill. Once full weight bearing is achieved comfortably, the rehabilitation provider should focus on restoration of a normal gait pattern. The quadriceps muscle is typically atrophied42 and inadequate quadriceps strength contributes to altered gait patterns following knee surgery.43 Persistent intra-articular effusion may contribute to quadriceps inhibition, loss of range of motion, joint pain, and gait abnormality.44 Notable reduction in quadriceps electromyographic activity occurs with as little of 10 mL of intra-articular fluid.42 Caution to avoid a valgus moment at the knee joint during stance phase, which can occur in an attempt to unload the knee joint by posting the foot of the surgically treated extremity lateral to the base of support.28 Progression of exercises is not permitted if the patient is unable to ambulate without a limp to ensure that increasing activities does not result in abnormal forces on the reconstruction grafts.
Immediate Range of Motion
Long periods of immobilization after anterior cruciate ligament reconstruction have been replaced by accelerated protocols to improve functional outcomes.45-47 Similarly, immediate range of motion is recommended following medial knee reconstruction. Animal studies have examined the metabolic and cellular effects of immobilization on the collateral ligaments and their authors have reported a detrimental impact on cellular metabolism.48,49 Another animal study evaluating the vulnerability of ligament grafts to creep assessed thirty-nine MCL autografts in a rabbit model with nineteen of the rabbits being immobilized post procedure. The authors of the study revealed that immobilization resulted in increased vulnerability of the ligament autografts to creep, postulating that following immobilization the increase in magnitude of susceptibility to creep will result in functionally significant elongation of the graft if exposed to higher tensile loads and over longer periods of time in vivo.50 Thornton et al51 also assessed the creep and creep recovery of fresh anatomic ligament autografts in an extra-articular environment using a rabbit model. The immobilized grafts (duration of immobilization, six weeks) had significantly greater creep compared to non-immobilized grafts at one year of healing (p < 0.05).
A safe range of motion which promotes joint mobility, yet protects the healing soft tissue graft, should be determined intra-operatively by the treating surgeon, and then communicated to the rehabilitation provider to ensure appropriate range of motion parameters immediately post repair or reconstruction.52 Knee stiffness after medial-sided repair or reconstruction is a known complication; therefore, initiation of passive ROM exercises is necessary to avoid capsular adhesion formation.2,28 Preferably, immediate passive range of motion is recommended to minimize the risk of arthrofibrosis,41 with the goal of attaining 0 to 90 degrees at two weeks post-operatively. Noyes et al53 found immediate passive range of motion following open and arthroscopic anterior cruciate ligament reconstruction surgery did not increase joint effusion, hemarthrosis, or soft tissue swelling; however, aggressive range of motion exercises should be avoided the first three weeks post-operatively to avoid placing tension on the reconstruction.53
After the two-week mark, range of motion should be progressively increased as tolerated with the goal of 130 degrees of passive knee flexion at six weeks post-operatively. Early knee motion has not been found to result in stretching of the graft, based on post-operative stress radiographs.16 Of note, even minor deficits in knee flexion and extension ROM are not well tolerated in athletes, as these limitations can negatively affect muscular strength development, impacting running, agility and jumping performance. Deficits larger than ten degrees of knee flexion have been associated with running speed reduction.54,55 Adhesion formation in the suprapatellar pouch and anterior interval can limit joint range of motion and increase joint contact pressures.56 For this reason, mobilization of the patellofemoral joint should be initiated immediately post surgery and continued for six weeks.
Phases of Rehabilitation Progression
The outlined rehabilitation protocol time points are provided as a guideline for phase progression; however, true readiness for phase advancement should be determined by achievement of the goals provided for each phase of rehabilitation (Appendix 1). Progression of exercises is not permitted at the authors’ institution if the patient has a persistent limp or gait abnormality to ensure advancing activities does not result in recurrent joint effusion. All exercises should be conducted under careful supervision and monitoring of proper lower extremity kinematics and alignment. Recurrent or persistent pain and swelling indicate improper phase progression.
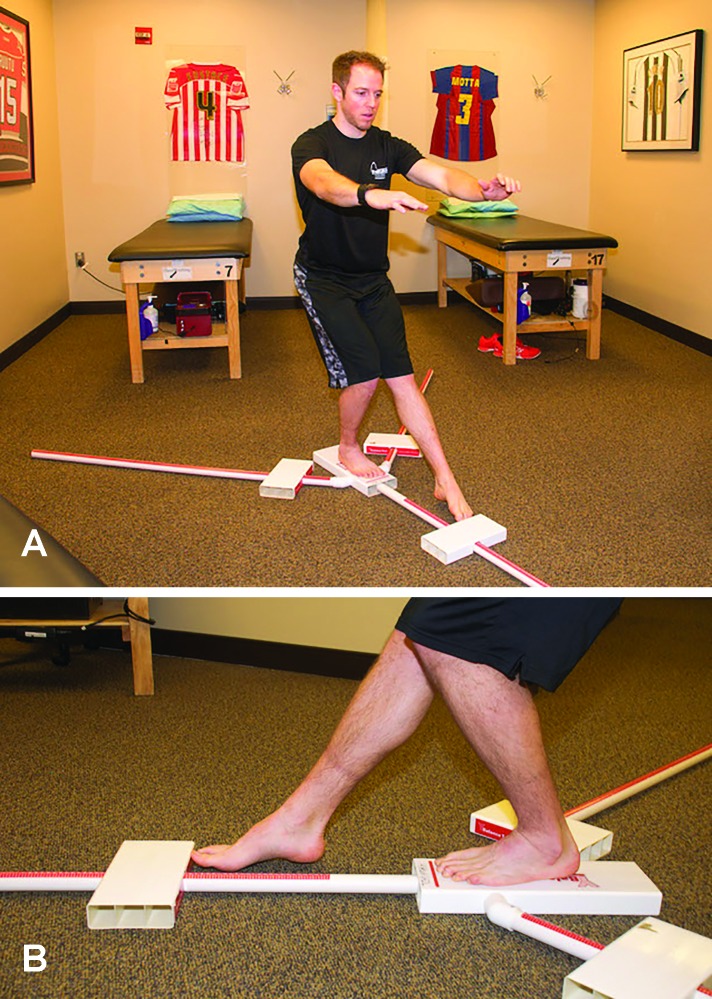
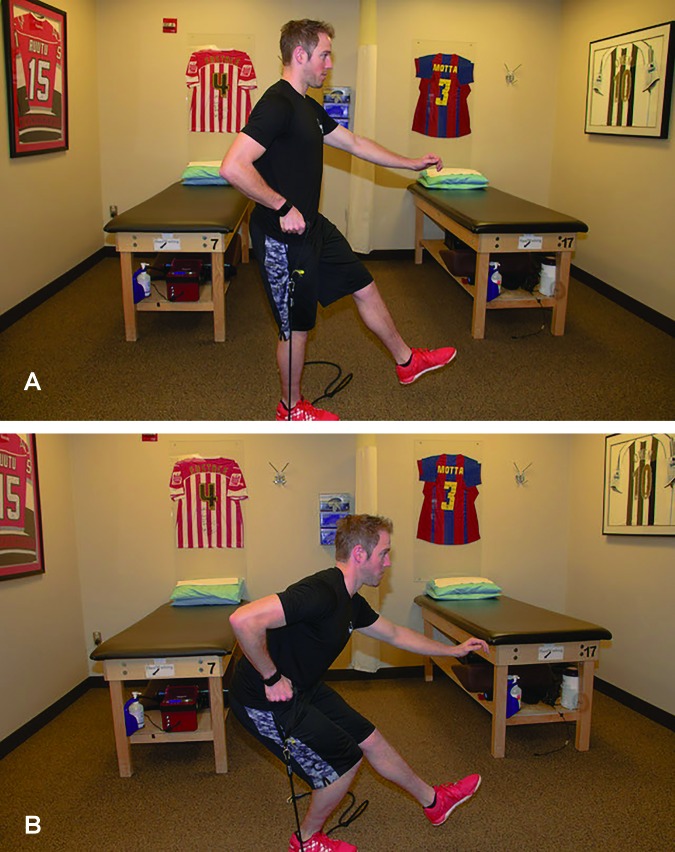
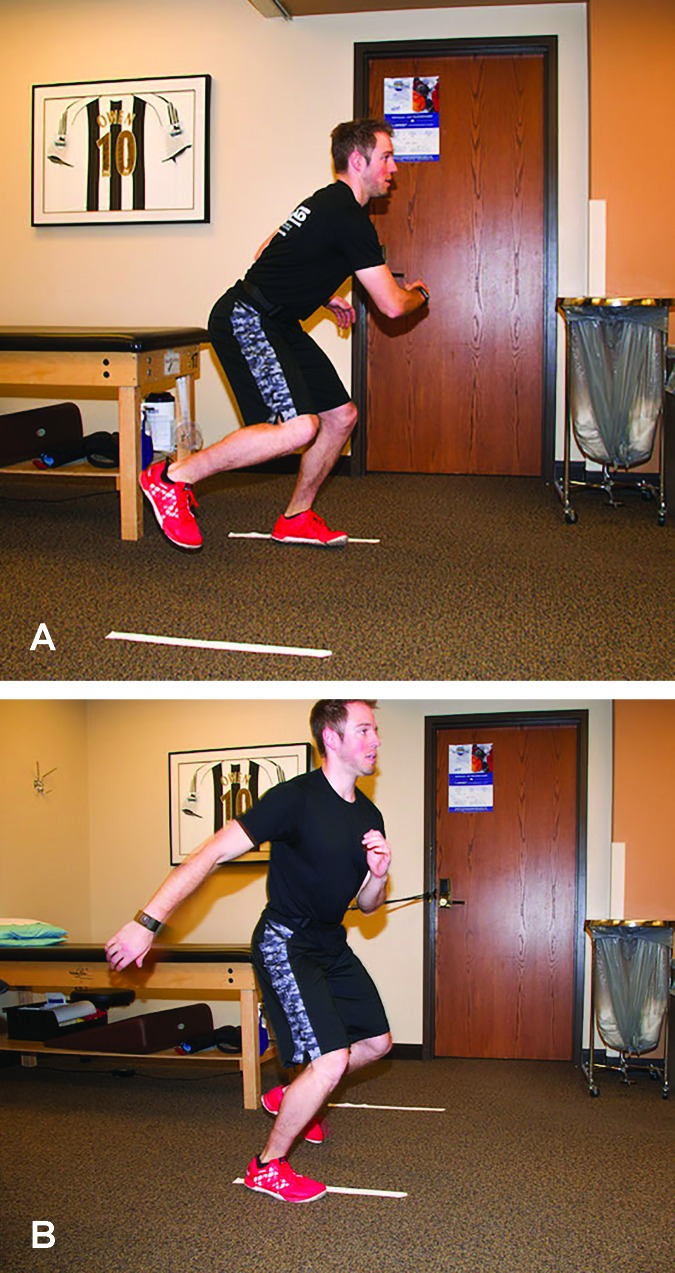
Additional measures and tests are included in the phase progression criteria. A large array of measures and tests are available, with the following chosen for their clinical utility, ease of administration and interpretation; the quadriceps index, the Y-Balance Test™ (YBT), the Vail Sport Test™, the modified agility T-test, and the single leg hop series. The quadriceps index is a measure of the relative isometric strength of the involved quadriceps in comparison to the uninvolved quadriceps and expressed as a percentage. Strength measurements are captured using a handheld dynamometer positioned on the distal tibia with the patient seated on the end of a treatment table with this hip and knee angle at 90 degrees. The YBT is a functional test performed to evaluate functional symmetry and performance, as well as assess risk for injury (Figures 2a, 2b). Performance of the YBT requires lower extremity and trunk/core strength, flexibility, neuromuscular control, range of motion, balance and proprioception, and can be performed relatively efficiently. The Vail Sport Test™ is a return to sports assessment that incorporates a series of dynamic multiplanar functional activities against the resistance of a sports cord (Figures 3a, 3b). The modified agility T-test focuses on identifying side-to-side deficits in multiplanar cutting and agility tasks (Figures 4a, 4b), while the single leg hop series is a commonly employed performance measure used to analyze limb symmetry and functional performance following knee surgery (Figures 5a, 5b).
Figure 2A, 2B.
The Y-Balance Test™ (YBT), a functional test performed to evaluate functional symmetry and performance, as well as assess risk for injury.
Figure 3A, 3B.
The Vail Sport Test™, a return to sports assessment that incorporates a series of dynamic multiplanar functional activities against the resistance of a sports cord.
Figure 4A, 4B.
The modified agility T-test focuses on identifying side-to-side deficits in multiplanar cutting and agility tasks.
Figure 5A, 5B.
The single leg hop series, a commonly employed functional performance measure used to capture limb asymmetries following knee surgery.
Muscle Initiation, Endurance, Strength, and Power
Quadriceps activation via quadriceps setting exercises, straight leg raises in the brace, hip extension and hip abduction exercises are encouraged immediately after surgery. Quadriceps activation is particularly important to combat the marked weakness of quadriceps muscles typically observed after knee surgery.42,57 Once the post-operative effusion has resolved, knee motion is greater than 115 degrees, the patient is full weight bearing without crutches, and straight leg raises can be performed without an extension lag, the patient may commence CKC strengthening. Progression of strengthening exercises, both in their mode and complexity, is dependent on many factors. The post-operative rehabilitation protocol outlined in Appendix 1 includes a suggested timeline, general goals for each phase, precautions to maintain, specific exercises to perform, and criteria for advancement to the next phase of rehabilitation. Worthy of inclusion, as well, is an overview of the guidelines on the resistance training variables. Traditional rehabilitation programs utilize basic progressive overload principles (stress to the muscle is progressively increased as it becomes capable of producing greater force, power, and endurance), however, the application of periodization in rehabilitation protocols has also been proposed.58
Periodization is defined as the planned manipulation of training variables (load, sets, and repetitions) to maximize training adaptations and prevent the onset of overtraining,59 with the goal of optimizing the neuromuscular systems to adapt to unaccustomed load or stressors. Lorenz and colleagues58 described both linear and nonlinear periodization protocols following anterior cruciate ligament reconstruction, which have been adapted and integrated into the post-operative rehabilitation protocol for grade III medial collateral ligament injury. In a linear program, phases depend on the time frame of rehabilitation, while a nonlinear program allows for increased flexibility by the treating physical therapist. Nonlinear programs permit the clinician to alter intensity, volume and training focus (endurance, strength, power) for a particular duration of time. The overall goals are similar in both programs. There is a paucity of data in rehabilitation research, however, in healthy trained and untrained athletes periodized strengthening programs elicit greater strength gains than non-periodized programs,58 and have been shown to be a safe method in older adults and those with pain.58,60
In addition to lower extremity strengthening, emphasis on proximal strength and core stability is necessary to promote appropriate joint reaction forces at the knee. Noehren et al,61 in their analysis of hip and trunk neuromuscular control, found patients who underwent anterior cruciate ligament reconstruction had significantly greater trunk ipsilateral lean, forward lean, and higher errors on trunk stability testing compared to healthy controls. The average time between reconstruction and testing was 222.2 + /- 44 days. Further, weakness in hip abduction during dynamic activities can place a valgus stress across the knee, excessively loading the reconstructed complex.62 Core stability training has also been shown to result in a significant reduction in strength asymmetries during jump testing.63
Neuromuscular Reeducation
Mechanoreceptors within the ligament and joint capsule are damaged with medial sided ligament injury, and knee proprioception is reduced, hampering response to perturbations.64 Balance and proprioception exercises should begin shortly after full weight bearing,65 initially with bilateral upper extremity support. Lower extremity proprioception and balance activity progression includes single-leg exercises, followed by the inclusion of less stable surfaces. Finally, dynamic, multi-directional drills are implemented. Throughout the progression, strict attention to lower extremity alignment is emphasized, with special attention on the avoidance of a valgus moment at the knee.
Return to Jogging, Plyometric & Agility Training
Once the patient achieves appropriate lower extremity strength, range of motion and proprioception, as outlined in the simple measures reported below, a running progression program may begin. Patients must demonstrate good control in concentric and eccentric phases during strength training exercises and preserve proper lower extremity alignment during neuromuscular reeducation drills, which generally occurs between sixteen and twenty weeks post-operatively. Readiness may be determined with two simple tests; the patient's ability to tolerate one to two miles of brisk walking without a limp and demonstrate balance and control while performing a single-leg squat to approximately 90 degrees of knee flexion.28,66 The single-leg squat test is a reliable tool to evaluate frontal plane motion of the knee and is a valid clinical measure to assess lower extremity movement quality.67
Readiness for body weight plyometric and agility exercises is determined by comparison of strength and functional hopping. Initially, basic double-leg plyometric drills are recommended, followed by progression to single-leg activities and more challenging, dynamic drills. Upper extremity support or modifications may be utilized to assist transition to more difficult exercises. The patient must achieve 75% of quadriceps strength and, on the functional hop test, score >75% relative to the unaffected side.19 Attention to landing mechanics is of particular importance during plyometric and jumping exercises to avoid excessive valgus forces through the knee joint. Well-studied in the setting of anterior cruciate ligament reconstruction, poor landing technique has been attributed to secondary tears.68-71 Sufficient hip and core strength is required to maintain proper landing kinematics.62
Return to Play
Multiple patient factors are considered prior to an athlete's return to play, including their recovery of strength compared to the contralateral lower extremity, clinical and objective knee stability, and desired activity or sport. At approximately twenty weeks post-operatively, an assessment of readiness to return to full activity can be conducted. Assessment may be performed with sport-specific functional tests, such as the Vail Sport Test™; however, no single test exists to meet the functional and activity needs of each patient, necessitating an individualized approach by the treating surgeon and the rehabilitation provider. Preferred sport or activity is influenced by geography; for example, flat land versus mountain-based activity, and the demands these activities place on the operative extremity must be considered. Further, functional testing provides an inaccurate marker for risk of injury because tests are performed under non-fatigued conditions.72 Fatigue protocols may be introduced to return to sport tests, realizing the difficultly replicating the fatigue experienced in a competition or performance-based environment. A return to full activity is appropriate when the patient can demonstrate appropriate strength on functional testing and proper alignment and control with dynamic activities, as well as objective knee stability on clinical examination. Repeat valgus stress radiographs may be obtained to further confirm stability.
SUMMARY
The vast majority of MCL injuries may be managed non-operatively, however, a select group of patients who sustain MCL complex injuries benefit from operative intervention, specifically anatomic repair or reconstruction. Successful return to activity relies on strict adherence to the post-operative rehabilitation protocol. The authors’ rehabilitation protocol has been provided that is informed by available scientific evidence and is responsive to an individual's ability to safely advance through rehabilitation phase progressions.
Table 1.
| ROM/Muscular initiation | Time/reps + sets | Frequency |
|---|---|---|
| Wall slides- for repeated passive flexion and extension | 10 min | 3x/day |
| Seated flexion at end of bed (if unable to wall slide) | 10 min | 3x/day |
| Patellofemoral mobilization | 10 min | 3x/day |
| Quad sets | 3 x 15 | 3x/day |
| Hamstring sets | 3 x 15 | 3x/day |
| Stationary bike - no resistance, starting at week 7 | 10 min | 2x/day |
Table 2.
| Muscular endurance | Sets | Reps | Rest |
|---|---|---|---|
| Double leg leg press+ | 3 | 15 | 45s |
| Static lunge hold | 3 | maximum | 45s |
| Single leg deadlift+ | 3 | 15 | 45s |
Squat progression-body weight
|
3 | 30 | 45s |
| Tuck squat | 3 | maximum | 45s |
| Double leg bridge | 3 | 15 | 45s |
Patient-specific levels of resistance are added to exercises as appropriate, to allow for completion of the outlined exercise parameters and maximal development of the desired physiological characteristic.
Table 3.
| Muscular strength | Sets | Reps | Rest |
|---|---|---|---|
| Single leg leg press+ | 3-4 | 12 | 2min |
| Reverse lunge with dumb bells+ | 3-4 | 12 | 2min |
| Single leg squat | 3-4 | maximum | 2min |
| Single leg deadlift with kettle bells+ | 3-4 | 12 | 2min |
| Balance squat with dumb bells+ | 3-4 | 12 | 2min |
| Crab walk with resistance band+ | 3-4 | maximum | 2min |
Patient-specific levels of resistance are added to exercises as appropriate, to allow for completion of the outlined exercise parameters and maximal development of the desired physiological characteristic
Table 4.
| Muscular Power | Sets | Reps | Rest |
|---|---|---|---|
| Single leg leg press+ | 3 | 8 | 3min |
| Reverse lunge into hip drive | 3 | 8 | 3min |
| Lateral agility with sports cord | 3 | 60s | 3min |
| Bulgarian jump squat | 3 | 8 | 3min |
| Double leg 8 inch box jump up | 3 | 8 | 3min |
ROM = Range of motion, min = minutes, s = seconds.
Resistance is increased for single leg leg press beyond that performed in the muscular strength phase. By increasing exercise intensity, the desired physiological characteristic is maximally developed
APPENDIX 1
REFERENCES
- 1.Miyamoto RG, Bosco JA, Sherman OH. Treatment of medial collateral ligament injuries. J Am Acad Orthop Surg. 2009;17(3):152-161. [DOI] [PubMed] [Google Scholar]
- 2.Phisitkul P, James SL, Wolf BR, Amendola A. MCL injuries of the knee: current concepts review. Iowa Orthop J. 2006;26:77-90. [PMC free article] [PubMed] [Google Scholar]
- 3.Giannotti BF, Rudy T, Graziano J. The non-surgical management of isolated medial collateral ligament injuries of the knee. Sports Med Arthrosc. 2006;14(2):74-77. [DOI] [PubMed] [Google Scholar]
- 4.Indelicato P. Isolated Medial Collateral Ligament Injuries in the Knee. J Am Acad Orthop Surg. 1995;3(1):9-14. [DOI] [PubMed] [Google Scholar]
- 5.Roach CJ, Haley CA, Cameron KL, Pallis M, Svoboda SJ, Owens BD. The epidemiology of medial collateral ligament sprains in young athletes. Am J Sports Med. 2014;42(5):1103-1109. [DOI] [PubMed] [Google Scholar]
- 6.Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. [DOI] [PubMed] [Google Scholar]
- 7.Frank C, Woo SL, Amiel D, Harwood F, Gomez M, Akeson W. Medial collateral ligament healing. A multidisciplinary assessment in rabbits. Am J Sports Med. 1983;11(6):379-389. [DOI] [PubMed] [Google Scholar]
- 8.Inoue M, McGurk-Burleson E, Hollis JM, Woo SL. Treatment of the medial collateral ligament injury. I: The importance of anterior cruciate ligament on the varus-valgus knee laxity. Am J Sports Med. 1987;15(1):15-21. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Pan T, Im H-J, Fu FH, Wang JHC. Differential properties of human ACL and MCL stem cells may be responsible for their differential healing capacity. BMC Med. 2011;9(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie J, Huang W, Jiang J, et al. Differential expressions of lysyl oxidase family in ACL and MCL fibroblasts after mechanical injury. Injury. 2013;44(7):893-900. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Wang C, Yin L, Xu C, Zhang Y, Sung K-LP. Interleukin-1 beta influences on lysyl oxidases and matrix metalloproteinases profile of injured anterior cruciate ligament and medial collateral ligament fibroblasts. Int Orthop. 2013;37(3):495-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie J, Wang C, Huang D-Y, et al. TGF-beta1 induces the different expressions of lysyl oxidases and matrix metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after mechanical injury. J Biomech. 2013;46(5):890-898. [DOI] [PubMed] [Google Scholar]
- 13.Frank CB, Loitz BJ, Shrive NG. Injury location affects ligament healing. A morphologic and mechanical study of the healing rabbit medial collateral ligament. Acta Orthop Scand. 1995;66(5):455-462. [DOI] [PubMed] [Google Scholar]
- 14.Wilson TC, Satterfield WH, Johnson DL. Medial collateral ligament “tibial” injuries: indication for acute repair. Orthopedics. 2004;27(4):389-393. [DOI] [PubMed] [Google Scholar]
- 15.Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. [DOI] [PubMed] [Google Scholar]
- 16.LaPrade RF, Wijdicks CA. Surgical technique: development of an anatomic medial knee reconstruction. Clin Orthop Relat Res. 2012;470(3):806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coobs BR, Wijdicks CA, Armitage BM, et al. An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med. 2010;38(2):339-347. [DOI] [PubMed] [Google Scholar]
- 18.LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The Anatomy of the Medial Part of the Knee. J Bone Joint Surg Am. 2007;89(9):2000-2010. [DOI] [PubMed] [Google Scholar]
- 19.Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. 10.2106/JBJS.I.01229. [DOI] [PubMed] [Google Scholar]
- 20.Grood ES, Noyes FR, Butler DL, Suntay WJ. Ligamentous and capsular restraints preventing straight medial and lateral laxity in intact human cadaver knees. J Bone Joint Surg Am. 1981;63(8):1257-1269. [PubMed] [Google Scholar]
- 21.Warren LA, Marshall JL, Girgis F. The prime static stabilizer of the medical side of the knee. J Bone Joint Surg Am. 1974;56(4):665-674. [PubMed] [Google Scholar]
- 22.Battaglia MJ, Lenhoff MW, Ehteshami JR, et al. Medial collateral ligament injuries and subsequent load on the anterior cruciate ligament: a biomechanical evaluation in a cadaveric model. Am J Sports Med. 2009;37(2):305-311. [DOI] [PubMed] [Google Scholar]
- 23.Wijdicks CA, Ewart DT, Nuckley DJ, Johansen S, Engebretsen L, LaPrade RF. Structural properties of the primary medial knee ligaments. Am J Sports Med. 2010;38(8):1638-1646. [DOI] [PubMed] [Google Scholar]
- 24.Gardiner JC, Weiss JA, Rosenberg TD. Strain in the human medial collateral ligament during valgus loading of the knee. Clin Orthop Relat Res. 2001;(391):266-274. [DOI] [PubMed] [Google Scholar]
- 25.Marchant MH, Tibor LM, Sekiya JK, Hardaker WT, Garrett WE, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113. [DOI] [PubMed] [Google Scholar]
- 26.Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159-172. [PubMed] [Google Scholar]
- 27.Indelicato Isolated Medial Collateral Ligament Injuries in the Knee. J Am Acad Orthop Surg. 1995;3(1):9-14. [DOI] [PubMed] [Google Scholar]
- 28.Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. [DOI] [PubMed] [Google Scholar]
- 29.LaPrade RF, Bernhardson AS, Griffith CJ, Macalena JA, Wijdicks CA. Correlation of valgus stress radiographs with medial knee ligament injuries: an in vitro biomechanical study. Am J Sports Med. 2010;38(2):330-338. [DOI] [PubMed] [Google Scholar]
- 30.Injuries AMACOTMAOSSOCOS. Standard nomenclature of athletic injuries. 1966.
- 31.Wilk KE, Andrews JR. Current concepts in the treatment of anterior cruciate ligament disruption. J Orthop Sports Phys Ther. 1992;15(6):279-293. [DOI] [PubMed] [Google Scholar]
- 32.Wilk KE, Reinold MM, Hooks TR. Recent advances in the rehabilitation of isolated and combined anterior cruciate ligament injuries. Orthop Clin North Am. 2003;34(1):107-137. [DOI] [PubMed] [Google Scholar]
- 33.Lephart SM, Pincivero DM, Giraldo JL, Fu FH. The role of proprioception in the management and rehabilitation of athletic injuries. Am J Sports Med. 1997;25(1):130-137. [DOI] [PubMed] [Google Scholar]
- 34.Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261. [DOI] [PubMed] [Google Scholar]
- 35.Hart LE. Ice for the treatment of acute soft-tissue injury: a review. Clin J Sport Med. 2004;14(5):320-321. [DOI] [PubMed] [Google Scholar]
- 36.Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127(1):57-65. [DOI] [PubMed] [Google Scholar]
- 37.Hubbard TJ, Denegar CR. Does Cryotherapy Improve Outcomes With Soft Tissue Injury? J Athl Train. 2004;39(3):278-279. [PMC free article] [PubMed] [Google Scholar]
- 38.Schröder D, Pässler HH. Combination of cold and compression after knee surgery. A prospective randomized study. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):158-165. [DOI] [PubMed] [Google Scholar]
- 39.Stöckle U, Hoffmann R, Schütz M, Fournier von C, Südkamp NP, Haas N. Fastest reduction of posttraumatic edema: continuous cryotherapy or intermittent impulse compression? Foot Ankle Int. 1997;18(7):432-438. [DOI] [PubMed] [Google Scholar]
- 40.Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778. [DOI] [PubMed] [Google Scholar]
- 41.LaPrade RF, Wijdicks CA. The management of injuries to the medial side of the knee. J Orthop Sports Phys Ther. 2012;42(3):221-233. [DOI] [PubMed] [Google Scholar]
- 42.Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. 2010;40(3):250-266. [DOI] [PubMed] [Google Scholar]
- 43.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2002;17(1):56-63. [DOI] [PubMed] [Google Scholar]
- 44.Rutherford DJ, Hubley-Kozey CL, Stanish WD. Knee effusion affects knee mechanics and muscle activity during gait in individuals with knee osteoarthritis. Osteoarthritis Cartilage. 2012;20(9):974-981. [DOI] [PubMed] [Google Scholar]
- 45.Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292-299. [DOI] [PubMed] [Google Scholar]
- 46.Wilk KE, Arrigo C. Current concepts in the rehabilitation of the athletic shoulder. J Orthop Sports Phys Ther. 1993;18(1):365-378. [DOI] [PubMed] [Google Scholar]
- 47.Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-336. [DOI] [PubMed] [Google Scholar]
- 48.Walsh S, Frank C, Hart D. Immobilization alters cell metabolism in an immature ligament. Clin Orthop Relat Res. 1992;(277):277-288. [PubMed] [Google Scholar]
- 49.Padgett LR, Dahners LE. Rigid immobilization alters matrix organization in the injured rat medial collateral ligament. J Orthop Res. 1992;10(6):895-900. [DOI] [PubMed] [Google Scholar]
- 50.Boorman RS, Shrive NG, Frank CB. Immobilization increases the vulnerability of rabbit medial collateral ligament autografts to creep. J Orthop Res. 1998;16(6):682-689. [DOI] [PubMed] [Google Scholar]
- 51.Thornton GM, Johnson JC, Maser RV, Marchuk LL, Shrive NG, Frank CB. Strength of medial structures of the knee joint are decreased by isolated injury to the medial collateral ligament and subsequent joint immobilization. J Orthop Res. 2005;23(5):1191-1198. [DOI] [PubMed] [Google Scholar]
- 52.LaPrade RF, Wijdicks CA. The management of injuries to the medial side of the knee. J Orthop Sports Phys Ther. 2012;42(3):221-233. [DOI] [PubMed] [Google Scholar]
- 53.Noyes FR, Mangine RE, Barber S. Early knee motion after open and arthroscopic anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15(2):149-160. [DOI] [PubMed] [Google Scholar]
- 54.Cosgarea AJ, DeHaven KE, Lovelock JE. The surgical treatment of arthrofibrosis of the knee. Am J Sports Med. 1994;22(2):184-191. [DOI] [PubMed] [Google Scholar]
- 55.Cosgarea AJ, Sebastianelli WJ, DeHaven KE. Prevention of arthrofibrosis after anterior cruciate ligament reconstruction using the central third patellar tendon autograft. Am J Sports Med. 1995;23(1):87-92. [DOI] [PubMed] [Google Scholar]
- 56.Ahmad CS, Kwak SD, Ateshian GA, Warden WH, Steadman JR, Mow VC. Effects of patellar tendon adhesion to the anterior tibia on knee mechanics. Am J Sports Med. 1998;26(5):715-724. [DOI] [PubMed] [Google Scholar]
- 57.Halinen J, Lindahl J, Hirvensalo E. Range of motion and quadriceps muscle power after early surgical treatment of acute combined anterior cruciate and grade-III medial collateral ligament injuries. A prospective randomized study. J Bone Joint Surg Am. 2009;91(6):1305-1312. [DOI] [PubMed] [Google Scholar]
- 58.Lorenz DS, Reiman MP, Walker JC. Periodization: current review and suggested implementation for athletic rehabilitation. Sports Health. 2010;2(6):509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buford TW, Rossi SJ. A comparison of periodization models during nine weeks with the volume and intensity to match the force. J Strength Cond Res. 2007;21(4):1245-50. [DOI] [PubMed] [Google Scholar]
- 60.Kell RT, Asmundson GJG. A comparison of two forms of periodized exercise rehabilitation programs in the management of chronic nonspecific low-back pain. J Strength Cond Res. 2009;23(2):513-523. [DOI] [PubMed] [Google Scholar]
- 61.Noehren B, Abraham A, Curry M, Johnson D, Ireland ML. Evaluation of proximal joint kinematics and muscle strength following ACL reconstruction surgery in female athletes. J Orthop Res. 2014;32(10):1305-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42-51. [DOI] [PubMed] [Google Scholar]
- 63.Iacono Dello A, Padulo J, Ayalon M. Core stability training on lower limb balance strength. J Sports Sci. July 2015:1-8. 10.1080/02640414.2015.1068437. [DOI] [PubMed] [Google Scholar]
- 64.Gould JA. Orthopaedic and Sports Physical Therapy. C.V. Mosby; 1990. [PubMed] [Google Scholar]
- 65.Romeyn RL, Jennings J, Davies GJ. Surgical treatment and rehabilitation of combined complex ligament injuries. N Am J Sports Phys Ther. 2008;3(4):212-225. [PMC free article] [PubMed] [Google Scholar]
- 66.LaPrade RF, Wijdicks CA. The management of injuries to the medial side of the knee. J Orthop Sports Phys Ther. 2012;42(3):221-233. [DOI] [PubMed] [Google Scholar]
- 67.Ageberg E, Bennell KL, Hunt MA, Simic M, Roos EM, Creaby MW. Validity and inter-rater reliability of medio-lateral knee motion observed during a single-limb mini squat. BMC Musculoskelet Disord. 2010;11(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dunn WR, Lyman S, Lincoln AE, Amoroso PJ, Wickiewicz T, Marx RG. The effect of anterior cruciate ligament reconstruction on the risk of knee reinjury. Am J Sports Med. 2004;32(8):1906-1914. [DOI] [PubMed] [Google Scholar]
- 69.Myer GD, Schmitt LC, Brent JL, et al. Utilization of Modified NFL Combine Testing to Identify Functional Deficits in Athletes Following ACL Reconstruction. J Orthop Sports Phys Ther. 2011;41(6):377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513-518. [DOI] [PubMed] [Google Scholar]
- 71.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Augustsson J, Thomeé R, Karlsson J. Ability of a new hop test to determine functional deficits after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):350-356. [DOI] [PubMed] [Google Scholar]