Abstract
AIM
To clarify the current state of practice for colonic diverticular bleeding (CDB) in Japan.
METHODS
We conducted multicenter questionnaire surveys of the practice for CDB including clinical settings (8 questions), diagnoses (8 questions), treatments (7 questions), and outcomes (4 questions) in 37 hospitals across Japan. The answers were compared between hospitals with high and low number of inpatient beds to investigate which factor influenced the answers.
RESULTS
Endoscopists at all 37 hospitals answered the questions, and the mean number of endoscopists at these hospitals was 12.7. Of all the hospitals, computed tomography was performed before colonoscopy in 67% of the hospitals. The rate of bowel preparation was 46.0%. Early colonoscopy was performed within 24 h in 43.2% of the hospitals. Of the hospitals, 83.8% performed clipping as first-line endoscopic therapy. More than half of the hospitals experienced less than 20% rebleeding events after endoscopic hemostasis. No significant difference was observed in the annual number of patients hospitalized for CDB between high- (≥ 700 beds) and low-volume hospitals. More emergency visits (P = 0.012) and endoscopists (P = 0.015), and less frequent participation of nursing staff in early colonoscopy (P = 0.045) were observed in the high-volume hospitals.
CONCLUSION
Some practices unique to Japan were found, such as performing computed tomography before colonoscopy, no bowel preparation, and clipping as first-line therapy. Although, the number of staff differed, the practices for CDB were common irrespective of hospital size.
Keywords: Colonic diverticular hemorrhage, Lower gastrointestinal bleeding, Computed tomography, Endoscopy, Bowel preparation
Core tip: Colonic diverticular bleeding (CDB) is increasing in Asia. There are no practice guidelines for CDB, and it is important to determine which recommendation is acceptable to a majority of hospitals. We conducted multicenter questionnaire surveys of 37 hospitals in Japan regarding management of CDB including clinical settings, diagnosis, treatment, and clinical outcomes, and made comparisons between hospitals with different patient volumes and between hospitals in different regions. Thus, practice styles unique to Japan such as performing computed tomography before colonoscopy, no bowel preparation, and clipping as first-line therapy were identified. However, management of CDB was common among hospitals irrespective of hospital size and region.
INTRODUCTION
Colonic diverticular bleeding (CDB) is a major cause of lower gastrointestinal bleeding, and is estimated to cause 25% to 40% of all cases of lower gastrointestinal bleeding[1-3]. In Japan, CDB was found in 427 (1.5%) of 28192 patients who underwent colonoscopy at an emergency hospital[4]. Its occurrence has increased in Japan as well as in Western countries[4-7]. CDB results in hemorrhagic shock requiring blood transfusion[8,9], and has a high recurrence rate of 20% within 1 year[10,11]. As a result, patients are often burdened by the frequent examinations, hospitalization, repeated blood transfusions, and a consequent decrease in their quality of life. Furthermore, these practices for CDB may be different between Western countries and Japan. For example, Western countries perform purged colonoscopy using polyethylene glycol as the first diagnostic procedure, and perform endoscopic hemostasis using clipping[12]. In contrast, Japanese hospitals have good access to computed tomography (CT)[13] and may select CT as the first diagnostic procedure. In addition, diagnostic tools, endoscopic environment, and treatment strategy may potentially differ among hospitals in Japan. Moreover, the practice for CDB may differ according to hospital patient volume and region, as is seen in the practice for other lower gastrointestinal disease[14,15]. Some studies have reported significant associations between hospital volume and clinical outcome, and between hospital region and diagnosis methods[14,15]. Today, there are no practice guidelines for CDB, and it is important to determine what recommendations would be acceptable to a large number of hospitals.
Therefore, we conducted a multicenter questionnaire survey of the practice for CDB in 37 hospitals across Japan to elucidate the current state of the clinical settings, diagnosis, treatment, and clinical outcomes of patients with CDB, and to compare these findings according to hospital volumes and regions.
MATERIALS AND METHODS
Contents of the questionnaire
First, 1 endoscopist (Doyama H) developed the questionnaire on practice for CDB. Then, 3 endoscopists (Ota R, Niikura R and Nagata N) reviewed and edited the questionnaire regarding the length, clarity, and contents. Finally, 27 survey questions on practice for CDB were developed. The questionnaire consisted of 4 parts (clinical settings, diagnosis, treatment, clinical outcomes) as follows. In part (I), there were 9 questions for clinical settings on: (1) the clinical database for CDB such as gastrointestinal bleeding database, inpatient database, or endoscopy database; (2) institution-specific strategy for CDB; (3) number of CDB admissions; (4) number of emergency ambulance visits; (5) number of endoscopists performing early colonoscopy within 24 h of patient arrival; (6) number of expert endoscopists with hemostatic technical skills; (7) nursing staff who monitored vital signs during bowel preparation; (8) nursing staff assisting early colonoscopy; and (9) use of a water-jet colonoscope. For part (II), there were 8 questions for diagnoses of CBD on (10) the first choice diagnostic examination; (11) early contrast-enhanced CT within 3 h of patient arrival; (12) early colonoscopy; (13) bowel preparation; (14) cap-assisted colonoscopy; (15) how to improve the identification of stigmata of recent hemorrhage (SRH); (16) availability of small bowel examinations in case of negative colonoscopy; and (17) modality for small bowel examinations. For part (III), there were 6 questions for treatment of CDB on (18) first-line endoscopic therapy; (19) selection of non-endoscopic therapy; (20) first-line therapy among non-endoscopic therapies; (21) how to prevent rebleeding; (22) discontinuation of antithrombotic drugs on admission; and (23) strategy for restarting antithrombotic drugs. In part (IV), there were 4 questions for clinical outcomes of CDB on (24) identification rate of SRH; (25) rebleeding rate after endoscopic hemostasis; (26) rebleeding rate after interventional radiology; and (27) rebleeding rate after barium impaction therapy.
Questionnaire survey
The questionnaire survey was conducted by e-mail that was sent to 1 or 2 endoscopists at each of the 37 hospitals with different numbers of inpatient beds and in different regions in Japan between May 2015 and June 2015. Selection of the hospitals was made by Fujishiro M, who knew that the representative endoscopists would be interested in this topic from his personal communications. To assess the reproducibility of questionnaire, we conducted a blinded secondary questionnaire survey 2 mo after using the same 16 questionnaire items. Selection of these questionnaire items was made by Niikura R and Nagata N. because these items were found to be related to the practice for CDB. These 37 hospitals were located in East or West Japan and have 100 to 1000 inpatient beds (Appendix).
Statistical analysis
The data from the first questionnaire survey were analyzed, and the intra-observer agreement between the first and second questionnaires was analyzed using kappa statistics. Kappa values were evaluated as follows: > 0.80, excellent agreement; > 0.60 to 0.80, good agreement; > 0.40 to 0.60, moderate; > 0.20 to 0.40, fair; and ≤ 0.20, poor[16].
A high-volume hospital was defined as one with over 700 beds, because the median number of beds in our data was 700 beds per hospital. Expert endoscopists were defined as those who were able to perform endoscopic hemostatic treatment by themselves. We evaluated the clinical settings, diagnosis methods, treatment, and outcomes between the groups of hospital separated by hospital volume and region (East Japan, West Japan) using a χ2 test or Fisher’s exact test as appropriate. Continuous variables were compared using the Mann-Whitney U test. We also evaluated the associations of the rates of SRH identification and rebleeding with type of procedure from questionnaire answers using a nonparametric trend test. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using the STATA version 13 software (StataCorp, College Station, TX, United States).
RESULTS
The number of beds per hospital in each region of Japan is shown in Figure 1. There were 18 high-volume hospitals (≥ 700 beds) and 19 low-volume hospitals. Twenty-one of the 37 (56.8%) hospitals were located in East Japan, and 16 hospitals (43.2%) were located in West Japan (Figure 1). All 37 hospitals completed the first questionnaires, and 35 of the hospitals completed the second questionnaires. Intra-observer agreement for each question between the first and second surveys was excellent (mean κ, 0.83, 95% confidence interval 0.78-0.87) (Supplementary Table 1).
Figure 1.
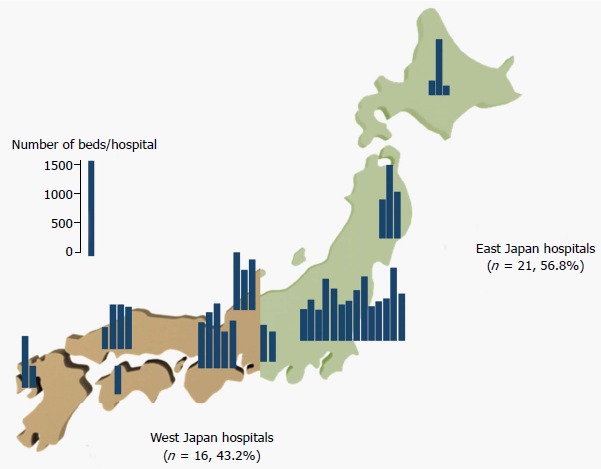
Number of beds per hospital in East and West Japan.
Table 1.
Questions and answers regarding clinical settings in 37 hospitals n (%)
| No. | Question | Answer (n = 37) | High volume (n = 18) | Low volume (n = 19) | P value | East Japan (n = 21) | West Japan (n = 16) | P value |
| 1 | Did you answer the questions based on a clinical database? | |||||||
| Yes | 31 (83.8) | 16 (51.6) | 15 (48.4) | 18 (58.1) | 13 (41.9) | |||
| No | 6 (16.2) | 2 (33.3) | 4 (66.7) | 0.660 | 3 (50.0) | 3 (50.0) | 1.000 | |
| 2 | Do you have a specific institutional strategy for CDB? | |||||||
| Yes | 5 (13.5) | 0 | 5 (100) | 2 (40.0) | 3 (60.0) | |||
| No | 32 (86.5) | 18 (56.3) | 14 (43.7) | 0.046 | 19 (59.4) | 13 (40.6) | 0.634 | |
| 3 | How many patients are hospitalized for CDB annually? | |||||||
| 1-10 | 12 (32.5) | 7 (58.3) | 5 (41.7) | 7 (58.3) | 5 (41.7) | |||
| 11-20 | 10 (27.0) | 4 (40.0) | 6 (60.0) | 4 (40.0) | 6 (60.0) | |||
| 21-30 | 5 (13.5) | 2 (40.0) | 3 (60.0) | 1 (20.0) | 4 (80.0) | |||
| ≥ 31 | 10 (27.0) | 2 (50.0) | 5 (50.0) | 0.824 | 9 (90) | 1 (10) | 0.035 | |
| 4 | How many emergency ambulance visits do you receive annually?1 | |||||||
| < 2000 | 15 (44.1) | 5 (33.3) | 10 (66.7) | 8 (53.3) | 7 (46.7) | |||
| 2000-6000 | 11 (32.3) | 4 (36.4) | 7 (63.6) | 5 (45.5) | 6 (54.5) | |||
| 6000-10000 | 6 (17.7) | 6 (100) | 0 | 3 (50.0) | 3 (50.0) | |||
| ≥ 10000 | 2 (5.9) | 0 | 2 (100) | 0.012 | 2 (100) | 0 | 0.724 | |
| 5 | How many endoscopists perform early colonoscopy within 24 h after patient arrival at your hospital? | |||||||
| 12.7 ± 9.4 | 17.0 ± 11.6 | 8.8 ± 4.4 | 0.015 | 10.4 ± 5.7 | 15.8 ± 12.5 | 0.296 | ||
| 6 | How many are expert endoscopists with endoscopic hemostasis technical skills are there at your hospital? | |||||||
| 10.1 ± 7.5 | 13.1 ± 9.5 | 7.3 ± 3.3 | 0.019 | 7.9 ± 3.3 | 13.0 ± 10.2 | 0.143 | ||
| 7 | Do you have nursing staff who monitor the patients’ vital signs during bowel preparation? | |||||||
| Yes | 33 (89.2) | 17 (51.5) | 16 (48.5) | 19 (57.6) | 14 (42.4) | |||
| No | 4 (10.8) | 1 (25.0) | 3 (75.0) | 0.604 | 2 (50.0) | 2 (50.0) | 1.000 | |
| 8 | Do you have nursing staff for early colonoscopy examinations within 24 h after patient arrival at the hospital? | |||||||
| Yes | 23 (62.2) | 8 (34.8) | 15 (65.2) | 13 (56.5) | 10 (43.5) | |||
| No | 14 (37.8) | 10 (71.4) | 4 (28.6) | 0.045 | 8 (57.1) | 6 (42.9) | 1.000 | |
| 9 | Do you have a water-jet colonoscope? | |||||||
| Yes | 34 (91.9) | 17 (50.0) | 17 (50.0) | 20 (58.9) | 14 (41.1) | |||
| No | 3 (8.1) | 1 (33.3) | 2 (66.7) | 1.000 | 1 (33.3) | 2 (66.7) | 0.568 | |
Missing data included. The values in parentheses are percentages, and continuous data are shown as mean ± standard deviation. CDB: Colonic diverticular bleeding.
Questionnaire items for clinical settings
Questions and answers regarding clinical settings are shown in Table 1. Of all the hospitals, 86.5% answered the questionnaire based on the clinical database of each hospital. Only 13.5% of hospitals had an institution-specific strategy for CDB. The number of CDB patients who received therapy, and the number of emergency ambulance visits, differed among hospitals. The mean number of endoscopists and expert endoscopists were 12.7 and 10.1, respectively. Of all the hospitals, 89.2% and 62.2% had nursing staff for monitoring vital signs during bowel preparation and early colonoscopy examination, respectively. Ninety-one percent of hospitals had a water-jet colonoscope.
Comparing hospital with high and low patient volumes, more emergency visits (P = 0.012), endoscopists (P = 0.015), and expert endoscopists (P = 0.019), and less institution-specific management for CDB (P = 0.046) and frequent participation of nursing staff in early colonoscopy (P = 0.045) were observed in high-volume hospitals (Table 1). No significant differences were observed in other questionnaire items such as number of patients hospitalized for CDB between the two groups (Table 1). Comparing hospitals in East and West Japan, a higher number of patients hospitalized for CDB was observed in East Japan hospitals (P = 0.035) (Table 1). No significant difference was observed in other questionnaire items between the two groups (Table 1).
Questionnaire items for diagnoses
Questions and answers regarding diagnosis are shown in Table 2. Of all the hospitals, 59.5% selected contrast-enhanced CT as first examination of choice. The rates of urgent CT, early colonoscopy, bowel preparation, cap-assisted colonoscopy were 61.1%, 43.2%, 46.0%, and 64.9%, respectively. Ninety-one percent of hospitals washed out with water to improve identification of SRH. There was a wide variation among hospitals in small bowel intestinal examination, but 85.3% of hospitals selected capsule endoscopy as the tool of choice when it was unable to diagnose definite CDB.
Table 2.
Questions and answers regarding diagnosis of colonic diverticular bleeding in 37 hospitals n (%)
| No. | Question | Answer (n = 37) | High volume (n = 18) | Low volume (n = 19) | P value | East Japan (n = 21) | West Japan (n = 16) | P value |
| 10 | What do you use as the first-line diagnostic method for hematochezia and suspected CDB? | |||||||
| Non-contrast-enhanced CT | 3 (8.1) | 0 | 3 (100) | 0 | 3 (100) | |||
| Contrast-enhanced CT | 22 (59.5) | 11 (50.0) | 11 (50.0) | 12 (54.6) | 10 (45.4) | |||
| Colonoscopy | 10 (27.0) | 6 (60.0) | 4 (40.0) | 7 (70.0) | 3 (30.0) | |||
| Contrast-enhanced CT and colonoscopy | 2 (5.4) | 1 (50.0) | 1 (50.0) | 0.359 | 2 (100) | 0 | 0.101 | |
| 11 | Can you perform urgent contrast-enhanced CT within 3 h after patient arrival at hospital?2 | |||||||
| Yes | 22 (61.1) | 12 (54.6) | 10 (45.4) | 13 (59.1) | 9 (40.9) | |||
| No | 14 (38.9) | 6 (42.9) | 8 (57.1) | 0.494 | 7 (50.0) | 7 (50.0) | 0.593 | |
| 12 | Can you perform early colonoscopy within 24 h after patient arrival at hospital? | |||||||
| Yes | 16 (43.2) | 9 (56.3) | 7 (43.7) | 10 (62.5) | 6 (37.5) | |||
| No | 21 (56.8) | 9 (42.9) | 12 (57.1) | 0.419 | 11 (52.4) | 10 (47.6) | 0.538 | |
| 13 | Do you request bowel preparation? | |||||||
| Yes | 17 (46.0) | 6 (35.3) | 11 (64.7) | 13 (76.5) | 4 (23.5) | |||
| No | 3 (8.1) | 3 (100) | 0 | 2 (66.7) | 1 (33.3) | |||
| Case by case | 17 (45.9) | 9 (52.9) | 8 (47.1) | 0.105 | 6 (35.3) | 11 (64.7) | 0.046 | |
| 14 | Do you use a cap-assisted colonoscopy for early colonoscopy? | |||||||
| Yes | 24 (64.9) | 11 (45.8) | 13 (54.2) | 15 (62.5) | 9 (37.5) | |||
| No | 13 (35.1) | 7 (53.9) | 6 (46.1) | 0.642 | 6 (46.2) | 7 (53.8) | 0.338 | |
| 15 | How do you perform colonoscopy to improve identification of SRH?1 | |||||||
| Cap-assisted colonoscopy | 17 (46.0) | 10 (58.8) | 7 (41.2) | 0.254 | 10 (58.8) | 7 (41.2) | 0.815 | |
| Long cap-assisted colonoscopy | 13 (35.1) | 6 (46.2) | 7 (53.8) | 0.823 | 7 (53.9) | 6 (46.1) | 0.793 | |
| Inverting diverticulum via suction of colonoscopy | 18 (48.7) | 11 (61.1) | 7 (38.9) | 0.140 | 11 (61.1) | 7 (38.9) | 0.603 | |
| Wash out with water | 36 (97.3) | 18 (50.0) | 18 (50.0) | 1.000 | 21 (58.3) | 15 (41.7) | 0.432 | |
| Colonoscopy by multiple doctors | 3 (8.1) | 1 (33.3) | 2 (66.7) | 1.000 | 2 (66.7) | 1 (33.3) | 1.000 | |
| Colonoscopy under X-ray | 3 (8.1) | 1 (33.3) | 2 (66.7) | 1.000 | 1 (33.3) | 2 (66.7) | 0.568 | |
| 16 | Do you examine the small bowel when you are unable to diagnose definite CDB by colonoscopy? | |||||||
| Yes | 18 (48.7) | 11 (61.1) | 7 (38.9) | 10 (55.6) | 8 (44.4) | |||
| No | 7 (18.9) | 1 (14.3) | 6 (85.7) | 4 (57.1) | 3 (42.9) | |||
| Case by case | 12 (32.4) | 6 (50.0) | 6 (50.0) | 0.145 | 7 (58.3) | 5 (41.7) | 1.000 | |
| 17 | Which modality do you select for the small bowel examination?2 | |||||||
| Capsule endoscopy | 29 (85.3) | 17 (58.6) | 12 (41.4) | 18 (62.1) | 11 (37.9) | |||
| Balloon-endoscopy | 2 (5.9) | 0 | 2 (100) | 1 (50.0) | 1 (50.0) | |||
| Case by case | 3 (8.8) | 1 (33.3) | 2 (66.7) | 0.301 | 1 (33.3) | 2 (66.7) | 0.776 | |
Duplicated data allowed;
Missing data included. Parenthesis shows percentage. CT: Computed tomography; CDB: Colonic diverticular bleeding; SRH: Stigmata of recent hemorrhage.
No significant differences between hospitals with high and low patient volumes were observed in all questionnaire items (Table 2). Comparing hospitals in East and West Japan, East Japan hospitals performed more frequent bowel preparation compared with West Japan hospitals (P = 0.046) (Table 2). No significant differences were observed in other questionnaire items between the two groups (Table 2).
Questionnaire items for treatments
Questions and answers regarding treatment are shown in Table 3. In endoscopic treatment, clipping, band ligation, and epinephrine injection were performed as first-line therapy in 83.8%, 13.5%, and 2.7% of hospitals. Seventy-three percent and 67% of hospitals selected non-endoscopic therapy for patients with rebleeding and hemorrhagic shock, and 77.4% of hospitals performed interventional radiology as first-line non-endoscopic therapy. Fifty-nine percent of hospitals discontinued antithrombotic drugs on admission and only 15% of hospitals had a strategy for restarting these drugs.
Table 3.
Questions and answers regarding treatments of colonic diverticular bleeding in 37 hospitals n (%)
| No. | Question | Answer (n = 37) | High volume (n = 18) | Low volume (n = 19) | P value | East Japan (n = 21) | West Japan (n = 16) | P value |
| 18 | What kind of endoscopic treatment do you perform as first-line therapy? | |||||||
| Clipping | 31 (83.8) | 15 (48.4) | 16 (51.6) | 1.000 | 17 (54.8) | 14 (45.2) | ||
| Endoscopic band ligation | 5 (13.5) | 3 (60.0) | 2 (40.0) | 3 (60.0) | 2 (40.0) | |||
| Epinephrine injection | 1 (2.7) | 0 | 1 (100) | 1 (100) | 0 | 1.000 | ||
| 19 | What kinds of patient undergo non-endoscopic therapy?1 | |||||||
| Patients with an unidentified bleeding source | 18 (48.7) | 10 (55.6) | 8 (44.4) | 0.413 | 8 (44.4) | 10 (55.6) | 0.141 | |
| Patients with rebleeding | 27 (73.0) | 15 (55.6) | 12 (44.4) | 0.269 | 17 (63.0) | 10 (37.0) | 0.274 | |
| Patients with hemorrhagic shock | 25 (67.6) | 15 (60.0) | 10 (40.0) | 0.079 | 13 (52.0) | 12 (48.0) | 0.491 | |
| 20 | What kind of non-endoscopic therapy do you perform as first-line therapy or when you are unable to identify SRH at endoscopy? | |||||||
| IVR | 24 (77.4) | 2 (50.0) | 2 (50.0) | 0.253 | 10 (41.7) | 14 (58.3) | ||
| Surgery | 3 (9.7) | 14 (58.3) | 10 (41.7) | 3 (100) | 0 | |||
| Barium impaction therapy | 4 (12.9) | 0 | 3 (100) | 3 (75.0) | 1 (25.0) | 0.145 | ||
| 21 | What kind of treatment do you perform to prevent rebleeding?1 | |||||||
| Treatment of diabetes mellitus | 0 | 0 | 0 | NA | 0 | 0 | NA | |
| Treatment of hypertension | 6 (17.1) | 2 (33.3) | 4 (66.7) | 0.658 | 3 (50.0) | 3 (50.0) | 1.000 | |
| Discontinuation NSAIDs | 14 (40.0) | 7 (50.0) | 7 (50.0) | 0.890 | 10 (71.4) | 4 (28.6) | 0.296 | |
| Discontinuation antithrombotic drugs | 22 (62.9) | 11 (50.0) | 11 (50.0) | 0.826 | 15 (68.2) | 7 (31.8) | 0.086 | |
| Administrating vitamin D | 0 | 0 | 0 | NA | 0 | 0 | NA | |
| Treatment of constipation | 14 (40.0) | 9 (64.3) | 5 (35.7) | 0.129 | 6 (42.9) | 8 (57.1) | 0.163 | |
| Administrating a low fiber diet | 5 (14.3) | 2 (40.0) | 3 (60.0) | 1.000 | 3 (60.0) | 2 (40.0) | 1.000 | |
| 22 | Do you discontinue antithrombotic drugs on admission? | |||||||
| Yes | 22 (59.5) | 10 (45.5) | 12 (54.5) | 12 (54.6) | 10 (46.4) | |||
| No | 12 (32.4) | 7 (58.3) | 5 (41.7) | 6 (50.0) | 6 (50.0) | |||
| Case by case | 3 (8.1) | 1 (33.3) | 2 (66.7) | 0.693 | 3 (100) | 0 | 0.398 | |
| 23 | Do you have a strategy for restarting antithrombotic drugs?2 | |||||||
| Yes | 4 (15.4) | 4 (100) | 0 | 0 | 4 (100) | |||
| No | 22 (84.6) | 6 (27.3) | 16 (72.7) | 0.014 | 15 (68.2) | 7 (31.8) | 0.022 | |
Duplicated data allowed;
Missing data included. Values in parentheses are percentages. IVR: Interventional radiology; NSAIDs: Non-steroidal anti-inflammatory drugs; SRH: Stigmata of recent bleeding.
Comparing hospitals with high and low patient volume, low-volume hospitals had more strategies for restarting antithrombotic drugs (P = 0.014) than low-volume hospitals (Table 3). No significant differences were observed in other questionnaire items between the two groups (Table 3). Comparing hospitals in East and West Japan, East Japan hospitals had less strategies for restarting antithrombotic drugs than West Japan hospitals (P = 0.022) (Table 3). No significant differences were observed in other questionnaire items between the two groups (Table 3).
Questionnaire items for clinical outcomes
Questions and answers regarding clinical outcomes are shown in Table 4. The rate of identification of SRH varied widely among hospitals. No significant association between SRH identification rate and type of procedure was observed from questionnaire answers (Table 5). Forty-one percent of hospitals experienced less than 20% rebleeding events after endoscopic hemostasis, interventional radiology, and barium impaction therapy. No significant association was observed between rebleeding rate and endoscopic treatments from questionnaire answers (Table 5). No significant differences between hospitals with high and low patient volumes were observed in all questionnaire items (Table 4). Comparing hospitals in East and West Japan, East Japan hospitals experienced less rebleeding events after barium impaction therapy than West Japan hospitals (P = 0.005). No significant differences were observed in other questionnaire items between the two groups (Table 4).
Table 4.
Questions and answers regarding clinical outcomes of colonic diverticular bleeding in 37 hospitals n (%)
| No. | Question | Answer (n = 37) | High volume (n = 18) | Low volume (n = 19) | P value | East Japan (n = 21) | West Japan (n = 16) | P value |
| 24 | How often do you identify SRH in patients who undergo colonoscopy?1 | |||||||
| 0%-20% | 15 (41.7) | 6 (40.0) | 9 (60.0) | 7 (46.7) | 8 (53.3) | |||
| 21%-40% | 16 (44.4) | 7 (43.8) | 9 (56.2) | 10 (62.5) | 6 (37.5) | |||
| 41%-60% | 4 (11.1) | 4 (100) | 0 | 3 (75.0) | 1 (25.0) | |||
| 61%-80% | 1 (2.8) | 0 | 1 (100) | 0 | 1 (100) | |||
| 81%-100% | 0 | 0 | 0 | 0.122 | 0 | 0 | 0.658 | |
| 25 | How often do you experience rebleeding events after endoscopic hemostasis?1 | |||||||
| 0%-20% | 22 (61.1) | 10 (45.5) | 12 (54.6) | 13 (59.1) | 9 (40.9) | |||
| 21%-40% | 10 (27.8) | 4 (40.0) | 6 (60.0) | 7 (70.0) | 3 (30.0) | |||
| 41%-60% | 3 (8.3) | 2 (66.7) | 1 (33.3) | 1 (33.3) | 2 (66.7) | |||
| 61%-80% | 1 (2.8) | 1 (100) | 0 | 0 | 1 (100) | |||
| 81%-100% | 0 | 0 | 0 | 0.721 | 0 | 0 | 0.458 | |
| 26 | How often do you experience rebleeding events after IVR?1 | |||||||
| 0%-20% | 27 (90.1) | 15 (55.6) | 12 (44.4) | 16 (59.3) | 11 (40.7) | |||
| 21%-40% | 0 | 0 | 0 | 0 | 0 | |||
| 41%-60% | 1 (3.3) | 0 | 1 (100) | 0 | 1 (100) | |||
| 61%-80% | 1 (3.3) | 1 | 1 (100) | 0 | 1 (100) | |||
| 81%-100% | 1 (3.3) | 1 (100) | 0 | 0.448 | 0 | 1 (100) | 0.090 | |
| 27 | How often do you experience rebleeding events after barium impaction therapy?1 | |||||||
| 0%-20% | 10 (71.6) | 6 (60.0) | 4 (40.0) | 9 (90.0) | 1 (10.0) | |||
| 21%-40% | 1 (7.1) | 0 | 1 (100) | 0 | 1 (100) | |||
| 41%-60% | 1 (7.1) | 0 | 1 (100) | 0 | 1 (100) | |||
| 61%-80% | 1 (7.1) | 1 (100) | 0 | 0 | 1 (100) | |||
| 81%-100% | 1 (7.1) | 0 | 1 (100) | 0.559 | 0 | 1 (100) | 0.005 | |
Missing data included. Values in parentheses are percentages. SRH: Stigmata of recent hemorrhage; IVR: Interventional radiology.
Table 5.
Association between procedures and outcomes n (%)
|
Answer |
SRH identification rate2 |
||||||
| Procedure1 (Question No. 15) | (n = 37) | 0%-20% (n = 15) | 21%-40% (n = 16) | 41%-60% (n = 4) | 61%-80% (n = 1) | 81%-100% (n = 0) | P for trend |
| Cap-assisted colonoscopy | 17 (46.0) | 4 (25.0) | 9 (56.3) | 2 (12.5) | 1 (6.2) | 0 | 0.081 |
| Long cap-assisted colonoscopy | 13 (35.1) | 6 (46.2) | 5 (38.5) | 2 (15.3) | 0 | 0 | 0.735 |
| Inverting diverticulum via suction of colonoscopy | 18 (48.7) | 5 (29.4) | 10 (58.8) | 2 (11.8) | 0 | 0 | 0.588 |
| Wash out with water | 36 (97.3) | 14 (40.0) | 16 (45.7) | 4 (11.4) | 1 (2.9) | 0 | 0.323 |
| Colonoscopy by multiple doctors | 3 (8.1) | 2 (66.7) | 1 (33.3) | 0 | 0 | 0 | 0.328 |
| Colonoscopy under X-ray | 3 (8.1) | 2 (66.7) | 1 (33.3) | 0 | 0 | 0 | 0.328 |
| Answer | Rebleeding rate2 | ||||||
| Endoscopic treatment (Question No. 18) | 0%-20% | 21%-40% | 41%-60% | 61%-80% | 81%-100% | P for trend | |
| (n = 37) | (n = 22) | (n = 10) | (n = 3) | (n = 1) | (n = 0) | ||
| Clipping | 31 (83.8) | 19 (63.3) | 8 (26.7) | 3 (10.0) | 0 | 0 | 0.290 |
| Endoscopic band ligation | 5 (13.5) | 2 (40.0) | 2 (40.0) | 0 | 1 (20.0) | 0 | 0.142 |
| Epinephrine injection | 1 (2.7) | 1 (100) | 0 | 0 | 0 | 0 | 0.489 |
Duplicated data allowed;
Missing data included. Values in parentheses are percentages. SRH: Stigmata of recent bleeding.
DISCUSSION
Our questionnaire-based study was the first investigation to evaluate the current practice for CDB such as clinical settings, diagnoses, treatments, and clinical outcomes in 37 hospitals nationwide in Japan. Although the clinical setting such as the number of endoscopists and nursing staff were different between hospitals with high and low patient volumes, the practice for CDB was almost the same throughout Japan, such as performing CT before colonoscopy, various procedures to improve SRH identification rate, and clipping as first-line endoscopic therapy, irrespective of hospital size.
In regard to clinical settings, a high number of emergency visits, endoscopists, and expert endoscopists were observed in high-volume hospitals compared with low-volume hospitals. CDB is a major cause of acute lower gastrointestinal bleeding, and CDB patients experience severe bleeding and require transfusion and intensive care because of their advanced age or comorbidities[8,17-19]. Therefore, the management of CDB patients requires an adequate number of medical staff and expert endoscopists, and a careful nursing system during the nighttime and weekend. However, there was no significant difference in the number of CDB patients who received treatment between high- and low-volume hospitals, which indicated that low-volume hospitals also need to treat CDB patients as well as high-volume hospitals regardless of the small number of endoscopists. Therefore, action is needed to handle an increasing number of CDB patients, such as transfer of CDB patients to core hospitals in each region.
In regard to diagnostic methods, most Japanese hospitals performed CT before colonoscopy for CDB diagnosis, and there were no significant differences between the groups separated by hospital volume and region. In contrast, Western countries may perform colonoscopy or scintigraphy, not CT[20]. This is probably because there were some studies from Japan that showed the usefulness of CT for the diagnosis of CDB, which had a sensitivity of 20.0%-42.9% and specificity of 78.6%-87.5%[13,21]. Only 46% of hospitals performed bowel preparation, and there was a significant difference between East and West Japan in this respect. This is probably because some physicians are concerned that bowel preparation potentially increases the risk of aspiration pneumonia, volume overload, and a change in vital signs with blood loss[22]. However, the presence of colonic diverticula with poor visualization was a risk factor for perforation in screening colonoscopy[23]. Recent studies have shown that bowel preparation during acute lower gastrointestinal bleeding did not increase adverse events compared with non-gastrointestinal bleeding[24], and bowel preparation for early colonoscopy was safe as well as for elective colonoscopy[25]. In addition, bowel preparation contributes to excellent SRH identification rates[24,26]. Therefore, we may need to expand awareness of the safety of full bowel preparation in CDB diagnosis in Japan. Moreover, the rate of early colonoscopy was 43.2%. Now, we are conducting a randomized control study to resolve these unclarified issues in the diagnostic methods (UMIN 000021129).
In endoscopic treatment, clipping, band ligation, and epinephrine injection were performed as first-line therapy in 83.8%, 13.5%, and 2.7% of cases, which might be different from Western countries[27]. Some reports have indicated that Western countries usually performed thermal contact therapy[18,26,28,29]; however, this therapy is not approved in Japan[30]. Several reports from Western countries showed that clipping was a useful hemostasis treatment[12,31,32], and clipping may be performed as a common endoscopic treatment for CDB patients. On the other hand, in Japan, endoscopic band ligation was reported as useful for hemostasis in CDB, and therapeutic options for CDB have been expanding in Japan[33].
There was very limited data on the strategy for antithrombotic drugs in patients with acute gastrointestinal bleeding. The American Society for Gastrointestinal Endoscopy guidelines reported[34] that endoscopic hemostasis was considered as a procedure with a high risk of bleeding, and recommended that: (1) patients requiring endoscopic hemostasis taking non-steroidal anti-inflammatory drugs or low-dose aspirin continue these medications; (2) those taking thienopyridine should have the medication discontinued; and (3) those taking anticoagulants should consider bridging therapy. In contrast, Japan and European countries have no guidelines on the management of antithrombotic drugs in patients with gastrointestinal bleeding. Only 15% of hospitals have a strategy for antithrombotic drugs, and the timing of discontinuation and restart of antithrombotic drugs were individualized. Physicians considered discontinuation of antithrombotic therapy in patients following a hospitalization for gastrointestinal bleeding[35,36]. Discontinued use of antithrombotic drugs may decrease the risk of gastrointestinal bleeding, but discontinuation of these drugs was associated with an increased risk of thrombosis and mortality[37,38]. Although there is no consensus, we believed that patients with antithrombotic drugs need to have these medications continued, or restarted as soon as possible if patients discontinued antithrombotic drugs.
Our study has several strengths. First, our data were obtained from a large number of hospitals, so the generalizability of the results is high. Second, we evaluated intra-observer agreement, and our data showed a high level of reproducibility. However, our study has limitations. Our study was based on data from a questionnaire, and not based on patient data, so caution should be exercised in the interpretation of our results. In addition, our study has the potential of selection bias.
In conclusion, compared with Western countries, some practice styles unique to Japan such as performing CT before colonoscopy, no bowel preparation, and clipping as first-line endoscopic therapy were found. Although the number of endoscopists and nursing staff were different, the practices for CDB were almost the same, irrespective of the size of the hospital in Japan.
ACKNOWLEDGMENTS
The authors wish to express their gratitude to Kazuya Matsumoto, Atsushi Imagawa, Kenichiro Nakachi, Mikitaka Iguchi, Kyoko Katakura, Teruhito Kishihara, Yorimasa Yamamoto, Takamitsu Sato, Tomoyuki Yada, Tomoki Fujita, Waku Hatta, Katsuya Endo, Tomoo Nakagawa, Koichi Nonaka, Kazuya Kitamura, Tetsuya Sumiyoshi, Taku Sakamoto, Kazuo Hara, Tsukasa Furuhata, Syu Hoteya, Shiro Oka, Tatsuya Mikami, Manabu Sawaya, Yoshito Hayashi, Takashi Otsuka, Yoshinori Morita, Naomi Kakushima, Kenji Ishido, Takuya Inoue, Tetsuro Honda, Maiko Tabuchi, Hitomi Minami, Tomoki Michida, Shinichi Hashimoto, and Kenkei Hasatani for their help with answering the questionnaire.
COMMENTS
Background
Colonic diverticular bleeding (CDB) is increasing in Asia however there are no practice guidelines for CDB. It is important to determine which recommendation is acceptable to a majority of hospitals.
Research frontiers
To clarify the current state of the clinical settings, diagnosis, treatment, and clinical outcomes of patients with CDB.
Innovations and breakthroughs
The authors conducted multicenter questionnaire surveys of 37 hospitals in Japan regarding management of CDB such as the clinical settings, diagnosis, treatment, and clinical outcomes, comparing them between hospitals with different patient volumes and between hospitals in different regions. As a result, some practice styles unique to Japan such as performing computed tomography before colonoscopy, no bowel preparation, and clipping as first-line therapy were found. However, the management of CDB was common among hospitals irrespective of hospital size and region.
Applications
These data were obtained from a large number of hospitals, so the generalizability of the results is high.
Peer-review
This multicenter trial by questionnaire is very useful for assessment of current state of diagnosis and treatment of CDB.
Footnotes
Institutional review board statement: Owing to the anonymous nature of the data in this retrospective questionnaire survey of endoscopists, institutional review board approval was waived.
Informed consent statement: Informed consent was waived because this study included no personal information about patients.
Conflict-of-interest statement: Dr. Mitsuhiro Fujishiro has received grant support from Hoya and Pentax.
Data sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: May 17, 2016
First decision: August 10, 2016
Article in press: November 2, 2016
P- Reviewer: Narasaka T S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
References
- 1.Steinmuller DR, Graneto D, Swift C, Novick AC, Streem SB, Cunningham RJ, Hodge E, Bretan P. Use of intravenous immunoglobulin prophylaxis for primary cytomegalovirus infection post living-related-donor renal transplantation. Transplant Proc. 1989;21:2069–2071. [PubMed] [Google Scholar]
- 2.Nagata N, Niikura R, Aoki T, Shimbo T, Kishida Y, Sekine K, Tanaka S, Okubo H, Watanabe K, Sakurai T, et al. Lower GI bleeding risk of nonsteroidal anti-inflammatory drugs and antiplatelet drug use alone and the effect of combined therapy. Gastrointest Endosc. 2014;80:1124–1131. doi: 10.1016/j.gie.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 3.Tsuruoka N, Iwakiri R, Hara M, Shirahama N, Sakata Y, Miyahara K, Eguchi Y, Shimoda R, Ogata S, Tsunada S, et al. NSAIDs are a significant risk factor for colonic diverticular hemorrhage in elder patients: evaluation by a case-control study. J Gastroenterol Hepatol. 2011;26:1047–1052. doi: 10.1111/j.1440-1746.2010.06610.x. [DOI] [PubMed] [Google Scholar]
- 4.Nagata N, Niikura R, Aoki T, Shimbo T, Itoh T, Goda Y, Suda R, Yano H, Akiyama J, Yanase M, et al. Increase in colonic diverticulosis and diverticular hemorrhage in an aging society: lessons from a 9-year colonoscopic study of 28,192 patients in Japan. Int J Colorectal Dis. 2014;29:379–385. doi: 10.1007/s00384-013-1808-4. [DOI] [PubMed] [Google Scholar]
- 5.Song JH, Kim YS, Lee JH, Ok KS, Ryu SH, Lee JH, Moon JS. Clinical characteristics of colonic diverticulosis in Korea: a prospective study. Korean J Intern Med. 2010;25:140–146. doi: 10.3904/kjim.2010.25.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharara AI, El-Halabi MM, Mansour NM, Malli A, Ghaith OA, Hashash JG, Maasri K, Soweid A, Barada K, Mourad FH, et al. Alcohol consumption is a risk factor for colonic diverticulosis. J Clin Gastroenterol. 2013;47:420–425. doi: 10.1097/MCG.0b013e31826be847. [DOI] [PubMed] [Google Scholar]
- 7.Peery AF, Barrett PR, Park D, Rogers AJ, Galanko JA, Martin CF, Sandler RS. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142:266–72.e1. doi: 10.1053/j.gastro.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuire HH. Bleeding colonic diverticula. A reappraisal of natural history and management. Ann Surg. 1994;220:653–656. doi: 10.1097/00000658-199411000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niikura R, Yasunaga H, Yamaji Y, Horiguchi H, Fushimi K, Yamada A, Hirata Y, Koike K. Factors affecting in-hospital mortality in patients with lower gastrointestinal tract bleeding: a retrospective study using a national database in Japan. J Gastroenterol. 2015;50:533–540. doi: 10.1007/s00535-014-0994-3. [DOI] [PubMed] [Google Scholar]
- 10.Niikura R, Nagata N, Yamada A, Akiyama J, Shimbo T, Uemura N. Recurrence of colonic diverticular bleeding and associated risk factors. Colorectal Dis. 2012;14:302–305. doi: 10.1111/j.1463-1318.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto T, Watabe H, Yamada A, Hirata Y, Yoshida H, Koike K. The association between arteriosclerosis related diseases and diverticular bleeding. Int J Colorectal Dis. 2012;27:1161–1166. doi: 10.1007/s00384-012-1491-x. [DOI] [PubMed] [Google Scholar]
- 12.Kaltenbach T, Watson R, Shah J, Friedland S, Sato T, Shergill A, McQuaid K, Soetikno R. Colonoscopy with clipping is useful in the diagnosis and treatment of diverticular bleeding. Clin Gastroenterol Hepatol. 2012;10:131–137. doi: 10.1016/j.cgh.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Nagata N, Niikura R, Aoki T, Moriyasu S, Sakurai T, Shimbo T, Shinozaki M, Sekine K, Okubo H, Watanabe K, et al. Role of urgent contrast-enhanced multidetector computed tomography for acute lower gastrointestinal bleeding in patients undergoing early colonoscopy. J Gastroenterol. 2015;50:1162–1172. doi: 10.1007/s00535-015-1069-9. [DOI] [PubMed] [Google Scholar]
- 14.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–725; discussion 726. doi: 10.1001/archsurg.138.7.721. [DOI] [PubMed] [Google Scholar]
- 15.Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 16.Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85:257–268. [PubMed] [Google Scholar]
- 17.Nagata N, Niikura R, Aoki T, Shimbo T, Sekine K, Okubo H, Watanabe K, Sakurai T, Yokoi C, Akiyama J, et al. Impact of discontinuing non-steroidal antiinflammatory drugs on long-term recurrence in colonic diverticular bleeding. World J Gastroenterol. 2015;21:1292–1298. doi: 10.3748/wjg.v21.i4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foutch PG. Diverticular bleeding: are nonsteroidal anti-inflammatory drugs risk factors for hemorrhage and can colonoscopy predict outcome for patients? Am J Gastroenterol. 1995;90:1779–1784. [PubMed] [Google Scholar]
- 19.Strate LL, Orav EJ, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch Intern Med. 2003;163:838–843. doi: 10.1001/archinte.163.7.838. [DOI] [PubMed] [Google Scholar]
- 20.Pasha SF, Shergill A, Acosta RD, Chandrasekhara V, Chathadi KV, Early D, Evans JA, Fisher D, Fonkalsrud L, Hwang JH, et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc. 2014;79:875–885. doi: 10.1016/j.gie.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Obana T, Fujita N, Sugita R, Hirasawa D, Sugawara T, Harada Y, Oohira T, Maeda Y, Koike Y, Suzuki K, et al. Prospective evaluation of contrast-enhanced computed tomography for the detection of colonic diverticular bleeding. Dig Dis Sci. 2013;58:1985–1990. doi: 10.1007/s10620-013-2629-6. [DOI] [PubMed] [Google Scholar]
- 22.Lhewa DY, Strate LL. Pros and cons of colonoscopy in management of acute lower gastrointestinal bleeding. World J Gastroenterol. 2012;18:1185–1190. doi: 10.3748/wjg.v18.i11.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loffeld RJ, Engel A, Dekkers PE. Incidence and causes of colonoscopic perforations: a single-center case series. Endoscopy. 2011;43:240–242. doi: 10.1055/s-0030-1255939. [DOI] [PubMed] [Google Scholar]
- 24.Niikura R, Nagata N, Aoki T, Shimbo T, Tanaka S, Sekine K, Kishida Y, Watanabe K, Sakurai T, Yokoi C, et al. Predictors for identification of stigmata of recent hemorrhage on colonic diverticula in lower gastrointestinal bleeding. J Clin Gastroenterol. 2015;49:e24–e30. doi: 10.1097/MCG.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 25.Nagata N, Niikura R, Sakurai T, Shimbo T, Aoki T, Moriyasu S, Sekine K, Okubo H, Imbe K, Watanabe K, et al. Safety and Effectiveness of Early Colonoscopy in Management of Acute Lower Gastrointestinal Bleeding on the Basis of Propensity Score Matching Analysis. Clin Gastroenterol Hepatol. 2016;14:558–564. doi: 10.1016/j.cgh.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78–82. doi: 10.1056/NEJM200001133420202. [DOI] [PubMed] [Google Scholar]
- 27.Strate LL, Naumann CR. The role of colonoscopy and radiological procedures in the management of acute lower intestinal bleeding. Clin Gastroenterol Hepatol. 2010;8:333–343; quiz e44. doi: 10.1016/j.cgh.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Bloomfeld RS, Rockey DC, Shetzline MA. Endoscopic therapy of acute diverticular hemorrhage. Am J Gastroenterol. 2001;96:2367–2372. doi: 10.1111/j.1572-0241.2001.04048.x. [DOI] [PubMed] [Google Scholar]
- 29.Green BT, Rockey DC, Portwood G, Tarnasky PR, Guarisco S, Branch MS, Leung J, Jowell P. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100:2395–2402. doi: 10.1111/j.1572-0241.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamada A, Niikura R, Yoshida S, Hirata Y, Koike K. Endoscopic management of colonic diverticular bleeding. Dig Endosc. 2015;27:720–725. doi: 10.1111/den.12534. [DOI] [PubMed] [Google Scholar]
- 31.Yen EF, Ladabaum U, Muthusamy VR, Cello JP, McQuaid KR, Shah JN. Colonoscopic treatment of acute diverticular hemorrhage using endoclips. Dig Dis Sci. 2008;53:2480–2485. doi: 10.1007/s10620-007-0151-4. [DOI] [PubMed] [Google Scholar]
- 32.Simpson PW, Nguyen MH, Lim JK, Soetikno RM. Use of endoclips in the treatment of massive colonic diverticular bleeding. Gastrointest Endosc. 2004;59:433–437. doi: 10.1016/s0016-5107(03)02711-1. [DOI] [PubMed] [Google Scholar]
- 33.Ishii N, Setoyama T, Deshpande GA, Omata F, Matsuda M, Suzuki S, Uemura M, Iizuka Y, Fukuda K, Suzuki K, et al. Endoscopic band ligation for colonic diverticular hemorrhage. Gastrointest Endosc. 2012;75:382–387. doi: 10.1016/j.gie.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 34.Anderson MA, Ben-Menachem T, Gan SI, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060–1070. doi: 10.1016/j.gie.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 35.White RH, McKittrick T, Takakuwa J, Callahan C, McDonell M, Fihn S. Management and prognosis of life-threatening bleeding during warfarin therapy. National Consortium of Anticoagulation Clinics. Arch Intern Med. 1996;156:1197–1201. [PubMed] [Google Scholar]
- 36.Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis. 2011;31:419–423. doi: 10.1007/s11239-010-0536-7. [DOI] [PubMed] [Google Scholar]
- 37.Witt DM, Delate T, Garcia DA, Clark NP, Hylek EM, Ageno W, Dentali F, Crowther MA. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172:1484–1491. doi: 10.1001/archinternmed.2012.4261. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez LA, Cea-Soriano L, Martín-Merino E, Johansson S. Discontinuation of low dose aspirin and risk of myocardial infarction: case-control study in UK primary care. BMJ. 2011;343:d4094. doi: 10.1136/bmj.d4094. [DOI] [PMC free article] [PubMed] [Google Scholar]


