Abstract
Background
Patients’ drug regimens often need to be changed when they pass from one care sector to another, but these changes sometimes pose a safety risk. To avoid such risks, a new inter-sector transition concept was developed incorporating discharge medication plans and counseling modules for the patients themselves and the doctors receiving them into their care.
Methods
A prospective interventional trial was carried out in two internal medicine wards of a general hospital. After data acquisition from the control group, the transition concept was developed and evaluated in an independent intervention group. The discharge medication plan and the first post-discharge prescription were compared to identify patients who had at least one medication change that increased the post-discharge risk of either failure to achieve the therapeutic goal (category A, first endpoint) or of patient’s lack of treatment adherence (category B). Gaps in care after discharge were also analyzed.
Results
200 consecutive patients were enrolled in the trial. In the intention-to-treat analysis, the percentage of patients with potentially jeopardizing medication changes in category A declined from 54% (54/100) in the control group to 15% (15/100) in the intervention group. (p<0.001). For medication changes in category B, there was a corresponding decline from 53% (53/100) to 7% (7/100) (p < 0.001). Gaps in care were seen in 28% (28/100) of control patients and 18% (18/100) of patients in the intervention group (p = 0.031).
Conclusion
The likelihood of a potentially jeopardizing medication change upon hospital discharge can be markedly reduced with the aid of a modular transition concept. Gaps in care can be closed in this way as well.
Almost all patients have their medications changed during a hospital stay (1). After discharge, further medication changes may result from necessary adjustments to treatment or new diagnoses. However, there is also a risk that patients are affected by unintended medication changes occurring after discharge, potentially leading to adverse drug events and hospital readmission (2– 4). Sending out discharge summaries with complete information about the discharge medications at an early point and advising patients on their discharge medications are among the key preventive strategies to optimize the safety of drug therapy at the interface between hospital and outpatient care (5– 9). However, such measures have not been widely adopted as yet in Germany.
The aim of this study was to develop and evaluate a modular transition concept (“Konstanz model“) with discharge medication plans for the physicians providing ongoing care as well as the patients plus structured discharge counseling in a model region of a tertiary care hospital.
Methods
Definitions
In eBox 1, definitions of the terms “potentially jeopardizing medication changes”, “first post-discharge prescription“, “care gap“, and “high-risk medications“ are provided. In eBox 2, examples of potentially jeopardizing medication changes are given.
eBOX 1. Definitions of (potentially jeopardizing) medication changes, care gaps and high-risk medications.
-
Medication change and first post-discharge prescription
Medication changes were determined between the discharge medications and the first medications prescribed by a community-based physician (first post-discharge prescription). A medication change was defined as any
discontinued,
newly prescribed, or
with regard to active ingredient, potency or method of administration—changed medication.
Over-the-counter medications and nutritional supplements were not included in the analysis.
-
Potentially jeopardizing medication changes
Medication changes which fell under the pre-defined categories A or B were considered as potentially jeopardizing. These were defined by an expert panel consisting of three clinicians (professional experience: 11 to 22 years) and four clinical pharmacists (professional experience: 5 to 35 years) as follows:
| Category A: | Medication changes potentially jeopardizing the therapeutic goal |
| These include medication changes in the subcategories A1–A3: A1 Non-continuation of a medication which is recommended in the discharge summary and indicated for the documented diagnosis A2 Prescription of a medication despite a clear recommendation in the discharge summary against it A3 Change in active ingredient, potency and/or method of administration despite a clear recommendation in the discharge summary against it |
| Category B: | Medication changes potentially jeopardizing the patient’s treatment adherence |
| These include medication changes in the subcategories B1 and B2: B1 First post-discharge prescription contains a medication not listed in the discharge summary, but which is reasonably indicated for the documented diagnosis B2 Change to a pharmaceutically equivalent medication “with pharmacologically comparable active ingredients or therapeutically comparable effect“ (section 115c sentence 1, German Social Insurance Code (SGB) V), for example change from atorvastatin to pravastatin or from losartan to valsartan Examples of medication changes are listed in eBox 2. |
-
Gap in care
A gap in care was deemed to have occurred if the administration of at least one medicine of the discharge medication plan which would have been indicated based on the patient’s diagnoses was not continued after the hospital stay so that a pharmacotherapy gap arose. It could not be excluded that patients had medicines of the discharge medication plan, which they already received before their hospital stay, available at home. Therefore, only those patients were analyzed for care gaps whose discharge medication plans included at least one new medication or medication with changed active ingredient so that a new prescription of this medication was definitely required.
-
High-risk medications
According to Saedder et al. (13), the following active substances and active substance groups with a particularly high potential for adverse drug events were defined as high-risk medications: methotrexate, theophylline, oral potassium, amiodarone, cardiac glycosides, oral and transdermal opioid analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), oral anticoagulants and oral anti-infectives.
eBOX 2. Examples of and notes to medication changes in the categories A and B between discharge medications and the first post-discharge prescription (gray)*.
| A | Medication changes potentially jeopardizing the therapeutic goal | |||
| A1 | Non-continuation of a medication which is recommended in the discharge summary and indicated for the patient’s diagnosis | |||
| Pre-hospital: | – | |||
| Inpatient: | Cefpodoxime (200 mg) 1–0–1 (started at day of discharge) | |||
| Discharge summary: | Cefpodoxime (200 mg) 1–0–1 for additional 5 days | |||
| (Diagnosis in the discharge summary: acute urinary tract infection) | ||||
| Post-discharge prescription: | – | |||
| Note: The antibiotic treatment started at the day of discharge is not continued even though it is still required, potentially jeopardizing the therapeutic goal (resolution of the urinary tract infection). | ||||
| A2 | Prescription of a medication against a clear recommendation in the discharge summary | ||
| Pre-hospital: | Candesartan (16 mg) 1–0–0 | ||
| Inpatient: | Candesartan (16 mg) 1–0–0 + HCT (25 mg) 1–0–0 | ||
| Discharge summary: | Candesartan (16 mg) 1–0–0 + HCT (25 mg) 1–0–0 | ||
| (Notes in the discharge summary: “Due to a marked deterioration in heart failure, we intensified the pharmacotherapy. Please continue treatment in this intensity.“) | |||
| Post-discharge prescription: | Candesartan (16 mg) 1–0–0 | ||
| Note: The recommended intended intensification of treatment is not continued after discharge from hospital, potentially jeopardizing the therapeutic goal (intensification of heart failure treatment). | |||
| A3 | Change in active ingredient, potency and/or method of administration against a clear recommendation in the discharge summary | |
| Pre-hospital: | – | |
| Inpatient: | Xarelto (15 mg) 1–0–1 (start one day before discharge) | |
| Discharge summary: | Xarelto (15 mg) 1–0–1 for 3 weeks, then 20 mg 1–0–0 | |
| (Diagnosis in the discharge summary: pulmonary embolism) | ||
| Post-discharge prescription: Xarelto (20 mg) 1–0–0 | ||
| Note: The anticoagulation treatment is not continued using the dosage recommended for pulmonary embolism. Instead of continuing the treatment with 15 mg 1–0–1, immediately 20 mg 1–0–0 is prescribed for further treatment. This may jeopardize the therapeutic goal (treatment of pulmonary embolism) | ||
| B | Medication changes potentially jeopardizing the patient’s treatment adherence | |
| B1 | First post-discharge prescription contains a medication not listed in the discharge summary, but which is clearly indicated for a documented diagnosis | |
| Pre-hospital: | Berodual inhaler 2 puffs, as required | |
| Inpatient: | Inhalation of Atrovent and salbutamol three times daily, according to regimen followed in the hospital | |
| Discharge summary: – (Information about previous illnesses in the discharge summary: Chronic obstructive pulmonary disease [COPD]) | ||
| Post-discharge prescription: Berodual inhaler 2 puffs, as required | ||
| Note: Treatment with the Berodual inhaler, which was used as a rescue medication before the hospital stay, is not required during the inpatient stay because during this time the patient receives temporary inhalation treatment with Atrovent and salbutamol. On compilation of the discharge medication plan, it is forgotten to include Berodual again which is indicated for the treatment of COPD. The referring doctor starts the patient again on Berodual which is indicated. The incomplete information in the discharge summary can jeopardize the patient’s treatment adherence because it is not clear for the patient whether or not Berodual was discontinued with intention. | ||
| B2 | Change to a pharmaceutically equivalent medication “with pharmacologically comparable active ingredients or therapeutically comparable effect“ (section 115c sentence 1, German Social Insurance Code (SGB) V [11]) | ||
| Pre-hospital: | Atorvastatin (10 mg) 0–0–1 | ||
| Inpatient: | Simvastatin (20 mg) 0–0–1 | ||
| Discharge summary: | Simvastatin (20 mg) 0–0–1 | ||
| Post-discharge prescription: | Atorvastatin (10 mg) 0–0–1 | ||
| Note: During the inpatient stay, the patient is switched to a statin which is included in the hospital’s medication list (simvastatin). On compilation of the discharge medications, the hospital-listed statin is included and it is missed to switch the patient back to the initially taken statin (atorvastatin). After discharge, the patient’s family doctor prescribes again atorvastatin which the patient originally has taken. Because it is missed to switch the patient back to the originally taken statin at the time the discharge medication plan is compiled, the patient’s treatment adherence can be jeopardized, since it is not clear to the patient that simvastatin, which is recommended in the discharge summary, is a pharmaceutically equivalent medication to the originally taken atorvastatin. | |||
Data sources: pre-hospital medication: electronic admission entry in the hospital information system; inpatient medication: last documented medication in handwritten patient chart; discharge medication: discharge summary; first post-discharge prescription: medication prescribed immediately after discharge by the referring physician (based on copies of prescriptions, medication packages, medication lists, and patients interviews
Study design
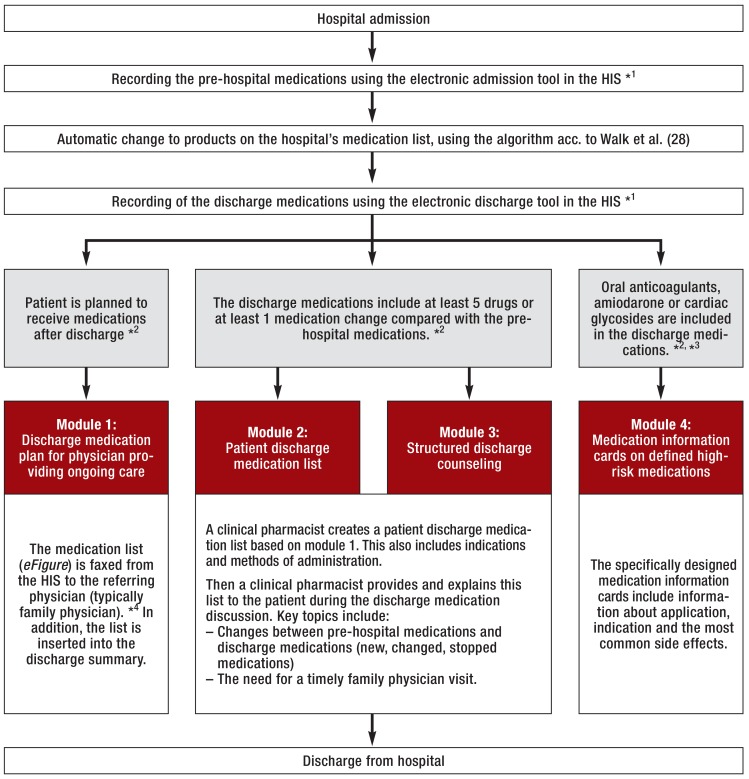
We conducted a prospective intervention study. Starting from November 2013, consecutive patients were included in the control group on working days over a period of 12 weeks. Based on the insights from the control group, the modules of the transition concept were then developed over a period of 15 months and implemented on the pilot wards. Subsequently, consecutive patients were included in the intervention group over a further 12-week period, using the same procedure as with the control group. Patients already included in the control group were not re-recruited for the intervention group. Researchers who conducted the study had no influence on the inclusion of patients at a specific point in time (control or intervention phase). Control group patients received the standard medical and nursing care on hospital admission and discharge. The standard care included a physician-obtained medication history on admission and a physician-led discharge discussion as well as handing out the preliminary discharge summary and enough medications until the next working day to the patient, as it is legally required or specified in relevant checklists (10, 11). For intervention group patients, a transition concept with four modules was used:
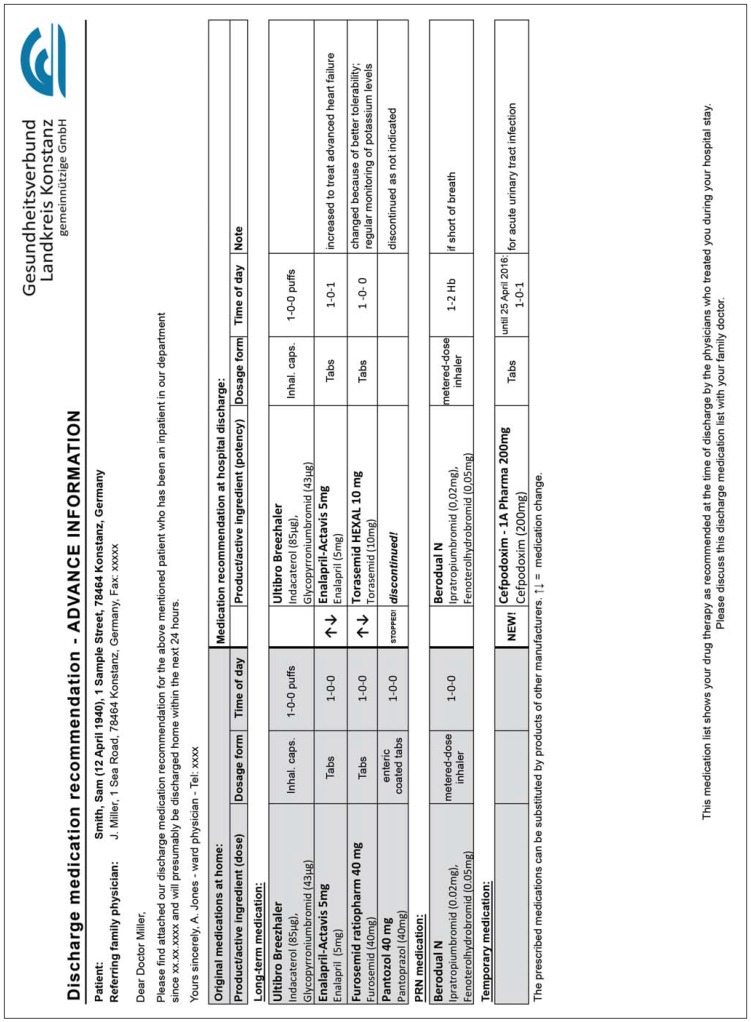
Module 1: a discharge medication plan for the referring physician providing ongoing care (eFigure)
Module 2: a patient discharge medication plan
Module 3: discharge counseling
Module 4: specific medication information cards on defined high-risk medications.
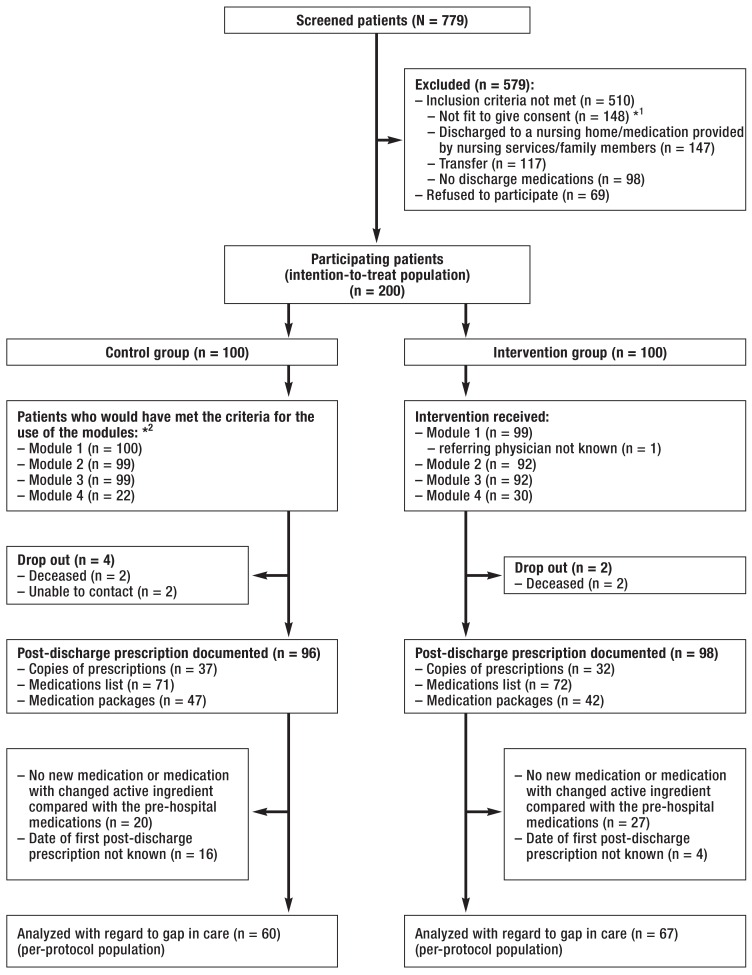
eFigure:
CONSORT flow chart
*1 The group of patients not fit to give their informed consent also includes patients with diagnosed dementia.
*2 The number of patients who would theoretically have met the criteria for the use of modules 1 to 4 (see Figure 1) is provided here.
The modules and the criteria for their use were defined in advance by an interdisciplinary expert panel composed of physicians and clinical pharmacists (Figure 1). Prior to the start of the study, an information event was held to educate the primary care physicians in the region about module 1 (discharge medication plans for referring physicians). The sole purpose of this event was to provide information to the community-based practitioners, covering the structure and the mailing of the medication plan; it was not intended to be a training event or an intervention.
Figure 1.
Process and modules of the transition concept as well as criteria for their use (gray); several modules (1–4) can be used in a single patient.
*1 HIS = hospital information system; admission and discharge tools were programmed by the hospital’s IT department in SAP/i.s.h.med.
*2 The criteria (gray) for the use of the modules were set prior to the start of the study by an expert panel of clinical pharmacists and physicians.
*3 Oral anticoagulants = phenprocoumon, dabigatran, rivaroxaban, apixaban; cardiac glycosides = beta-acetyldigoxin, digoxin, digitoxin
*4 Faxed only to verified provided fax numbers after written authorization by the recipient
Study setting and study population
Following the positive opinions of the ethics committees of the University of Leipzig and the State Medical Chamber of Baden–Württemberg (Stuttgart) (209/13 ff and 213/107 ff), the study was conducted in 2 medical departments (46 inpatient beds) in a tertiary care hospital. The inclusion criteria were met by all patients >18 years and fit to give their informed consent who were discharged home and had at least one medication on their discharge medication plan. Patients discharged to a nursing home, to another department or another hospital, and patients where family members or nursing services were solely responsible for the provision of medications after discharge were excluded from this study. All patients gave their written informed consent prior to participating in this study.
Primary and secondary outcomes
The primary outcome was the proportion of patients with at least one potentially jeopardizing category A medication change (potential threat to the therapeutic goal). The secondary outcomes were the proportion of patients with at least one potentially jeopardizing category B medication change (potential threat to treatment adherence by the patient) and the proportion of patients with a gap in care after hospital discharge.
Data collection methods
Medication changes were identified by means of a structured comparison of the discharge medications with the first post-discharge prescription. To determine the first post-discharge prescription, participating patients received written instructions at the time of discharge that they should—over a period of 4 weeks—keep copies of all filled prescriptions, medication lists or medication packages prescribed during this period. During a structured patient interview at week 4 after hospital discharge, the clinical pharmacist recorded the first post-discharge prescription based on the collected documents and medication packages.
To identify any gap in care, the discharge date documented in the hospital information system was compared with the date of the filling of the first post-discharge prescription at a pharmacy. This was determined using prescriptions copies and information provided by the patient during the interview. The exact time spent on discharge counseling in the intervention group was documented by the clinical pharmacist providing this service. Sociodemographic data as well as pre-hospital and inpatient medications were extracted from the hospital’s patient health records. Diagnoses and information about the course of the treatment were obtained from the discharge summary, while information about the patient’s educational level and employment status was collected during the interview.
Sample size calculation and statistical analysis
Based on the results of a pilot study, we assumed that in 50% of patients at least one potentially jeopardizing category A medication change (primary outcome) can be expected. For the intervention concept, an absolute risk reduction of at least 20% was regarded as a clinically relevant outcome. Assuming this risk constellation, we arrived at a sample size of at least 93 patients per group, given an independent control group and intervention group, using a two-sided chi square test with a significance level of a = 0.05 and a power of 1 – ß = 0.80 for the primary outcome.
The results are reported as medians with first and third quartile (Q25/Q75) or as percentages. If not stated otherwise, the results of the intention-to-treat (ITT) analysis are presented. Missing values were filled in based on the assumption of a worst-case scenario. Here, it was assumed for all patients with missing values in the control group that no medication changes and care gaps occurred. For intervention group patients with missing values, it was assumed that they were affected by medication changes and care gaps. Risk reductions are reported as absolute risk reductions (ARR). Differences between the groups were analyzed using the Mann–Whitney U test for non-normally distributed data and the chi-square test for proportional values (nominal, dichotomous data). A significance level of a = 0.05 was assumed. Backed by multiple statistical tests used for endpoint evaluation, the interpretation of the result was based on a Bonferroni-adjusted significance level of a‘ = 0.0038. Statistical calculations were performed using SPSS (Statistical Package for the Social Science, Version 20, IBM, USA) and Microsoft Excel (Microsoft Corporation, Version 2010, USA).
Results
Patient population and medical care structure
Of the altogether 779 patients treated in the participating departments during the recruitment period of this study, 269 met the inclusion criteria. Of these, 200 patients consented to participating in the study (ITT population). During the intervention phase, for 99 patients a discharge medication plan was sent to the referring physician providing ongoing care (Module 1 in Figure 1); in one patient, the physician providing ongoing care was not known. The modules 2 and 3 were used in 92 patients, while module 4 was used in 30 patients. The first post-discharge prescription was documented in 96 patients of the control group and 98 patients of the intervention group during the interview, based on copies of prescriptions (control: 37; intervention: 32), medication lists (control: 71; intervention: 72) and medication packages (control: 47, intervention: 42). The date of filling the first post-discharge prescription could be obtained and documented in 60 patients of the control group and 67 of the intervention group (per-protocol population; p = 0.390) (eFigure). No significant differences in patient characteristics were found between the control group and the intervention group (Table 1, eTable).
Table 1. Patient characteristics of the intention-to-treat (ITT) population (continued in the eTable).
| Control group | Intervention group | p value | |
| Patients in total, N (%) | 100 | 100 | |
| Median age in years (Q25/Q75) | 72 (62/81) | 73 (60/80) | 0.634 |
| Men, n (%) | 50 (50) | 46 (46) | 0.571 |
| School-leaving qualification, n (%) | |||
|
7 (7) | 5 (5) | 0.522 |
|
40 (40) | 44 (44) | 0.567 |
|
33 (33) | 33 (33) | 1.000 |
|
20 (20) | 18 (18) | 0.718 |
| Vocational qualification, n (%) | |||
|
15 (15) | 11 (11) | 0.400 |
|
72 (72) | 73 (73) | 0.874 |
|
13 (13) | 16 (16) | 0.547 |
| Employment status, n (%) | |||
|
27 (27) | 27 (27) | 1.000 |
|
72 (72) | 71 (71) | 0.876 |
|
1 (1) | 2 (2) | 0.561 |
| Duration of hospital stay in days, median (Q25/Q75) |
7 (4/13) | 7 (4/10) | 0.409 |
| Discharging pilot department, n (%)* | |||
| Medical Department I | 60 (60) | 57 (57) | 0.667 |
| Medical Department II | 40 (40) | 43 (40) | 0.667 |
| Most frequent admission diagnoses according to DRG Major Diagnostic Categories, n (%) Diseases and disorders… | |||
| …of the circulatory system | 23 (23) | 22 (22) | 0.866 |
| …of the respiratory system | 17 (17) | 27 (27) | 0.088 |
| …of the digestive system | 9 (9) | 11 (11) | 0.637 |
| …of the nervous system | 9 (9) | 6 (6) | 0.421 |
| …of metabolism | 5 (5) | 10 (10) | 0.179 |
| Number of discharge medications of all study patients | 737 | 672 | |
| Patients with at least one high-risk medication on their discharge medication plan, n (%) | 56 (56) | 54 (54) | 0.776 |
* Specialties within the Medical Department I: gastroenterology, oncology, nephrology; specialties within the Medical Department II: pulmonology, cardiology;
Q, quantile
eTable. Patient characteristics – continued (intention-to-treat population).
| Control group | Intervention group | p value | |
| Patients in total, N (%) | 100 | 100 | |
|
Most common active ingredients in the discharge medication, n (%)
|
49 (49%) 37 (37%) 28 (28%) 27 (27%) 25 (25%) 25 (25%) 22 (22%) 21 (21%) 18 (18%) 18 (18%) |
44 (44%) 28 (28%) 15 (15%) 26 (26%) 15 (15%) 18 (18%) 9 (9%) 24 (24%) 19 (19%) 18 (18%) |
|
|
Patients for whom modules would have been used (control group) or were used (intervention group), n (%)
|
100 (100%) 99 (99%) 99 (99%) 22 (22%) |
99 (99%) 92 (92%) 92 (92%) 30 (30%) |
0.316 0.017 0.017 0.197 |
Fifty family physicians were responsible for the control group and 60 for the intervention group, while 31 were responsible for both the control group and the intervention group. The same two chief physicians, the same 5 senior physicians as well as 14 registrars (changing due to rotations required by the residency program) were responsible for the recruited patients. The median time spent on structured discharge counseling (module 3) was 7 minutes per patient in the intervention group (quantile [Q]25/Q75: 5/7 minutes).
Potentially jeopardizing medication change after hospital discharge
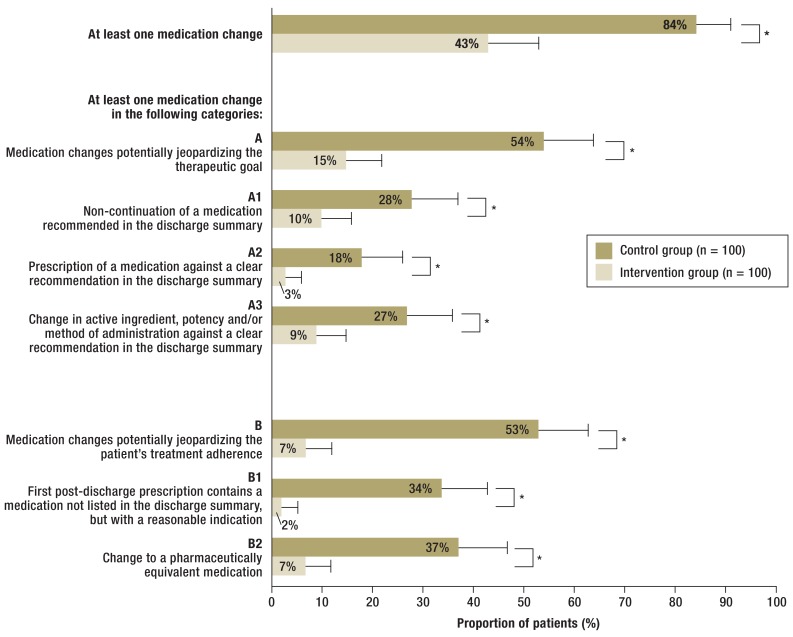
At least one medication change occurred in 84 of 100 patients of the control group and 43 of 100 patients of the intervention group (p<0.001). The proportion of patients with at least one potentially jeopardizing category A medication change (primary outcome) was 39 percentage points (Please note: This does not mean a reduction of 39 percent, but a reduction by 39 percentage points. See also the following percentages) lower than in the control group (control: 54/100 [54%] versus intervention: 15/100 [15%]; p<0.001). In 9 patients of the control group (9%), a high-risk medication was affected by a category A medication change (antibiotic [N = 4], cardiac glycoside [N = 2], opioid analgesic [N = 2], oral anticoagulant [N = 1]), but in no patient of the intervention group (p = 0.001). The proportion of patients with at least one potentially jeopardizing category B medication change (secondary outcome) was reduced by 46 percentage points (control: 53/100 [53%] versus intervention: 7/100 [7%]; p<0.001) (Figure 2). In 4 patients of the control group (4%), a high-risk medication was affected by a category B medication change (antibiotic [N = 2], opioid analgesic [N = 1], oral anticoagulant [N = 1]), but in no patient of the intervention group (p = 0.058).
Figure 2.
Proportion of patients of the intention-to-treat population with at least one medication change in the categories A or B, or the subcategories A1, A2, A3, B1, and B2; *p = 0.001 (statistically significant)
Gap in care after hospital discharge
Altogether in 79 of 100 patients of the control group and 72 of 100 patients of the intervention group, at least one new medication or medication with changed active ingredient was recommended compared with the pre-hospital medications (p = 0.250). The proportion of patients with a gap in care was by 10 percentage points lower in the intervention group compared with the control group (control: 28/100 [28%] versus intervention: 18/100 [18%], p = 0.031). The discharge medications of 36 of the 100 patients of the control group and of 29 of the 100 (29%) patients of the intervention group included new high-risk medications or high-risk medications with a changed active ingredient compared with the pre-hospital medications (p = 0.512). Among these patients, the proportion of individuals with a gap in care was reduced by 21 percentage points (control: 17/36 [47%] versus intervention: 8/29 [26%], p = 0.054) (Table 2).
Table 2. Proportion of patients with a gap in care after discharge from hospital and median duration of the care gap (intention-to-treat population).
| Control group | Intervention group | p value | |
| Patients in total, N | 100 | 100 | |
|
28 (28) | 18 (18) | 0.031 |
|
2 (1/7) | 1 (1/3) | 0.013 |
| Patients with new or—regarding active ingredient—changed high-risk medications among discharge medications, N | 36 | 29 | 0.512 |
|
17 (47) | 8 (26) | 0.054 |
|
2 (2/6) | 1 (1/2) | 0.039 |
Q, quantile
Discussion
In our study, at least every second patient receiving standard care had a change in medication which could have jeopardized the therapeutic goal of drug therapy after discharge (category A). In addition, almost one third of the patients in need of a new prescription after discharge experienced a gap in care. The modular transition concept proofed to be an effective preventive strategy, as it reduced the proportion of patients with potentially jeopardizing medication changes by 39 percentage points and that of patients with care gaps by 10 percentage points.
The success of the modular transition concept may be explained by the following factors: First, when the model project was developed, priority was given to strengthening the cross-sectoral communication between the hospital and the community-based referring physicians. Thus, a long-standing request of the primary-care physician community to receive a discharge medication plan at an early point in time, which has already been described in numerous studies, was addressed with this model (8, 12). Second, our study builds on the insights derived from earlier projects which revealed that medication lists and patient information sessions helped to prevent adverse drug events (5– 7). By integrating these two strategies in our modular transition concept, we achieved that especially category A medication changes, which have the potential to jeopardize the therapeutic goal, occurred less frequently. Furthermore, as the result of the intervention, no more medication changes involving high-risk medications associated with a significant threat to patient safety were observed (13, 14). In addition, components of medication reconciliation, which has already been successfully implemented in other countries, were included in our model (15– 17). For example, full data on both pre-hospital and the discharge medications were entered in a structured way into the hospital information system, using dedicated hospital admission and discharge software tools. The discharge medications were selected based on the pre-hospital medications. By taking this approach, it was ensured that in the case of medicines with a therapeutically equivalent effect, e.g. statins, the medications prescribed prior to the hospital stay were included in the discharge medication plan, instead of the drugs listed in the hospital. This process optimization helped to achieve that medication changes potentially leading to reduced treatment adherence (category B) only affected 7% of the patients in the intervention group. Even though these medication changes could primarily be considered acceptable from a therapeutic perspective, they may lead to medication-related problems after discharge, especially when there is a lack of patient information or little health-related knowledge (18, 19).
In addition, the implemented transition concept reduced the proportions of patients with a gap in care after discharge by 10 percentage points. In case high-risk medications were among the discharge medications, a reduction by 21 percentage points was observed. Despite improvements achieved with our concept, 18% of patients in the intervention group still experienced a gap in care, even though the need to immediately see their primary care physicians had clearly been pointed out to them during discharge counseling. This shows that many patients after having been discharged from hospital do not visit their referring physician in a timely manner. Other approaches are required to ensure the continuity of care with regard to drug therapy after hospital discharge. Here, the German E-Health Act and the introduction of a discharge prescription represent important steps towards a nation-wide implementation (20, 21).
We developed our transition concept with the sole aim to prevent potentially jeopardizing medication changes. As evidenced by previous studies, such medication changes can serve as important indicators for the quality of care at interfaces, as they are directly related to both adverse drug events and hospital readmissions (14, 22). It was not within the scope of this study to evaluate any necessary and planned medication changes after discharge.
This modular transition concept is notable in that each of the modules can be tailored to the needs of individual patients, using criteria defined by an expert panel. Thus, these modules are of particular clinical relevance to patient safety (14, 23). Consequently, the “Konstanz model“ does not only significantly reduce the risk of potentially jeopardizing medication changes and care gaps, but represents an efficient solution which can be applied to other hospitals and regions as well.
Furthermore, future studies should include close multi-professional management of patients after discharge to ensure that a long-term effect is achieved. For example, a recent study showed that patients with coronary heart disease who after discharge from hospital underwent an intensive multi-professional rehabilitation program had a lower mortality rate compared with patients who did not participate in such a program (24). Lastly, new insights into drug therapy safety should increasingly be taken into consideration when medications are prescribed at care interfaces and solutions should be devolved based on cross-sectoral approaches (25).
Limitations
This study was not a randomized controlled trial. Moreover, there was a 15-month interval between the control phase and the intervention phase during which the intervention strategies were developed and implemented. Despite similar patient and medical care structures and the fact that, in the opinion of the study-supporting expert panel, organizational changes during the two phases of the study could largely be excluded, it cannot be ruled out that during this interval other changes had an impact on the outcome. This monocentric study was performed in two internal medicine departments of a tertiary care hospital. Thus, it needs to be established whether the study results can be applied to other departments. The first post-discharge prescription was determined based on patient-collected copies of prescriptions, medication lists and medication packages, as well as information obtained during the interview at week 4 after discharge from hospital. Consequently, there is a potential for recall bias which needs to be taken into account in the interpretation of the results. However, with no change in methodology, its potential impact on the control group and the intervention group can be expected to be similar.
Finally, the definitions of medication changes and care gaps used in this study vary to some extent from those used in other studies (14, 26, 27), potentially limiting comparability. The categorization of medication changes is based on an expert decision. Necessary, planned medication changes, adverse drug events and factors potentially influencing treatment outcome were not investigated in this study.
Supplementary Material
Key Messages.
More than half of all patients were affected by potentially jeopardizing medication changes which may put the therapeutic goal of a pharmacotherapy after discharge from hospital at risk.
Gaps in care occurred in almost one third of patients who required either new or different medications compared with the pre-hospital list.
The modules of the “Konstanz model“ comprised of a discharge medication plan sent early to the referring physician, a patient discharge medication plan, a structured discharge medication discussion, as well as medication information cards on high-risk medications.
With this modular transition concept, the proportion of patients affected by potentially jeopardizing medication changes was reduced by 39 percentage points (absolute risk reduction).
After implementation of the modular transition concept, 18% of patients were affected by gaps in care.
eFigure:
Example of a discharge medication plan for the referring physician providing ongoing care
Acknowledgments
We would like to thank all participating patients, physicians, nursing staff, and clinical pharmacists for their support. We extend our thanks to Mr. Martin Kiewitz and Dr. Reinhold Funk for their IT support and the Pharmacists’ Association of Baden–Württemberg for their professional and financial support.
Contact for the Konstanz Model: Claudia Greißing, pharmacist.
Pharmacy Department, Konstanz Hospital, Luisenstraße 7, 78464 Konstanz, Germany. Phone: +49-7531/8011060, claudia.greissing@klinikum-konstanz.de
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
Claudia Greißing and Prof. Bertsche declare that they received third-party funding from the Pharmacists’ Association of Baden-Württemberg to support the conduct of this study.
The remaining authors declare that no conflict of interest exists.
References
- 1.Grimmsmann T, Schwabe U, Himmel W. The influence of hospitalisation on drug prescription in primary care—a large-scale follow-up study. Eur J Clin Pharmacol. 2007;63:783–790. doi: 10.1007/s00228-007-0325-1. [DOI] [PubMed] [Google Scholar]
- 2.Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005;173:510–515. doi: 10.1503/cmaj.045311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840–847. doi: 10.1001/jama.2011.1206. [DOI] [PubMed] [Google Scholar]
- 4.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20:317–323. doi: 10.1111/j.1525-1497.2005.30390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166:955–964. doi: 10.1001/archinte.166.9.955. [DOI] [PubMed] [Google Scholar]
- 6.Hohmann C, Neumann-Haefelin T, Klotz JM, Freidank A, Radziwill R. Adherence to hospital discharge medication in patients with ischemic stroke: a prospective, interventional 2-phase study. Stroke. 2013;44:522–524. doi: 10.1161/STROKEAHA.112.678847. [DOI] [PubMed] [Google Scholar]
- 7.Send AFJ, Schwab M, Gauss A, Rudofsky G, Haefeli WE, Seidling HM. Pilot study to assess the influence of an enhanced medication plan on patient knowledge at hospital discharge. Eur J Clin Pharmacol. 2014;70:1243–1250. doi: 10.1007/s00228-014-1723-9. [DOI] [PubMed] [Google Scholar]
- 8.Adam H, Niebling W, Schott G. Die Informationen zur Arzneimitteltherapie im Arztbrief: Was erwarten Hausärzte? DMW. 2015;140:e74–e79. doi: 10.1055/s-0041-101401. [DOI] [PubMed] [Google Scholar]
- 9.Mahler C, Jank S, Pruszydlo MG, et al. HeiCare: Ein Projekt zur Verbesserung der sektorenübergreifenden Arzneimittelkommunikation. DMW. 2011;136:2239–2244. doi: 10.1055/s-0031-1292036. [DOI] [PubMed] [Google Scholar]
- 10.Ärztliches Zentrum für Qualität in der Medizin. Checklisten für das ärztliche Schnittstellenmanagement zwischen den Versorgungssektoren; 1. Auflage, März 2012. www.aezq.de/mdb/edocs/pdf/info/checklisten-schnittstellenmanagement.pdf (last accessed on 5 January 2016) [Google Scholar]
- 11.Sozialgesetzbuch (SGB) Fünftes Buch (V) § 115c. [Google Scholar]
- 12.Karapinar F, van den Bemt PM, Zoer J, Nijpels G, Borgsteede SD. Informational needs of general practitioners regarding discharge medication: content, timing and pharmacotherapeutic advice. Pharm World Sci. 2010;32:172–178. doi: 10.1007/s11096-009-9363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saedder EA, Brock B, Nielsen LP, Bonnerup DK, Lisby M. Identifying high-risk medication: a systematic literature review. Eur J Clin Pharmacol. 2014;70:637–645. doi: 10.1007/s00228-014-1668-z. [DOI] [PubMed] [Google Scholar]
- 14.Coleman EA, Smith JD, Raha D, Min S. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165:1842–1847. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 15.Greenwald JL, Halasyamani LK, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant, and implementable: a consensus statementon key principles and necessary first steps. Jt Comm J Qual Patient Saf. 2010;36:504–513. doi: 10.1016/s1553-7250(10)36074-0. [DOI] [PubMed] [Google Scholar]
- 16.Accreditation Canada Canadian Institute for Health Information, Canadian Patient Safety Institute, Institute for Safe Medication Practices Canada. Medication reconciliation in Canada: Raising the bar. Progress to date and the course ahead. Ottawa 2012. https://accreditation.ca/sites/default/files/med-rec-en.pdf (last accessed on 27 January 2016) [Google Scholar]
- 17.Leotsakos A, Zheng H, Croteau R, et al. Standardization in patient safety: The WHO High 5s project. Int J Qual Health Care. 2014;26:109–116. doi: 10.1093/intqhc/mzu010. [DOI] [PubMed] [Google Scholar]
- 18.Lindquist LA, Go L, Fleisher J, Jain N, Friesema E, Baker DW. Relationship of health literacy to intentional and unintentional non-adherence of hospital discharge medications. J Gen Intern Med. 2012;27:173–178. doi: 10.1007/s11606-011-1886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulino EI, Bouvy ML, Gastelurrutia MA, Guerreiro M, Buurma H. Drug related problems identified by European community pharmacists in patients discharged from hospital. Pharm World Sci. 2004;26:353–360. [PubMed] [Google Scholar]
- 20.Gesetz zur Stärkung der Versorgung in der gesetzlichen Krankenversicherung (GKV-Versorgungsstärkungsgesetz - GKV-VSG) Bundesgesetzblatt 2015 Teil I Nr. 30, ausgegeben zu Bonn am 22. Juli 2015 [Google Scholar]
- 21.Gesetz für die sichere digitale Kommunikation und Anwendungen im Gesundheitswesen sowie zur Änderung weiterer Gesetze. Bundesgesetzblatt 2015 Teil I Nr. 54, ausgegeben zu Bonn am 28. Dezember 2015 [Google Scholar]
- 22.Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23:1414–1422. doi: 10.1007/s11606-008-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armor BL, Wight AJ, Carter SM. Evaluation of adverse drug events and medication discrepancies in transitions of care between hospital discharge and primary care follow-up. J Pharm Pract. 2016;29:132–137. doi: 10.1177/0897190014549836. [DOI] [PubMed] [Google Scholar]
- 24.Schlitt A, Wischmann P, Wienke A, et al. Rehabilitation in patients with coronary heart disease: Participation and its effect on prognosis. Dtsch Arztebl Int. 2015;112:527–534. doi: 10.3238/arztebl.2015.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schächtele S, Tümena T, Gaßmann K, Fromm MF, Maas R. Implementation of warnings from Dear Doctor Letters (Rote-Hand-Briefe): an analysis of medication data from a large cohort of elderly patients. Dtsch Arztebl Int. 2014;111:255–263. doi: 10.3238/arztebl.2014.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unroe KT, Pfeiffenberger T, Riegelhaupt S, Jastrzembski J, Lokhnygina Y, Colón-Emeric C. Inpatient medication reconciliation at admission and discharge: a retrospective cohort study of age and other risk factors for medication discrepancies. Am J Geriatr Pharmacother. 2010;8:115–126. doi: 10.1016/j.amjopharm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42:1373–1379. doi: 10.1345/aph.1L190. [DOI] [PubMed] [Google Scholar]
- 28.Walk SU, Bertsche T, Kaltschmidt J, et al. Rule-based standardised switching of drugs at the interface between primary and tertiary care. Eur J Clin Pharmacol. 2008;64:319–327. doi: 10.1007/s00228-007-0402-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.