Abstract
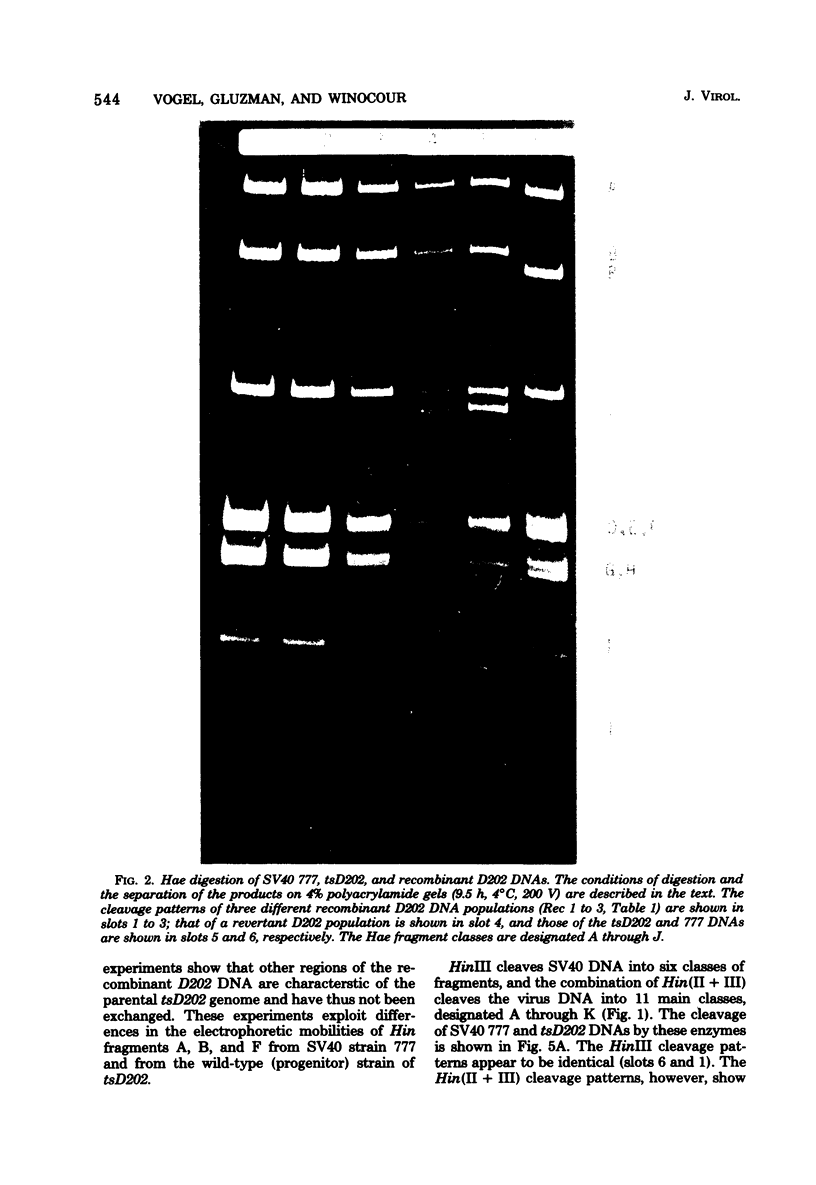
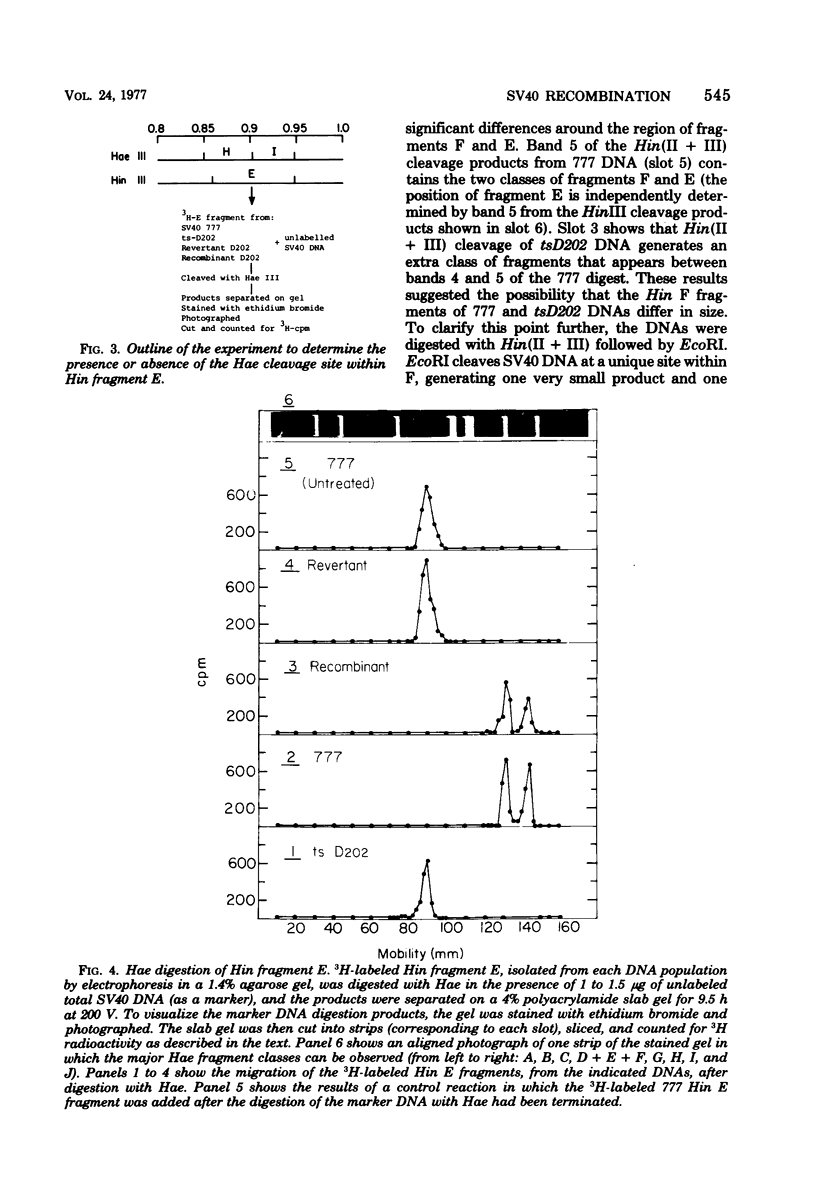
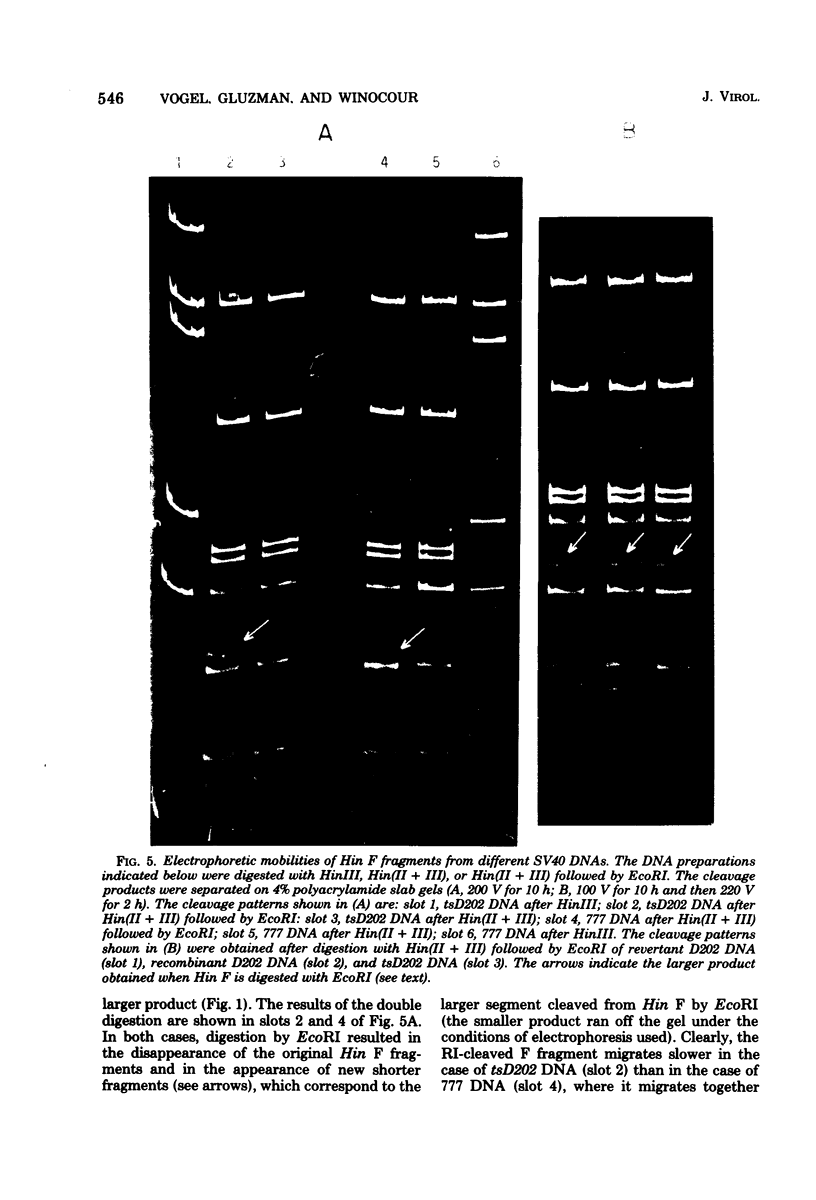
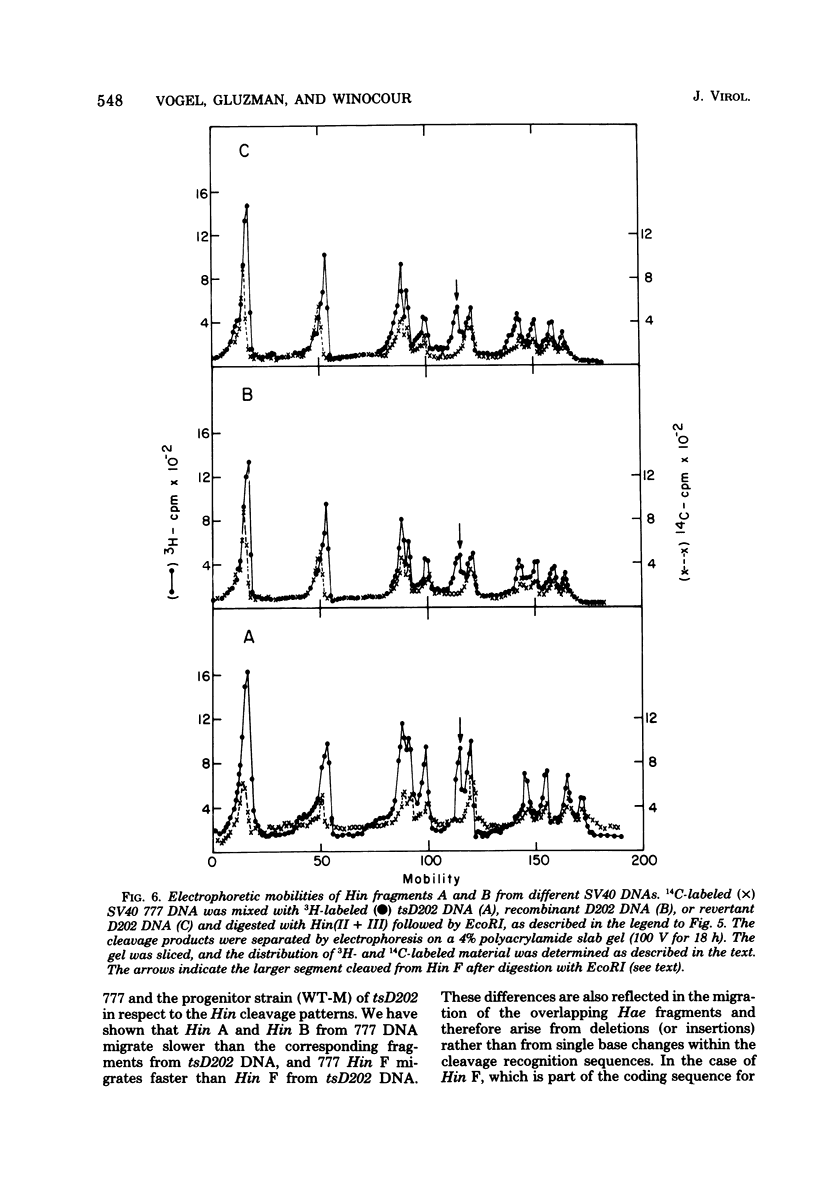
The genome of the simian virus 40 (SV40) temperature-sensitive (ts) mutant tsD202 rescued by passage on transformed permissive monkey lines (see accompanying paper [Y. Gluzman et al., J. Virol. 24:534-540, 1977]) was analyzed by restriction endonuclease cleavage mapping to obtain biochemical evidence that the rescue of the ts phenotype results from recombination with the resident SV40 genome of the transformed cell. It was demonstrated that the endonuclease R· HaeIII cleavage site, which is located at 0.9 map unit in the standard viral genome (and which is in the proximity of the known map position of the tsD lesion), is missing in the DNAs of the parental tsD202 virus and of three independent revertants of tsD202. In contrast, this cleavage site was shown to be present in the DNAs of four out of five independently derived rescued D202 populations and in the DNA of the SV40 strain, 777, used to transform the monkey cells. Comparison of the endonuclease R· Hin(II + III) cleavage patterns of SV40 strain 777 DNA and tsD202 DNA revealed differences in the electrophoretic mobilities of Hin fragments A, B, and F. However, the corresponding Hin fragments from all four rescued D202 genomes were identical in their mobilities to those of tsD202 DNA, indicating that these regions of the rescued D202 genome are characteristic of the tsD202 parent. We conclude, therefore, that the genome of the rescued D202 virus is a true recombinant, since it contains restriction endonuclease cleavage sites characteristic of both parents, the endogenous resident SV40 genome of the transformed monkey cells and the exogenous tsD202 mutant.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan R. K., Botstein D. Genetics of bacteriophage P22. I. Isolation of prophage deletions which affect immunity to superinfection. Virology. 1972 Jul;49(1):257–267. doi: 10.1016/s0042-6822(72)80027-8. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Danna K., Nathans D. Specific cleavage of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2913–2917. doi: 10.1073/pnas.68.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs D. R., Rachmeler M., Kit S. Recombination between temperature-sensitive mutants of simian virus 40. Virology. 1974 Jan;57(1):161–174. doi: 10.1016/0042-6822(74)90117-2. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Davison J., Oren M., Winocour E. Properties of permissive monkey cells transformed by UV-irradiated simian virus 40. J Virol. 1977 May;22(2):256–266. doi: 10.1128/jvi.22.2.256-266.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Kuff E. L., Winocour E. Recombination between endogenous and exogenous simian virus 40 genes. I. Rescue of a simian virus 40 temperature-sensitive mutant by passage in permissive transformed monkey lines. J Virol. 1977 Nov;24(2):534–540. doi: 10.1128/jvi.24.2.534-540.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Khoury G., Takemoto K. K., Martin M. A. Polynucleotide sequences common to the genomes of simian virus 40 and the human papovaviruses JC and BK. Virology. 1976 Aug;73(1):303–307. doi: 10.1016/0042-6822(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Deletion mutants of simian virus 40 generated by enzymatic excision of DNA segments from the viral genome. J Mol Biol. 1974 Oct 15;89(1):179–193. doi: 10.1016/0022-2836(74)90169-7. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping the genes of simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):53–60. doi: 10.1101/sqb.1974.039.01.010. [DOI] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Mapping of mutational alterations in DNA with S1 nuclease: the location of deletions, insertions and temperature-sensitive mutations in SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):61–67. doi: 10.1101/sqb.1974.039.01.011. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Nathans D. Letter: A suggested nomenclature for bacterial host modification and restriction systems and their enzymes. J Mol Biol. 1973 Dec 15;81(3):419–423. doi: 10.1016/0022-2836(73)90152-6. [DOI] [PubMed] [Google Scholar]
- Temin H. M. The cellular and molecular biology of RNA tumor viruses, especially avian leukosis-sarcoma viruses, and their relatives. Adv Cancer Res. 1974;19(0):47–104. doi: 10.1016/s0065-230x(08)60052-4. [DOI] [PubMed] [Google Scholar]
- Van de Voorde A., Contreras R., Rogiers R., Fiers W. The initiation region of the SV40 VP1 gene. Cell. 1976 Sep;9(1):117–120. doi: 10.1016/0092-8674(76)90057-x. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Yang R. C., Van de Voorde A., Fiers W. Cleavage map of the simian-virus-40 genome by the restriction endonuclease III of Haemopholus aegyptius. Eur J Biochem. 1976 Jan 2;61(1):101–117. doi: 10.1111/j.1432-1033.1976.tb10002.x. [DOI] [PubMed] [Google Scholar]
- Young R. W., Fulhorst H. W. Recovery of S35 radioactivity from protein-bearing polyacrylamide gel. Anal Biochem. 1965 May;11(2):389–391. doi: 10.1016/0003-2697(65)90030-8. [DOI] [PubMed] [Google Scholar]