Abstract
Vitamin D is a prohormone nutrient, which is involved in skeletal and extra-skeletal functions. Iron is another essential nutrient that is necessary for the production of red blood cells and oxygen transport. This element plays important roles in enzymatic systems including those required for Vitamin D activation. To the best of our knowledge, there is no exclusive review on the relationship between iron deficiency anemia (IDA), as the most prevalent type of anemia, and Vitamin D deficiency and the effect of recovery from iron deficiency on Vitamin D status. The aim of this study was to conduct a systematic search of observational and clinical trials in this field. The databases of PubMed, ProQuest, Cochrane Library, ISI Web of Knowledge, and SCOPUS were searched comprehensively. English-language human studies conducted on iron deficient patients or interventions on the effect of iron therapy on Vitamin D were extracted (n = 10). Our initial search yielded 938 articles. A total of 23 papers met the inclusion criteria. Thirteen studies were excluded because they were not relevant or not defining anemia types. The final analysis was performed on ten articles (3 cross-sectional and 7 interventional studies). Observational data indicated a positive relationship between iron status and Vitamin D, while trials did not support the effectiveness of iron supplementation on improving Vitamin D status. The mechanism underlying this association may involve the reduction of the activation of hydroxylases that yield calcitriol. Future randomized controlled trials with large sample sizes and proper designs are needed to highlight underlying mechanisms.
Keywords: Anemia, iron, iron-deficiency anemia, Vitamin D, Vitamin D deficiency
INTRODUCTION
Deficiencies in both Vitamin D and iron are recognized as two major public health concerns in the globe. Nearly, 30%–50% of all age groups are Vitamin D deficient worldwide.[1] Sun exposure is the most important source of Vitamin D for most people. The effect of sun exposure on Vitamin D synthesis depends on skin pigmentation, body size, and aging.[2] Photosynthesized Vitamin D is transported to the liver by the Vitamin D binding protein to pass the first hydroxylation. The second hydroxylation in kidneys converts it to its biologically active form, 1,25-hydroxy Vitamin D (1,25(OH)2D). Serum phosphorus, calcium, and fibroblast growth factor (FGF-23) are the key regulators of the renal production of 1,25(OH)2 D.[3] Although the most popular role of Vitamin D in the body is bone health, it has a wide range of functions. Vitamin D deficiency (VDD) is related to infant mortality, cardiovascular diseases, cancer, total mortality, diabetes, mood disorders, and increased risk of infections like tuberculosis and AIDS.[4,5] When the concentration of 25(OH)D3 is <20 ng/ml (50 nmol/L), VDD exists. A level of ≥30 ng/ml (≥75 nmol/L) is considered normal. Vitamin D insufficiency has been defined as 25(OH)D between 21 and 29 ng/ml.[6]
It has estimated that 2–3 billion individuals suffer from anemia worldwide.[7] IDA is the most prevalent type of anemia. Data from US NHANES 1976–1980, have been used to estimate iron deficiency based on the prevalence of anemia in countries with a high prevalence of anemia and iron deficiency. Accordingly, when anemia is prevalent in 20% of population, iron deficiency prevalence will be 50%, and when it is >40%, some degree of iron deficiency exists in whole population.[8,9] IDA is associated with maternal mortality, prenatal infant loss, and prematurity, immune status and morbidity from infection, physical capacity and work performance, cognitive performance, and behavior.
Anemia and VDD have been observed simultaneously.[10] Some recent studies blame IDA for VDD because of their linked metabolism.[11,12,13] The results of studies in this area are inconsistent due to heterogeneity in study objectives and lack of determining the etiology of anemia. There are also several trials evaluating the effect of iron intake on Vitamin D concentration as their primary or secondary outcomes,[14,15,16,17,18,19,20] but there is no exclusive review on the effect of iron deficiency or its replenishment on Vitamin D status.
To increase our understanding of this association, synthesize of the research, and generate new insights into coexistence of micronutrient deficiencies, we conducted a systematic review of the published literature investigating the development of VDD due to iron deficiency.
METHODS
Identification of studies
The PRISMA statement was used for reporting the present systematic review.[21] Articles indexed in PubMed, ProQuest, ISI Web of Science, Cochrane Library, and SCOPUS were searched using the following MeSH terms: Anemia, iron-deficiency anemia, 25(OH)D, and VDD. We looked for these terms in the abstract, title, or keywords. No limits were used. In addition, articles referenced by those identified in this search were reviewed for relevance.
The search results were imported to endnote to find duplicates. Titles and abstracts were examined by two independent reviewers. Inclusion criteria were (1) the articles written in the English, (2) observational studies were conducted on iron deficient patients or interventions demonstrating the effect of iron therapy on Vitamin D (i.e., those observational studies on anemia without specifying its type and review articles were not included), and (3) human studies. Only papers met the inclusion criteria were reviewed [Figure 1]. Because of the limited number of eligible studies, we did not define a strict age range.
Figure 1.
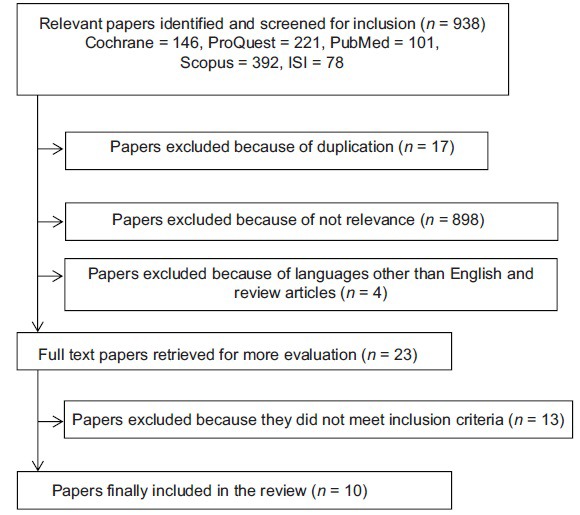
Flowchart describing the process of the review
Observational studies included in this review were scored by the Strengthening the Reporting of Observational Studies in Epidemiology: Explanation and Elaboration (STROBE) criteria[22] and trials were scored according to the Black and Downs’ checklist.[23] The STROBE checklist items relate to design, setting, participants, confounders, bias, sample size, statistical analysis, outcome measures, results, limitations, and generalizability of the study. The scoring system is as follows: 0 (not done), 1 (done partially), and 2 (done well). Score range for this tool is between 0 (lowest) to 40 (highest quality). Downs's checklist is composed of 27 items for evaluating the risk of bias, based on the adjustment of the confounders, adverse events of the intervention, patient loss, blindness, interventions compliance, and randomization. Score range is between 0 and 31 for the Black and Downs.
For each study met eligibility criteria, the first authors’ name, publication year, study location, number and age of volunteers, intervention (for trials), the most relevant results, and quality score were abstracted [Tables 1 and 2].
Table 1.
Cross-sectional studies investigating the relationship between iron deficiency anemia and Vitamin D deficiency
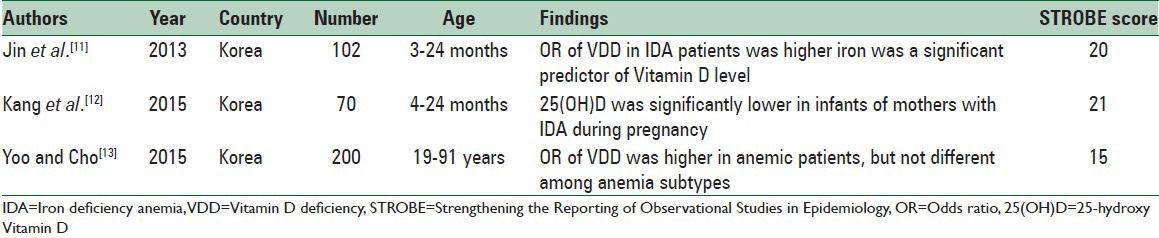
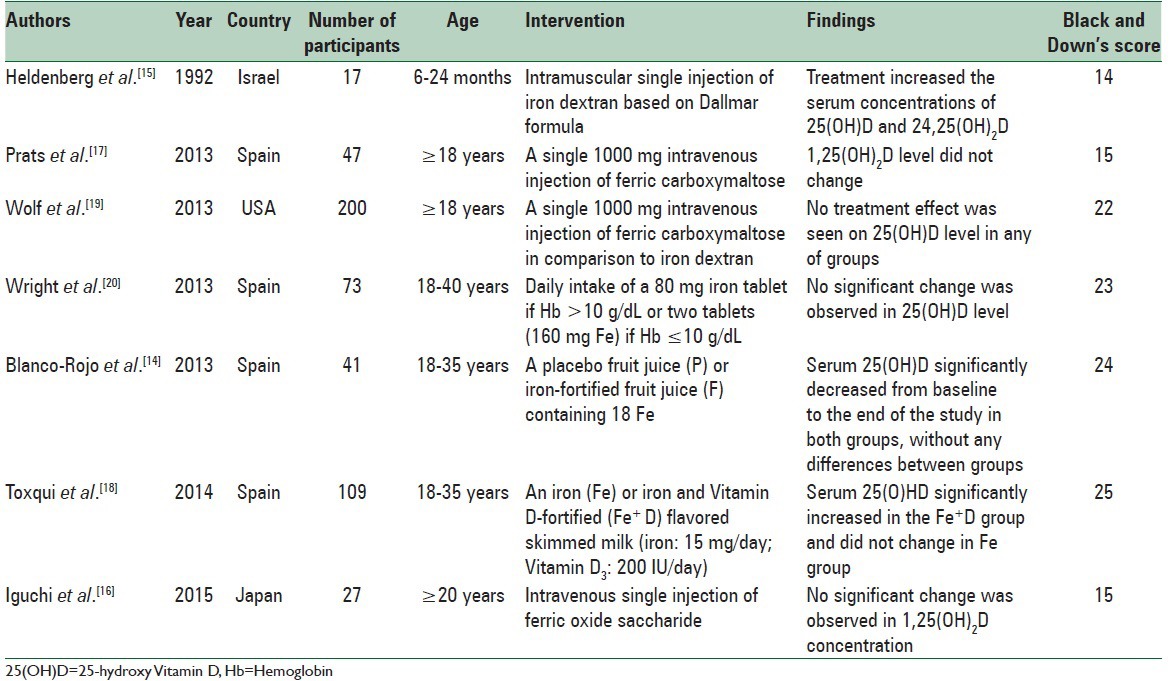
Table 2.
Interventional studies investigating the effect of iron supplementation on Vitamin D concentration
RESULTS
Our initial search yielded 938 articles. After duplicates were removed (n = 17), 898 articles were excluded because of not relevance and finally 23 articles identified for further assessment. One article that was published in language other than English and three reviews were also excluded. We also excluded studies in which iron status was not defined separately (n = 7). One trial did not describe the effect of the intervention on Vitamin D specifically. Finally, 3 observational studies and 7 trials were included.
Study characteristics
Of these 10 articles, 90% of them were published in recent years,[11,12,13,14,15,16,17,18,19,20] between 2013 and 2015. All cross-sectional studies were conducted in Korea.[11,12,13] Over half of the trials were conducted in Spain;[14,17,18,20] one in the United States;[19] one in Japan;[16] and one in Israel.[15] We divided studies into two groups to specify the results (1) observational studies [Table 1] and (2) interventional studies that evaluated the effect of iron supplementation on Vitamin D status [Table 2]. We also described the relationship between iron and Vitamin D in the second category, whenever it was mentioned.
Observational studies
Table 1 shows the characteristics of cross-sectional studies evaluating the relationship between iron and Vitamin D. All three included articles[11,12,13] revealed that 25(OH)D was lower in anemic cases. Kang et al.[12] showed that 25(OH)D level was significantly lower in infants of mothers with medical history of anemia during pregnancy (P = 0.011). Although VDD had an odds ratio (OR) of 4.74 for the presence of iron deficiency, it was nonsignificant. Similarly, Jin et al.[11] demonstrated that VDD was significantly more common in anemic children (OR = 4.115, 95% confidence interval [CI] = 1.665–10.171). Serum iron was an important predictor of 25(OH)D (P = 0.005). The most impressive limitations of these studies were small sample size and selection bias. Yoo and Cho[13] showed that VDD was significantly more seen in anemic individuals (OR = 3.316, 95% CI = 2.265–4.854). This study was also limited by small sample size, seasonal variation, and coexisting of other health problems in participants. The results of these studies were represented after adjustment for age, gender, estimated glomerular filtration rate, and transferrin saturation. The risk of bias in all studies was related to insufficient description of study design, potential source of bias, and generalizability of the results.
Interventional studies
Study design and baseline participant characteristics are presented in Table 2. Quality scores for these studies ranged from 14 to 25 for the Down. Vitamin D was not the main outcome in 3 studies.[16,17,19] Four of the studies investigated other metabolites of Vitamin D.[15,16,17,19] Only two studies were parallel-group double-blind randomized clinical trials.[18,20] A total of 411 individuals were recruited, with 371 participants remaining at the end of study. Over 50% of trials enrolled premenopausal women;[14,18,19,20] two enrolled elderlies;[16,17] and one enrolling 6–24 months infants.[15] Type, dosage and duration of supplementation varied. Four trials used single dosage of intravenous or intramuscular iron[15,16,17,19] and a follow-up of 5–12 weeks. Two studies used fortified products for 16 weeks.[18,20] One trial reported daily intake of ferrous sulfate tablets which the dose and duration were related to recovery from IDA.[14]
Pure iron supplementation did not significantly affect the serum concentration of any of the Vitamin D metabolites in most interventions.[16,17,19,20] Heldenberg et al. observed significant increases in both 25(OH)D and 24,25-dihydroxy Vitamin D levels in a group of infants with IDA and VDD.[15] Although children took Vitamin D as a routine treatment, they were Vitamin D deficient and showed positive results after iron injection. In a group of iron-deficient women consuming a placebo fruit juice (P) or iron-fortified fruit juice (F), 25(OH)D decreased in both groups (P < 0.001).[14] Toxqui et al. provided an iron (Fe group) or iron and Vitamin D-fortified (Fe plus D group) skimmed milk for iron-deficient women.[18] After 16 weeks, 25(OH)D significantly increased in the Fe+ D group (P < 0.001) without any change in the Fe group. Quality assessment using Down's checklist indicated missing data about confounders, blinding, and randomization.
The relationship between iron and Vitamin D was assessed in three studies.[14,15,20] A significant positive correlation was found between serum iron and baseline Vitamin D concentration, hematocrit, transferrin saturation in two studies.[14,20] In one study, infants with low 25(OH)D and low 24,25(OH)2 D had lower hemoglobin (Hb) and transferrin saturation.[15]
DISCUSSION
In the present review, a possible association was demonstrated between iron and Vitamin D levels. Our results indicated that iron supplementation has no statistically significant effect on the improvement of VDD. Future trials should be conducted to elucidate different dimensions of this relationship.
All observational studies reported positive correlation between iron and Vitamin D in spite of some differences in adjusted cofounders, VDD and ID cut off points, age ranges, and health status of populations. This was also confirmed in two interventional studies. There are potential confounders influencing the relationship between iron and Vitamin D including body mass index (BMI), age, dietary calcium and fat intake, ethnicity, some diseases and medications, inflammation, oxidative stress, and altitude which should be considered in future research. The most important ones are BMI and inflammation. Vitamin D is a fat soluble nutrient which is stored in body fat; so the amount of fat tissue can affect its concentration.[24] Iron marker, ferritin, is an acute-phase protein that increases in inflammatory conditions and this can result in IDA underestimation.[25] It is worth noting that unfortunately IDA detection was not defined accurately and number of iron deficient patients was very small in comparison with total sample size. Increased age leads to a decreased Vitamin D concentration.[26] Premenopausal women are at increased risk of anemia because of menstruation, but menopaused females progress anemia because of inflammation and nutritional deficiencies.[27] The final problem is cross-sectional nature of studies. Comparison of iron deficient patients with healthy controls can shed light on the mentioned relationship. However, the number of interventional studies is not efficient enough to make an accurate judgment.
Epidemiological data have shown that bone health is related to 25(OH)D status. Desirable range of 25(OH)D for fracture prevention is more than 75 nmol/L. It has been postulated that chronic iron deficiency increases bone resorption.[28] One of the proposed mechanisms is Vitamin D deactivation.
When iron depletion takes place in tissues, the activity of iron containing enzymes decrease.[29] As mentioned before, Vitamin D is activated in the body by two sequential steps. In the first step, 25(OH)D3 is produced in the liver. A kind of cytochrome P450, CYP2R1, is responsible for this stage. The second hydroxylation happens in kidneys and some other tissues by the virtue of CYP27B1 to form 1,25(OH)2D3. CYP2R1 requires NADPH-cytochrome P450 reductase to function properly. CYP27B1 needs two other compounds: Ferredoxin reductase and ferredoxin.[30] Both of these enzymes contain heme group. Hence, it seems that Vitamin D metabolism is dependent on iron and its deficiency might disturb Vitamin D activation.
There are some evidences demonstrating an opposite association. On the other hand, VDD may results in anemia. A number of mechanisms have been suggested to explain this finding: (a) VDD contributes to decreasing local calcitriol production in bone marrow and increases membrane permeability of calcium. As a result, erythropoiesis declines. In vitro studies have shown that this happens at mRNA and protein levels;[31,32] (b) hyperparathyroidism due to VDD raises proliferation of erythroid progenitor cells;[33] and (c) VDD is associated with higher hepcidin level in the body. This pro-inflammatory mediator is involved in anemia of chronic disease.[34] Previous studies have reported beneficial effects of supplementation with Vitamin D on the reduction of erythropoiesis stimulating agents requirements in patients with chronic kidney disease and increased Hb concentrations.[35,36] In a 16-week randomized controlled trial, Toxqui et al. tested the effectiveness of iron versus iron and Vitamin D-fortified milk on improving iron status in 109 iron-deficient women.[37] The group consuming Fe+ D, had higher levels of erythrocytes, hematocrit, and Hb at week 8 compared to the Fe group. However, most of these studies did not focus on a specific type of anemia in a healthy population. Further clinical trials are needed to explore the direction of the Vitamin D and iron association.
Almost all reviewed studies showed that iron supplementation had no effect on 25(OH)D level. However, most of these studies were inadequately powered regarding design (e.g., lack of blinding, randomization, and control group). Mostly, Vitamin D status is assessed by measuring 25(OH)D using ultraviolet detection after its separation by normal phase high-performance liquid chromatography.[38] None of the studies used this method; this might be involved in the lack of discovering any beneficial effect of supplementation. It may also be due to the level of iron depletion and its renewal. There are several stages for iron deficiency: (a) Latent iron deficiency; (b) iron deficient erythropoiesis; (c) IDA; and (d) functional iron deficiency.[39] In the third stage, Hb level decreases but mean corpuscular volume and mean corpuscular hemoglobin do not fall until the deficiency becomes chronic. It has shown that iron-containing enzymes including cytochromes are affected in this phase. Maybe lack of the effect of iron supplementation is caused by inadequate levels of iron deficiency to influence 25-hydroxylase. Just three interventions had recruited participants with IDA,[15,17,20] but only one of those showed positive results.[15] Increased Vitamin D concentration was also observed in the other two studies, although it was not significant.
To the best of our knowledge, this is the first systematic review to address IDA and VDD relationship. This review is strengthened by the inclusion of both cross-sectional studies and trials. However, this review is also limited by the small number of studies, with many of the included trials not originally designed for the primary outcomes intended for this review. The primary objective of some of these trials was to investigate the effect of iron on FGF-23.[16,17,19] Different types of outcome measures reported, Vitamin D dosages, duration of supplementation, and participants are the other factors producing heterogeneity. Several studies were conducted in Korean population with low generalization of the results and papers included were from very diverse age-strata. To further elucidate the role of iron supplementation on Vitamin D, future randomized controlled trials should focus on improving the quality of the study design.
CONCLUSIONS
This systematic review pointed out that the majority of the studies confirmed the existence of a relationship between iron and Vitamin D; but present data do not support a beneficial effect of iron supplementation in the management of VDD. Further studies with large sample sizes and proper designs are needed to highlight underlying cellular, molecular, and genetic mechanisms and to generate good quality evidence.
Financial support and sponsorship
This study was supported by Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to thank the authors of the reviewed articles who supplied their paper free of charge.
REFERENCES
- 1.Palacios C, Gonzalez L. Is Vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144(Pt A):138–45. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. Sunlight and Vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–88S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 3.Watson R. Handbook of Vitamin D in Human Health: Prevention, Treatment and Toxicity. The Netherlands: Wageningen Academic Publishing; 2013. [Google Scholar]
- 4.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of Vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–8. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality – A review of recent evidence. Autoimmun Rev. 2013;12:976–89. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald C, Mildon A, Neequaye M, Namarika R, Yiannakis M. Women's Health in the Majority World: Issues and Initiatives. New York: Nova Science Publishers Inc; 2007. Anemia-can its widespread prevalence among women in developing countries be impacted? A case study: Effectiveness of a large-scale, integrated, multiple-intervention nutrition program on decreasing anemia in Ghanaian and Malawian women; pp. 65–107. [Google Scholar]
- 8.Dallman PR, Yip R, Johnson C. Prevalence and causes of anemia in the United States, 1976 to 1980. Am J Clin Nutr. 1984;39:437–45. doi: 10.1093/ajcn/39.3.437. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Iron deficiency anemia: Assessment, prevention and control: A guide for program managers. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 10.Coutard A, Garlantézec R, Estivin S, Andro M, Gentric A. Association of Vitamin D deficiency and anemia in a hospitalized geriatric population: Denutrition as a confounding factor. Ann Hematol. 2013;92:615–9. doi: 10.1007/s00277-012-1633-9. [DOI] [PubMed] [Google Scholar]
- 11.Jin HJ, Lee JH, Kim MK. The prevalence of Vitamin D deficiency in iron-deficient and normal children under the age of 24 months. Blood Res. 2013;48:40–5. doi: 10.5045/br.2013.48.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang YS, Kim JH, Ahn EH, Yoo EG, Kim MK. Iron and Vitamin D status in breastfed infants and their mothers. Korean J Pediatr. 2015;58:283–7. doi: 10.3345/kjp.2015.58.8.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo EH, Cho HJ. Prevalence of 25-hydroxyvitamin D deficiency in Korean patients with anemia. J Clin Lab Anal. 2015;29:129–34. doi: 10.1002/jcla.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco-Rojo R, Pérez-Granados AM, Toxqui L, Zazo P, de la Piedra C, Vaquero MP. Relationship between Vitamin D deficiency, bone remodelling and iron status in iron-deficient young women consuming an iron-fortified food. Eur J Nutr. 2013;52:695–703. doi: 10.1007/s00394-012-0375-8. [DOI] [PubMed] [Google Scholar]
- 15.Heldenberg D, Tenenbaum G, Weisman Y. Effect of iron on serum 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D concentrations. Am J Clin Nutr. 1992;56:533–6. doi: 10.1093/ajcn/56.3.533. [DOI] [PubMed] [Google Scholar]
- 16.Iguchi A, Kazama JJ, Yamamoto S, Yoshita K, Watanabe Y, Iino N, et al. Administration of ferric citrate hydrate decreases circulating FGF23 levels independently of serum phosphate levels in hemodialysis patients with iron deficiency. Nephron. 2015;131:161–6. doi: 10.1159/000440968. [DOI] [PubMed] [Google Scholar]
- 17.Prats M, Font R, García C, Cabré C, Jariod M, Vea AM. Effect of ferric carboxymaltose on serum phosphate and C-terminal FGF23 levels in non-dialysis chronic kidney disease patients: Post-hoc analysis of a prospective study. BMC Nephrol. 2013;14:167. doi: 10.1186/1471-2369-14-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toxqui L, Pérez-Granados AM, Blanco-Rojo R, Wright I, de la Piedra C, Vaquero MP. Low iron status as a factor of increased bone resorption and effects of an iron and Vitamin D-fortified skimmed milk on bone remodelling in young Spanish women. Eur J Nutr. 2014;53:441–8. doi: 10.1007/s00394-013-0544-4. [DOI] [PubMed] [Google Scholar]
- 19.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28:1793–803. doi: 10.1002/jbmr.1923. [DOI] [PubMed] [Google Scholar]
- 20.Wright I, Blanco-Rojo R, Fernández MC, Toxqui L, Moreno G, Pérez-Granados AM, et al. Bone remodelling is reduced by recovery from iron-deficiency anaemia in premenopausal women. J Physiol Biochem. 2013;69:889–96. doi: 10.1007/s13105-013-0266-3. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9. [Google Scholar]
- 23.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zitt E, Sprenger-Mähr H, Mündle M, Lhotta K. Efficacy and safety of body weight-adapted oral cholecalciferol substitution in dialysis patients with Vitamin D deficiency. BMC Nephrol. 2015;16:128. doi: 10.1186/s12882-015-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bárány P. Inflammation, serum C-reactive protein, and erythropoietin resistance. Nephrol Dial Transplant. 2001;16:224–7. doi: 10.1093/ndt/16.2.224. [DOI] [PubMed] [Google Scholar]
- 26.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 27.Han SS, Kim M, Kim H, Lee SM, Oh YJ, Lee JP, et al. Non-linear relationship between serum 25-hydroxyvitamin D and hemoglobin in Korean females: The Korean National Health and Nutrition Examination Survey 2010-2011. PLoS One. 2013;8:e72605. doi: 10.1371/journal.pone.0072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsumata S, Katsumata-Tsuboi R, Uehara M, Suzuki K. Severe iron deficiency decreases both bone formation and bone resorption in rats. J Nutr. 2009;139:238–43. doi: 10.3945/jn.108.093757. [DOI] [PubMed] [Google Scholar]
- 29.Dallman PR. Biochemical basis for the manifestations of iron deficiency. Annu Rev Nutr. 1986;6:13–40. doi: 10.1146/annurev.nu.06.070186.000305. [DOI] [PubMed] [Google Scholar]
- 30.Jones G, Prosser DE. The activating enzymes of Vitamin D metabolism (25-and 1a-hydroxylases) In: Feldman D, Pike JW, Adams AJ, editors. Vitamin D. 3rd ed. New York: Elsevier; 2011. pp. 23–42. [Google Scholar]
- 31.Zittermann A, Jungvogel A, Prokop S, Kuhn J, Dreier J, Fuchs U, et al. Vitamin D deficiency is an independent predictor of anemia in end-stage heart failure. Clin Res Cardiol. 2011;100:781–8. doi: 10.1007/s00392-011-0312-5. [DOI] [PubMed] [Google Scholar]
- 32.Ernst JB, Becker T, Kuhn J, Gummert JF, Zittermann A. Independent association of circulating Vitamin D metabolites with anemia risk in patients scheduled for cardiac surgery. PLoS One. 2015;10:e0124751. doi: 10.1371/journal.pone.0124751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma S, Jain R, Dabla PK. The role of 25-hydroxy Vitamin D deficiency in iron deficient children of North India. Indian J Clin Biochem. 2015;30:313–7. doi: 10.1007/s12291-014-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fialho A, Fialho A, Kochhar G, Shen B. Association between Vitamin D deficiency and anemia in inflammatory bowel disease patients with ileostomy. J Coloproctol. 2015;35:139–45. [Google Scholar]
- 35.Riccio E, Sabbatini M, Bruzzese D, Capuano I, Migliaccio S, Andreucci M, et al. Effect of paricalcitol vs. calcitriol on hemoglobin levels in chronic kidney disease patients: A randomized trial. PLoS One. 2015;10:e0118174. doi: 10.1371/journal.pone.0118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin CL, Hung CC, Yang CT, Huang CC. Improved anemia and reduced erythropoietin need by medical or surgical intervention of secondary hyperparathyroidism in hemodialysis patients. Ren Fail. 2004;26:289–95. doi: 10.1081/jdi-120039528. [DOI] [PubMed] [Google Scholar]
- 37.Toxqui L, Pérez-Granados AM, Blanco-Rojo R, Wright I, González-Vizcayno C, Vaquero MP. Effects of an iron or iron and Vitamin D-fortified flavored skim milk on iron metabolism: A randomized controlled double-blind trial in iron-deficient women. J Am Coll Nutr. 2013;32:312–20. doi: 10.1080/07315724.2013.826116. [DOI] [PubMed] [Google Scholar]
- 38.Fraser WD, Milan AM. Vitamin D assays: Past and present debates, difficulties, and developments. Calcif Tissue Int. 2013;92:118–27. doi: 10.1007/s00223-012-9693-3. [DOI] [PubMed] [Google Scholar]
- 39.Crichton R. Iron Metabolism: From Molecular Mechanisms to Clinical Consequences. Chichester: John Wiley and Sons; 2009. [Google Scholar]


