Abstract
Reward motivation has been shown to modulate episodic memory processes in order to support future adaptive behavior. However, for a memory system to be truly adaptive, it should enhance memory for rewarded events as well as for neutral events that may seem inconsequential at the time of encoding but can gain importance later. Here, we investigated the influence of reward motivation on retroactive memory enhancement selectively for conceptually related information. We found behavioral evidence that reward retroactively enhances memory at a 24-h memory test, but not at an immediate memory test, suggesting a role for post-encoding mechanisms of consolidation.
Increasing the salience of an event, such as associating it with a reward, can enhance memory. Theoretical models propose that memory for these salient events are enhanced to support future adaptive behavior (Shohamy and Adcock 2010). For a memory system to be truly adaptive, however, memory should be enhanced not only for rewarding events, but also for other conceptually related events—even events encountered before the rewarded event. Thus, it may be critical for the brain to store details of events that may seem inconsequential at the time of encoding, but gain significance afterward. Here, we test whether there is a retroactive memory enhancement for items that belonged to the same category of items that are then subsequently rewarded, but are not rewarded at the time of encoding. Further, we test whether reward-associated memory enhancement is associated with post-encoding mechanisms of consolidation.
The tag-and-capture hypothesis provides a mechanism by which weak memories, such as neutral events, are strengthened by salient events occurring after encoding (Redondo and Morris 2011). This model predicts that encoding neutral items activates a synaptic “tag,” and salient events occurring after encoding promote protein-synthesis mediated consolidation of the previously encoded memory trace (Frey and Morris 1998). In turn, memories of “unimportant” events are strengthened if they are followed by salient events. Rodent research has shown that post-encoding presentations of salient events like reward or novelty can enhance memory for previously encoded information (Moncada and Viola 2007; de Carvalho Myskiw et al. 2013; Salvetti et al. 2014). While these studies provide a foundation for understanding these retroactive memory enhancements, they do not characterize the extent to which these memory enhancements generalize. Is the memory for all information preceding an event enhanced or is there a preferential memory enhancement for related information?
Work from our laboratory has previously addressed this question in the aversive domain (Dunsmoor et al. 2015). In a prior study, we demonstrated that fear conditioning can retroactively enhance memory for related information that was incidentally encoding, and these memory enhancements are supported by mechanisms of memory consolidation. This prior study was limited to the aversive domain, however, leaving open questions about the role of appetitive behavior in retroactive memory enhancements. Human research has supported a role for consolidation in guiding reward's influence on incidental memory encoding. For example, retroactive memory enhancements have been demonstrated when neutral items are immediately followed by an unrelated reward cue during incidental encoding (Murayama and Kitagami 2014). It remains unknown, however, whether these reward-related effects on consolidation are specific to items immediately followed by reward or if they generalize to conceptually related items.
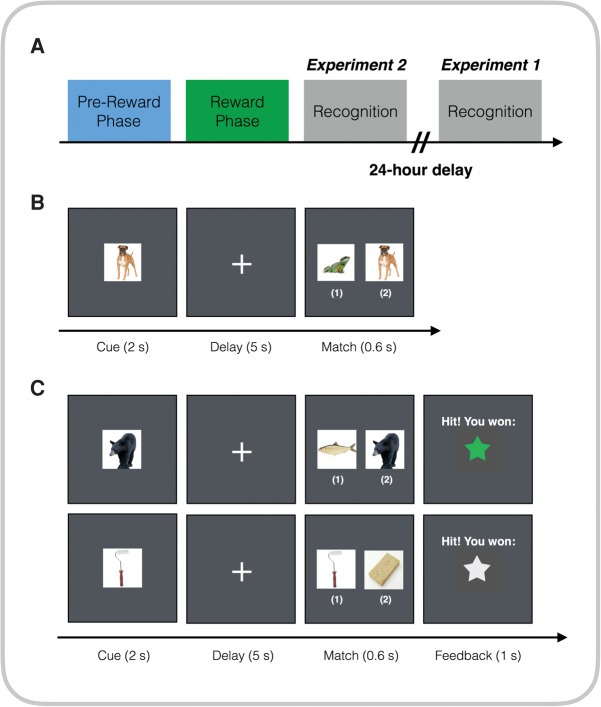
In this study, we tested the effect of reward on retroactive enhancement of memories for conceptually related items. To test this hypothesis, participants completed a two-phase, incidental encoding session followed by a surprise memory test. During the encoding session, participants first completed a nonrewarded delayed match-to-sample task (Fig. 1). On each trial, participants were presented with an image of either an animal or a tool (2 sec), and after a delay (5 sec), they were presented with the same image along with a trial-unique, foil image from the same image category. Participants were instructed to indicate which image matched the preceding image (i.e., the target) within 600 msec. Critically, during this first phase participants did not receive feedback or any performance-based reward.
Figure 1.
Experimental design. (A) Participants went through a prereward phase followed by a reward phase before completing a recognition memory test either immediately (Experiment 2) or after a 24-h delay (Experiment 1). (B) Prereward phase: Participants performed a delayed match-to-sample task. On each trial, they viewed a stimulus for 2 sec followed image pairs after a 5-sec delay. They then indicated which of those images matched the preceding target stimulus within 600 msec (“1” for the image on the left, “2” for the image on the right). No reward was given during this phase. (C) Reward phase: Participants re-did the task in prereward phase with different images followed by performance feedback (green or white stars when they correctly identified the target stimulus and did so within the time limit). The color of the stars was associated with a particular image category (animals or tools). Green stars indicated a higher reward than the white stars. In the given example, animals are associated with a higher reward than tools.
Following the prereward phase task, participants completed the reward phase task, which was identical to the prereward task except reward could be earned by an instrumental response on the delayed match-to-sample task indicated by feedback in the form of green or white stars for correct performance (see Supplemental Methods for details). Participants were notified that a performance of 90% or more on the “green star” trials would result in a $20 bonus, whereas a performance of 90% or more on the “white star” trials would result in a $1 bonus. Critically, the color of the star was always associated with a single image category (animals, tools); however, participants were not instructed of these relationships. Rather, they were expected to learn the association between the color of the stars and the image category by themselves. Assignment of the low and high reward to the image categories was counterbalanced across participants.
In Experiment 1 (n = 24, see Supplemental Methods for details), participants completed a surprise memory test for images shown during the prereward and reward phase at a 24-h delay. The memory test included 120 images of animals and 120 images of tools, half of which were target images from the prereward and reward phase and half of which were novel foil images. On each trial, participants had 6 sec to indicate whether they had previously seen the displayed image on a confidence rating scale from 1 to 4 (“Definitely yes,” “Maybe yes,” “Maybe no,” and “Definitely no”). We only report on high confidence memory (i.e., “Definitely yes” or “Definitely no”), however, results replicate when using all memory responses. To test whether memory differences across conditions were associated with post-encoding consolidation, Experiment 2 consisted of a paradigm identical to Experiment 1 except that memory was tested immediately to curtail post-encoding consolidation. Significant results during the delayed, but not immediate, memory test would indicate that memory benefits were facilitated in part by post-encoding mechanisms of consolidation.
We first analyzed performance on the delayed match-to-sample task. During the prereward phase, accuracy was greater than chance (Experiment 1: mean(se) = 0.937(0.05), P < 0.001; Experiment 2: mean(se) = 0.953(0.04) P < 0.001; Table 1), and did not differ by stimulus category (animals > tools; Experiment 1: t(22) = −1.16, P = 0.26; Experiment 2: t(24) = 1.10, P = 0.28; Supplemental Table S1A,B). Similarly in the reward phase, accuracy was significantly above chance in both high and low reward conditions (Experiment 1: high reward: mean(se) = 0.975(0.03), P < 0.001; low reward: mean(se) = 0.98(0.03), P < 0.001; Experiment 2: high reward: mean(se) = 0.975(0.03), P < 0.001; low reward: mean(se) = 0.98(0.03), P < 0.001; Table 1), and did not differ by stimulus category (Experiment 1: t(22) = 0.81, p = 0.43; Experiment 2: t(24) = −0.89, P = 0.38; Supplemental Table S1A,B).
Table 1.
Accuracy and RT for delayed match-to-sample task
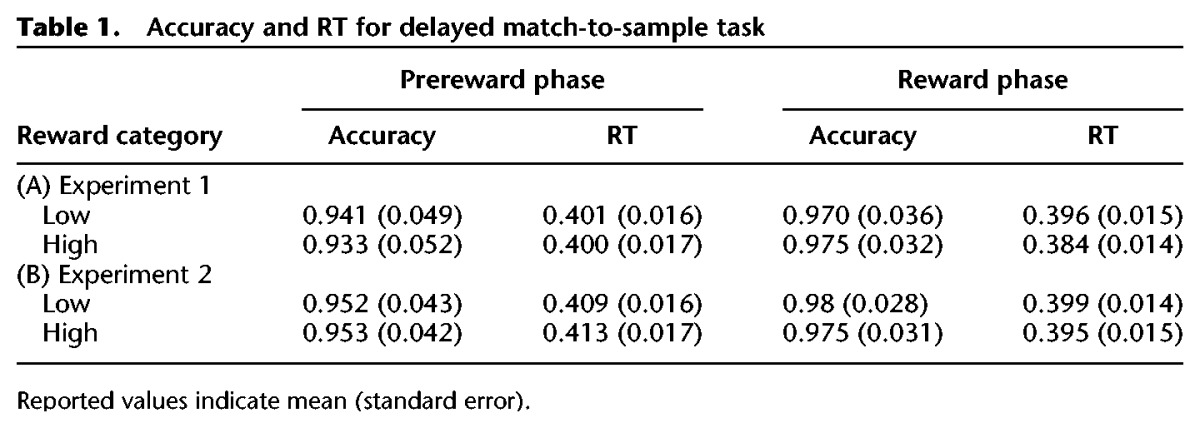
Next, we analyzed performance on the surprise memory test. In Experiment 1, participants returned after a 24-h delay to perform the memory test. We found that reward motivation enhanced memory for stimuli presented during the reward phase, such that there was better recognition memory for high compared with low reward stimuli (high confidence: t(23) = 3.43, P = 0.002; all-memory: t(23) = 2.74, P = 0.01; Fig. 2). Next, we tested memory for stimuli presented during the prereward phase. Critically, these stimuli were never directly associated with reward, as they were presented before reward incentives were ever mentioned in the experiment. Rather, the presented stimuli were only drawn from the same category as the ones that would be subsequently rewarded. As predicted, we found a retroactive enhancement of memory for stimuli that were related to the category subsequently rewarded. Specifically, there was better memory for stimuli that shared the same category as high versus low reward stimuli (t(23) = 2.98,P = 0.007; Fig. 2).
Figure 2.
At a 24-h, surprise memory test participants had significantly greater recognition memory for items associated with high versus low reward during the reward-phase (Green, R− = low reward, R+ = high reward). There was also a retroactive enhancement of memory for items in the prereward phase that were drawn from the same category of items that were subsequently associated with high versus low reward (Blue, R− = low reward, R+ = high reward).
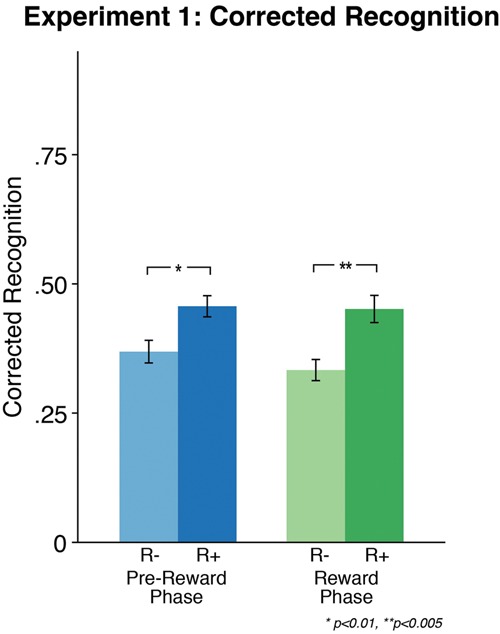
In Experiment 2 (n = 25, see Supplemental Methods for details), participants completed their recognition memory test immediately after the reward phase (∼5 min). Unlike Experiment 1, we found that reward motivation did not influence immediate memory, in that there were no differences in recognition memory across high and low reward stimuli (t(24) = 0.85, P = 0.4; Fig. 3). Direct comparisons across experiments showed a larger effect of reward on memory during the 24-h versus immediate memory test (F(47) = 3.44, P = 0.03, one-tailed). Next, we tested memory for stimuli presented during prereward phase, and found that there was no difference in immediate memory for stimuli that were related to the category subsequently rewarded (t(24) = 0.003, P = 0.99; Fig. 3). Direct comparisons across experiments showed a significantly larger retroactive enhancement of reward on memory for related stimuli at the 24-h versus immediate memory test (F(47) = 3.44, P = 0.03, one-tailed).
Figure 3.
At the immediate, surprise memory test, there were no significant differences between items associated with high versus low reward during reward phase (green), or items drawn from high versus low reward categories during the prereward phase (blue).
To provide a more rigorous test of these effects, we ran a three-factor ANOVA with phase (prereward, reward) and category (high reward, low reward) as within-subject factors, and delay (immediate, 24-h) as a between-subject factor. Critically, this analysis revealed a significant delay × category interaction (F(1,47) = 5.19, P = 0.03) indicating better memory for items drawn from the high versus low reward categories in the delayed versus immediate condition. These findings support that the benefits of reward on memory and retroactive memory only emerge after a delay. This analysis also revealed a main effect of delay (i.e., worse performance in the delayed versus immediate group; F(1,47) = 30.86, P < 0.001), a main effect of category (i.e., better memory for rewarded versus nonrewarded categories, F(1,47) = 9.13, P = 0.004), and a delay × phase interaction (i.e., better memory for the reward versus prereward phase in the delay versus immediate group independent of category, F(1,47) = 5.58, P = 0.02). Notably, performance differences across groups during the delayed match-to-sample task could not account for differences in reward's influence on memory performance (see Supplemental Material for control analyses).
The present study shows that, at a 24-h surprise memory test, individuals had memory enhancements both for images directly associated with reward and images drawn from the same category of information subsequently rewarded. For example, when animal memoranda were associated with high monetary bonus during the reward phase, there was also enhanced memory for animal memoranda presented before any reward learning ever occurred. These results are especially interesting because these items were never themselves associated with reward. These effects were evident only on a long-term memory test, as no differences in memory were evident at an immediate test. This suggests that reward's influence on memory, including retroactive memory, is supported by post-encoding mechanisms of consolidation.
These patterns of findings provide strong support for models of synaptic tag-and-capture, which have been detailed in animal models (Frey and Morris 1998; Redondo and Morris 2011). These models predict that memory for neutral items—such as nonrewarded items—can retroactively be enhanced when followed by a more salient event. Here, we show that similar retroactive memory enhancements described in these rodent models are evident in humans. Critically, however, we extend these models by demonstrating that memory enhancements are limited to items that are conceptually related to the salient events. Thus, our findings build upon the prior literature to specify that semantic distance may be a key factor determining the extant of retroactive memory enhancements by reward.
Previous research has demonstrated that reward facilitates memory by targeting mechanisms of post-encoding consolidation. In the animal literature, the introduction of rewards after encoding enhances memory for previously presented information during delayed, but not immediate, tests of memory (Wang and Morris 2010), a behavioral hallmark of consolidation-related memory enhancements. Similarly, in humans, the role of consolidation in retroactive memory enhancements during incidental encoding has been shown for memoranda presented in close temporal proximity to rewards (i.e., on the order of seconds; Murayama and Kitagami 2014). Our study extends these findings by showing that retroactive memory enhancements can happen at longer temporal delays, on the order of minutes, and the influence of reward on this process extends to information that is semantically related to the experience. Notably, a recent study failed to demonstrate a retroactive memory benefits by reward motivation using a similar design (Oyarzún et al. 2016). However, there was a key difference in the design of the studies in how reward motivation was elicited. The prior study implemented a classical conditioning procedure, whereas, we implemented a performance-contingent operant conditioning procedure (i.e., correct performance results in a monetary bonus). The pattern of findings across these studies raises the possibility that differences in how individuals earn reward (i.e., passive versus active) significantly influences retroactive memory.
This study complements prior work from our laboratory demonstrating a similar pattern of results in the aversive domain (Dunsmoor et al. 2015). Similar to reward, fear facilitated retroactive memory benefits for related items during a delayed memory test. While retroactive memory enhancements were similar, there were notable differences in the pattern of immediate memory benefits across studies. In the aversive domain, there were immediate memory benefits for items presented during fear conditioning, whereas, there were no immediate memory benefits for reward-related items. Prior work in the field of emotional memory has suggested that different mechanisms may support immediate versus delayed memory benefits. Namely, immediate memory benefits are thought to result from attentional mechanisms whereas delayed memory benefits are thought to result from post-encoding consolidation (Kensinger and Corkin 2004; Talmi et al. 2008). In light of these findings, we propose that a similar mechanism of post-encoding consolidation may support retroactive memory benefits in rewarding and aversive contexts (detailed below), however, different mechanisms may be supporting encoding and immediate memory benefits in aversive and rewarding contexts. In line with this interpretation, recent research has shown that discrete neural systems are engaged during incidental encoding in rewarding versus punishing contexts (Murty et al. 2016a).
Our findings demonstrate that reward motivation can retroactively enhance memory for related information via post-encoding mechanisms of consolidation. Open questions remain, however, as to how these memory enhancements are instantiated in the brain. One mechanism by which information is stabilized in memory via post-encoding processes is systems-level memory consolidation (McClelland et al. 1995; Nadel et al. 2000; Sutherland and McNaughton 2000). Within this framework, post-encoding interactions between hippocampus and cortex, particularly sensory cortex associated with encoding, stabilizes information in long-term memory. Theoretically, reward could selectively facilitate systems-level consolidation with sensory cortex associated with reward, and these processes could generalize to information previously encoded within the cortex prior to the introduction of reward incentives. For example, when images of animals are associated with reward, there would be a facilitation of post-encoding interactions between the hippocampus and category-selective cortex selective to processing animal memoranda that would in turn stabilize information in long-term memory. Critically, memory traces of animal images explicitly associated with reward (i.e., memoranda from the reward phase), as well as previously encoded memory traces of animal images (i.e., memoranda from the prereward phase) would both benefit from this mechanism. Interestingly, research has demonstrated that rewarded information can be preferentially “reactivated” following encoding (Singer and Frank 2009; Gomperts et al. 2015; Valdés et al. 2015; Gruber et al. 2016), which represents a putative mechanism supporting systems-level consolidation (Carr et al. 2011; Squire et al. 2015). Further, emotional learning, albeit in the aversive domain, has been shown to restructure representations of objects in category-selective cortex (i.e., animal versus tool cortex) associated with reinforcement (Dunsmoor et al. 2014). Finally, reward motivation, albeit in an intentional encoding paradigm, has been shown to selectively facilitate post-encoding interactions of the ventral tegmental area, the source of dopamine neurons, and the hippocampus with category-selective cortex in service of better memory (Murty et al. 2016b). Future work, however, will have to validate this proposed mechanism of retroactive memory enhancements by reward.
In conclusion, our work provides evidence that rewards can selectively and retroactively enhance memory for conceptually related information. Further, we show that these retroactive memory enhancements are supported by post-encoding mechanisms of consolidation. Together these results extend prior findings that aversive events can retroactively enhance memory and further corroborate rodent models of synaptic tag-and-capture. Finally, our findings extend current conceptualizations of the role of systems-level consolidation in reward-mediated memory enhancements.
Supplementary Material
Acknowledgments
We thank A. Tompary and V. Man for helpful discussions and comments. We also thank our financial supporters, NIMH grant R01 MH074692.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.042978.116.
References
- Carr MF, Jadhav SP, Frank LM. 2011. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci 14: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Myskiw J, Benetti F, Izquierdo I. 2013. Behavioral tagging of extinction learning. Proc Natl Acad Sci 110: 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Kragel PA, Martin A, LaBar KS. 2014. Aversive learning modulates cortical representations of object categories. Cereb Cortex 24: 2859–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Murty VP, Davachi L, Phelps EA. 2015. Emotional learning selectively and retroactively strengthens memories for related events. Nature 520: 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RGM. 1998. Weak before strong: dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology 37: 545–552. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Kloosterman F, Wilson MA. 2015. VTA neurons coordinate with the hippocampal reactivation of spatial experience. Elife 4 10.7554/eLife.05360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber MJ, Ritchey M, Wang S-F, Doss MK, Ranganath C. 2016. Post-learning hippocampal dynamics promote preferential retention of rewarding events. Neuron 89: 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. 2004. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci 101: 3310–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. 1995. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102: 419–457. [DOI] [PubMed] [Google Scholar]
- Moncada D, Viola H. 2007. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J Neurosci 27: 7476–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama K, Kitagami S. 2014. Consolidation power of extrinsic rewards: reward cues enhance long-term memory for irrelevant past events. J Exp Psychol Gen 143: 15–20. [DOI] [PubMed] [Google Scholar]
- Murty VP, LaBar KS, Adcock RA. 2016a. Distinct medial temporal networks encode surprise during motivation by reward versus punishment. Neurobiol Learn Mem 134: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Tompary A, Adcock RA, Davachi L. 2016b. Selectivity in post-encoding connectivity with high-level visual cortex is associated with reward-motivated memory. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Samsonovich A, Ryan L, Moscovitch M. 2000. Multiple trace theory of human memory: computational, neuroimaging, and neuropsychological results. Hippocampus 10: 352–368. [DOI] [PubMed] [Google Scholar]
- Oyarzún JP, Packard PA, de Diego-Balaguer R, Fuentemilla L. 2016. Motivated encoding selectively promotes memory for future inconsequential semantically-related events. Neurobiol Learn Mem 133: 1–6. [DOI] [PubMed] [Google Scholar]
- Redondo RL, Morris RGM. 2011. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci 12: 17–30. [DOI] [PubMed] [Google Scholar]
- Salvetti B, Morris RGM, Wang S-H. 2014. The role of rewarding and novel events in facilitating memory persistence in a separate spatial memory task. Learn Mem 21: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. 2010. Dopamine and adaptive memory. Trends Cogn Sci 14: 464–472. [DOI] [PubMed] [Google Scholar]
- Singer AC, Frank LM. 2009. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron 64: 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Genzel L, Wixted JT, Morris RG. 2015. Memory consolidation. Cold Spring Harb Perspect Biol 7: a021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GR, McNaughton B. 2000. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol 10: 180–186. [DOI] [PubMed] [Google Scholar]
- Talmi D, Anderson AK, Riggs L, Caplan JB, Moscovitch M. 2008. Immediate memory consequences of the effect of emotion on attention to pictures. Learn Mem 15: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés JL, McNaughton BL, Fellous J-M. 2015. Offline reactivation of experience-dependent neuronal firing patterns in the rat ventral tegmental area. J Neurophysiol 114: 1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-H, Morris RGM. 2010. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol 61: 49–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.