Key Points
KIR haplotype A is an independent risk factor for the progression of MDS to AML.
Abstract
Myelodysplastic syndromes (MDSs) are a group of hematopoietic disorders affecting the myeloid lineage, characterized by cytopenias and clonal evolution to acute myeloid leukemia (AML). We hypothesized that natural killer (NK) cells and their activating killer immunoglobulin-like receptors (aKIRs) influence the immune surveillance and clinical outcome of patients with MDSs. Here, we first examined the distribution of aKIR genes and haplotype in 2 independent cohorts of MDS and AML patients. The median number of aKIR genes was lower in MDS patients than healthy controls (2 vs 3 genes; P = .001), and lower in patients with secondary AML (progressed from MDSs) compared with de novo AML patients (2 vs 3; P = .008) and healthy controls (2 vs 3; P = .006). In a multivariate analysis, the presence of KIR haplotype A (characterized by low aKIR content 0-1) independently predicted a higher risk of conversion to AML (relative risk [RR] with 95% confidence interval [CI], 2.67 [1.13-6.71]; P = .02) and worse adjusted progression-free survival (RR with 95% CI, 2.96 [1.59-5.52]; P = .001) and overall survival (2.25 [1.17-4.31]; P = .02), compared with KIR haplotype B (multiple aKIR genes). These novel findings may help to identify MDS patients with a high risk of disease progression who would likely benefit from adoptive NK-cell therapy.
Introduction
Immune surveillance, an important mechanism of cancer control, is partly mediated by natural killer (NK) cells, a key component of the innate immune system.1 Recent studies implicate NK cells in the control of myelodysplastic syndromes (MDSs), a heterogeneous spectrum of clonal hematopoietic disorders affecting the myeloid lineage, characterized by cytopenias and transformation to acute myeloid leukemia (AML).1,2 Whereas higher NK-cell frequencies have been reported in patients with low-risk MDSs,3 in high-risk cases, NK cells are reduced, with decreased expression of activating receptors and impaired cytotoxicity.4,5
Each NK cell can express both activating killer immunoglobulin-like receptors (aKIRs) and inhibitory KIRs that interact to regulate NK-effector function.2,6,7 There is striking heterogeneity in the number of inherited aKIR genes, varying from 0 to 6.8 Based on the number and distribution of KIR genes, individuals are classed along 2 broad haplotypes. Haplotype A comprises 5 inhibitory genes and the single activating gene KIR2DS4, whereas haplotype B incorporates various combinations of aKIR (up to 5) and inhibitory KIR genes. The number of aKIR genes inherited by individuals is linked to risk for cancer development.9-16 Prognostic systems used for MDSs rely on karyotypic and clinical features to stratify patients into risk groups. Because over half of all MDS patients have a normal karyotype and highly variable clinical phenotypes, we considered that the aKIR gene repertoire may help predict clinical outcome in this disease. Thus, we studied variations in aKIR gene content and haplotype in MDSs and their relationship to AML progression and survival in 2 independent patient cohorts.
Study design
Patients
MDS cohort.
We studied 108 MDS patients treated at the MD Anderson Cancer Center (MDACC) from May 2008 to August 2013. Patients were classified according to the International Prognostic Scoring System (IPSS) for MDSs,17 cytogenetic risk group, and World Health Organization (WHO) classification (Table 1). Median age was 68.4 years (range, 18.1-88.4 years); 32% were female. Median follow-up for surviving patients was 33.3 months (range, 3-206 months). Controls were 139 healthy hematopoietic stem cell (HSC) donors at MDACC (supplemental Table 1, available on the Blood Web site).
Table 1.
Four-year cumulative incidence of progression to AML and 4-year probability of PFS and OS according to patient characteristics
| Variable | n | Univariate analysis 4-y probability | Multivariate analysis RR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| AML | PFS | OS | AML | PFS | OS | ||
| Age, y* | P = .34 | P = .03 | P = .02 | P = .02 | |||
| ≤70 | 53 | 27.2 | 55.8 | 57.0 | 1 | ||
| >70 | 55 | 17.6 | 28.0 | 28.7 | 1.97 (1.08-3.59) | ||
| Sex | P = .79 | P = .71 | P = .91 | ||||
| Female | 34 | 20.3 | 44.2 | 43.6 | |||
| Male | 74 | 23.6 | 41.7 | 43.6 | |||
| WHO type | P = .07 | P = .01 | P = .01 | ||||
| RA | 11 | 29.3 | 60.6 | 60.0 | |||
| RARS | 8 | 0.0 | 85.7 | 85.7 | |||
| RCMD | 35 | 11.9 | 44.9 | 47.5 | |||
| RCMD-RS | 5 | 20.0 | 60.0 | 60.0 | |||
| RAEB-1 | 25 | 42.6 | 21.4 | 23.2 | |||
| RAEB-2 | 18 | 36.7 | 13.8 | 13.8 | |||
| MDS-U | 4 | 0.0 | 100.0 | 100.0 | |||
| del(5q) | 2 | 0.0 | 100.0 | 100.0 | |||
| WHO type | P = .07 | P < .001 | P < .001 | ||||
| RAEB-1 and 2 | 43 | 36.3 | 18.5 | 18.7 | |||
| Others | 65 | 13.0 | 59.8 | 61.0 | |||
| IPSS | P = .003 | P < .001 | P < .001 | P = .01 | P = .01 | ||
| Low | 48 | 5.6 | 71.3 | 74.0 | 1 | 1 | |
| Intermediate-1 | 22 | 34.2 | 39.5 | 38.4 | 2.34 (0.97-5.58) | 2.35 (0.95-5.80) | |
| Intermediate-2 | 10 | 22.2 | 22.2 | 22.2 | 5.08 (1.67-15.42) | 4.39 (1.48-13.01) | |
| High | 28 | 40.9 | 4.3 | 4.8 | 6.10 (2.01-18.52) | 5.74 (1.94-17.04) | |
| Cytogenetic risk group | P = .001 | P < .001 | P < .001 | P = .003 | P = .03 | P = .004 | |
| Low | 69 | 12.1 | 62.6 | 63.9 | 1 | 1 | 1 |
| Intermediate | 14 | 30.8 | 15.9 | 11.9 | 2.87 (0.84-9.83) | 3.76 (1.48-9.54) | 5.46 (2.09-14.27) |
| High | 25 | 47.3 | 4.8 | 5.7 | 5.29 (2.01-13.95) | 2.07 (0.75-5.72) | 2.40 (0.90-6.40) |
| No. of activating KIR genes | P = .87 | P = .06 | P = .09 | ||||
| 0-1 | 65 | 22.7 | 36.7 | 39.2 | |||
| ≥2 | 43 | 22.6 | 52.5 | 51.3 | |||
| Activating KIR gene haplotype | P = .02 | P = .02 | P = .10 | P = .02 | P = .001 | P = .02 | |
| Haplotype B† | 80 | 16.8 | 48.9 | 48.2 | 1 | 1 | 1 |
| Haplotype A | 28 | 37.1 | 27.3 | 31.2 | 2.67 (1.13-6.31) | 2.96 (1.59-5.52) | 2.25 (1.17-4.31) |
| HLA-C group‡ | P = .50 | P = .78 | P = .90 | ||||
| HLA-C1/x§ | 93 | 24.4 | 44.2 | 44.4 | |||
| HLA-C2/2 | 13 | 15.4 | 35.2 | 39.6 | |||
MDS-U, MDS unclassified; RA, refractory anemia; RAEB, refractory anemia with excess blasts; RARS, refractory anemia with ring sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RS, refractory cytopenia with multilineage dysplasia and ringed sideroblasts.
The median age was 68.4 years (range, 18.1-88.4 years).
Two patients had missing data.
Includes HLA-C1/C1 and HLA-C1/C2.
Haplotype B patients were further characterized as haplotype B centromeric and telomeric according to KIR gene content. They had similar outcomes, namely progression to AML (17.0% vs 16.0%; P = .96); PFS (45.2% vs 37.1%; P = .78); OS (45.8% vs 57.5%; P = .82).
AML cohort.
A second study group consisted of 499 adults with AML, consecutively enrolled in the Medical Research Council (MRC-10/15)-AML trials in the United Kingdom between 1988 and 200918,19 with available DNA (supplemental Table 2). Median age was 44 years (range, 13-69 years); 51% were female. The median follow-up for surviving patients was 88 months (range, 7-263 months). DNA from 253 HSC donors served as controls (supplemental Table 3).
All patients consented to the study in accord with the Declaration of Helsinki, and local ethics approval was obtained before sample collection. AML patients received anthracycline and cytarabine-based chemotherapy according to published protocols.18,19
KIR genotyping
KIR genotyping to identify the presence or absence of KIR genes of interest was performed by polymerase chain reaction with sequence-specific primers.20 Reverse specific oligonucleotide probe methodology was used for confirmatory typing. The KIR B haplotype was assigned if 1 or more KIR B defining loci were present (supplemental Table 4).
Statistical methods
Probabilities of overall survival (OS) and progression-free survival (PFS) were calculated by the Kaplan-Meier method.21 Probabilities of progression to AML were calculated by the cumulative incidence procedure. Associations between categorical (KIR haplotype and number of aKIRs) and ordered variables (patient age group, presenting white blood cell count, cytogenetics) were tested with the Cochran-Armitage test. χ2 tests were used to test associations between categorical (KIR haplotype and number of activating KIRs) and other categorical variables (sex, AML type, stem cell transplant). Variables found to be significant at the P < .15 level were included in the multivariate analysis, where OS and PFS were examined with a Cox regression model and AML progression by Fine-Gray regression analysis.22,23 Categorical data were compared with the Fisher exact test and quantitative data with the Mann-Whitney U test. Relative risks (RRs) are reported with 95% confidence intervals (CIs). All P values are 2-sided.
Results and discussion
MDS patients have lower numbers of aKIR genes
Because aKIR gene content determines both KIR haplotype and functional diversity among NK cells, and is linked to the pathogenesis of childhood acute lymphoblastic leukemia,9 we first compared the number of aKIR genes in 108 MDS patients treated at MDACC with that in 139 HSC donors from the same institution. The median number of aKIR genes was significantly lower in MDS patients than controls (2 [range, 0-6] vs 3 [range 0-6]; P = .001). Interestingly, the KIR haplotype distribution did not differ significantly between these 2 groups (haplotype A, 28 of 108 [25.9] vs 27 of 139 [19.4] healthy donors; P = .28).
We next asked whether the lower number of aKIR genes observed in our study was characteristic of all myeloid malignancies or specific to MDSs. Thus, we compared the number of aKIR genes among healthy controls (n = 253), patients with secondary AML (transformed from MDS/previous therapy) (n = 37), and patients with de novo AML (n = 462) treated in the MRC-UK trials. The 37 patients with transformed AML had a median of 2 aKIR genes (range, 0-6) compared with a median of 3 (range, 0-6) for both the patients with de novo AML (P = .008) and healthy controls (P = .006). The distribution of KIR haplotype A was similar between the de novo AML cohort and healthy controls (141 of 462 [30.5] vs 85 of 253 [34.6]; P = .25), with no apparent influence of KIR haplotype on the probability of relapse (P = .3) or death (P = .8) in patients with de novo AML. In contrast, the proportion of patients with haplotype A was significantly increased in the transformed AML cohort (19 of 37 [51.4]; P = .01), suggesting a higher risk of AML progression in MDS patients with haplotype A. Of note, we found no significant associations between baseline patient or healthy donor characteristics and the number of aKIRs or KIR haplotype (supplemental Tables 1-3 and 5).
KIR haplotype is an independent predictor for MDS-AML transformation
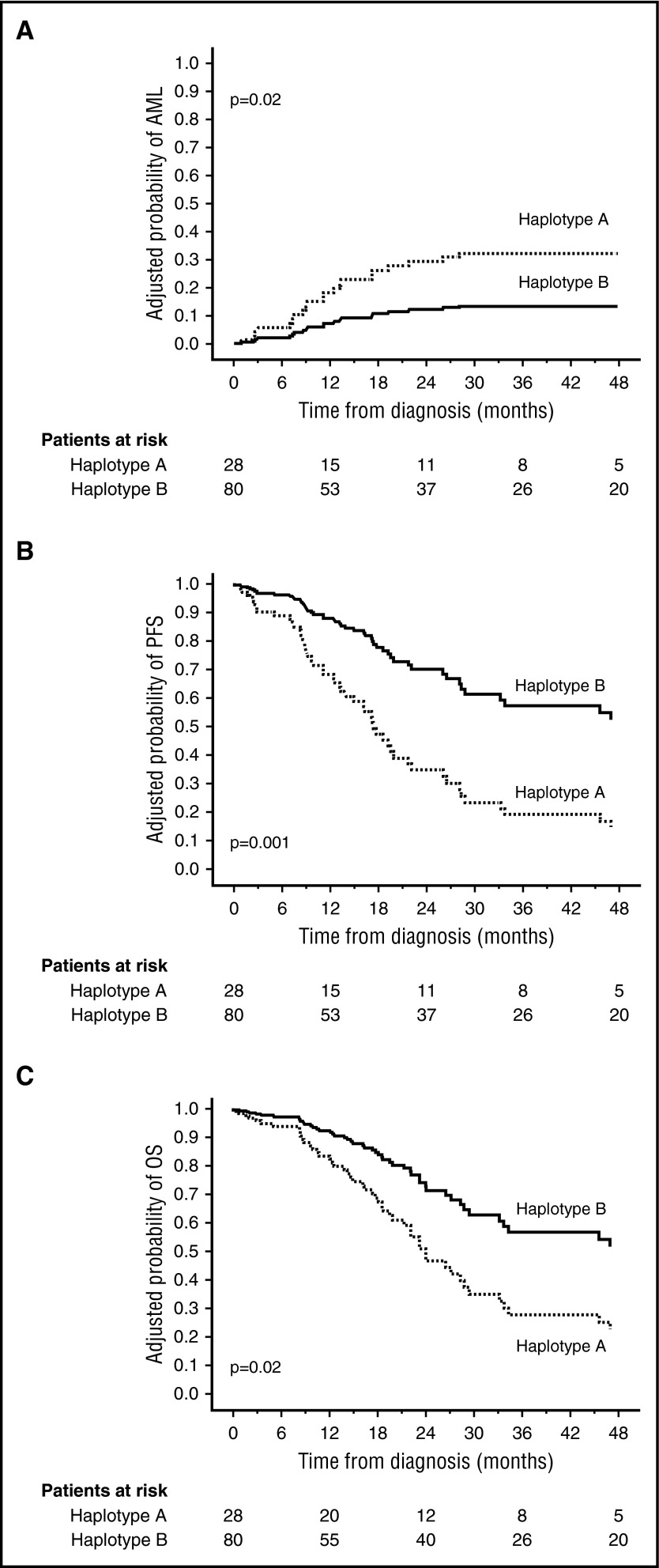
We next examined the influence of KIR haplotype on the risk of AML transformation in the MDACC cohort of 108 MDS patients. The 28 KIR haplotype A patients had a higher 4-year probability of progression to AML and lower 4-year PFS and OS than the 80 KIR haplotype B patients: 37.1 vs 16.8 (P = .02), 27.3 vs 48.9 (P = .02), and 31.2 vs 48.2, (P = .10). Other factors impacting on these end points in univariate analysis were age, WHO type, IPSS, and cytogenetic risk group (Table 1).
Multivariate analysis including all variables with P < .15 identified only cytogenetic risk group and KIR haplotype as independent predictors of MDS progression to AML (Table 1). The relative risk of MDS-AML transformation in patients with haplotype A vs haplotype B was 2.67 (1.13-6.31) (P = .02). Similarly, only cytogenetic risk group, IPSS, and KIR haplotype independently predicted survival. MDS patients with haplotype A had worse adjusted PFS (RR with 95% CI, 2.96 [1.59-5.52]; P = .001) and OS (2.25 [1.17-4.31]; P = .02) compared with KIR haplotype B (multiple aKIR genes) (Figure 1).
Figure 1.
KIR haplotype predicts outcome of MDS patients. Adjusted probabilities for progression to (A) AML, (B) PFS, and (C) OS according to KIR haplotype. Patients were classified according to the KIR haplotype and the probabilities for the outcomes were adjusted by the other variables found to be independent predictors in the multivariate models.
Although the number of patients analyzed is limited, this study shows an intriguing and previously unrecognized association between aKIR gene content and MDS-AML transformation: patients with MDSs progressing to AML have fewer aKIRs than healthy controls or patients with de novo AML. Moreover, MDS patients with lower aKIR content, as seen in haplotype A, have a greater probability of disease progression and death than do those with haplotype B. Even after adjusting for competing covariates, such as cytogenetic risk group and IPSS, haplotype A was strongly associated with progression to AML and worse survival.
What biological mechanisms could account for these results? One possibility is that MDSs often follow an indolent course, beginning with genetic and epigenetic changes that may promote progression to AML.17 Thus, inefficient immune surveillance from NK cells with low aKIR gene content may fail to block the expansion of the transforming MDS clones, progressing eventually to overt high-risk MDSs or AML. By contrast, efficient immune surveillance may not play an equally important role in the control of diseases with rapid growth kinetics such as de novo AML, a notion supported by our observation of the same numbers of aKIR genes and haplotype distribution in de novo AML patients and healthy individuals. If confirmed in larger numbers of MDS patients with haplotype A, our findings would provide a compelling rationale for the use of adoptive NK-cell therapy for this subgroup.
Acknowledgments
The authors thank the patients who agreed to participate in this study, the teams of nurses, pharmacists, mid-level practitioners, and physicians for their patient care.
This work was supported in part by MD Anderson Cancer Center Leukemia Specialized Program of Research Excellence grant CA 100632 from the National Institutes of Health, National Cancer Institute and the generous philanthropic contributions to The University of Texas MD Anderson Moon Shots Program.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.S. performed experiments and designed, interpreted, analyzed, and wrote the manuscript; D.M. interpreted, analyzed, and wrote the manuscript; C.S., K.C., J.G.S., M.D., H.S., N.S., and T.S. performed experiments and analyzed and commented on the manuscript; A.J.B., R.H., D.L., R.G., R.M., E.J.S., H.K., and G.G.-M. interpreted, analyzed, and commented on the manuscript; C.A. and R.C. supplied samples and commented on the manuscript; and K.R. designed and directed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katayoun Rezvani, Stem Cell Transplantation and Cellular Therapy, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77004; e-mail: krezvani@mdanderson.org.
References
- 1.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12(4):239-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal S, van de Loosdrecht AA, Alhan C, Ossenkoppele GJ, Westers TM, Bontkes HJ. Role of immune responses in the pathogenesis of low-risk MDS and high-risk MDS: implications for immunotherapy. Br J Haematol. 2011;153(5):568-581. [DOI] [PubMed] [Google Scholar]
- 4.Epling-Burnette PK, Bai F, Painter JS, et al. . Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109(11):4816-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiladjian JJ, Bourgeois E, Lobe I, et al. . Cytolytic function and survival of natural killer cells are severely altered in myelodysplastic syndromes. Leukemia. 2006;20(3):463-470. [DOI] [PubMed] [Google Scholar]
- 6.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yawata M, Yawata N, Draghi M, Partheniou F, Little AM, Parham P. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood. 2008;112(6):2369-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yawata M, Yawata N, Abi-Rached L, Parham P. Variation within the human killer cell immunoglobulin-like receptor (KIR) gene family. Crit Rev Immunol. 2002;22(5-6):463-482. [PubMed] [Google Scholar]
- 9.Almalte Z, Samarani S, Iannello A, et al. . Novel associations between activating killer-cell immunoglobulin-like receptor genes and childhood leukemia. Blood. 2011;118(5):1323-1328. [DOI] [PubMed] [Google Scholar]
- 10.Cooley S, Trachtenberg E, Bergemann TL, et al. . Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooley S, Weisdorf DJ, Guethlein LA, et al. . Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Impola U, Turpeinen H, Alakulppi N, et al. . Donor haplotype B of NK KIR receptor reduces the relapse risk in HLA-identical sibling hematopoietic stem cell transplantation of AML patients. Front Immunol. 2014;5:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancusi A, Ruggeri L, Urbani E, et al. . Haploidentical hematopoietic transplantation from KIR ligand-mismatched donors with activating KIRs reduces nonrelapse mortality. Blood. 2015;125(20):3173-3182. [DOI] [PubMed] [Google Scholar]
- 14.Oevermann L, Michaelis SU, Mezger M, et al. . KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood. 2014;124(17):2744-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venstrom JM, Pittari G, Gooley TA, et al. . HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367(9):805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Wang B, Ye S, et al. . Killer cell immunoglobulin-like receptor gene polymorphisms in patients with leukemia: possible association with susceptibility to the disease. Leuk Res. 2010;34(1):55-58. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Manero G. Myelodysplastic syndromes: 2015 update on diagnosis, risk-stratification and management. Am J Hematol. 2015;90(9):831-841. [DOI] [PubMed] [Google Scholar]
- 18.Burnett AK, Hills RK, Milligan D, et al. . Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369-377. [DOI] [PubMed] [Google Scholar]
- 19.Grimwade D, Walker H, Oliver F, et al. ; The Medical Research Council Adult and Children’s Leukaemia Working Parties. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92(7):2322-2333. [PubMed] [Google Scholar]
- 20.Stringaris K, Adams S, Uribe M, et al. . Donor KIR genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant. 2010;16(9):1257-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 22.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;32(2):187-220. [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. [Google Scholar]