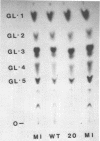
Abstract
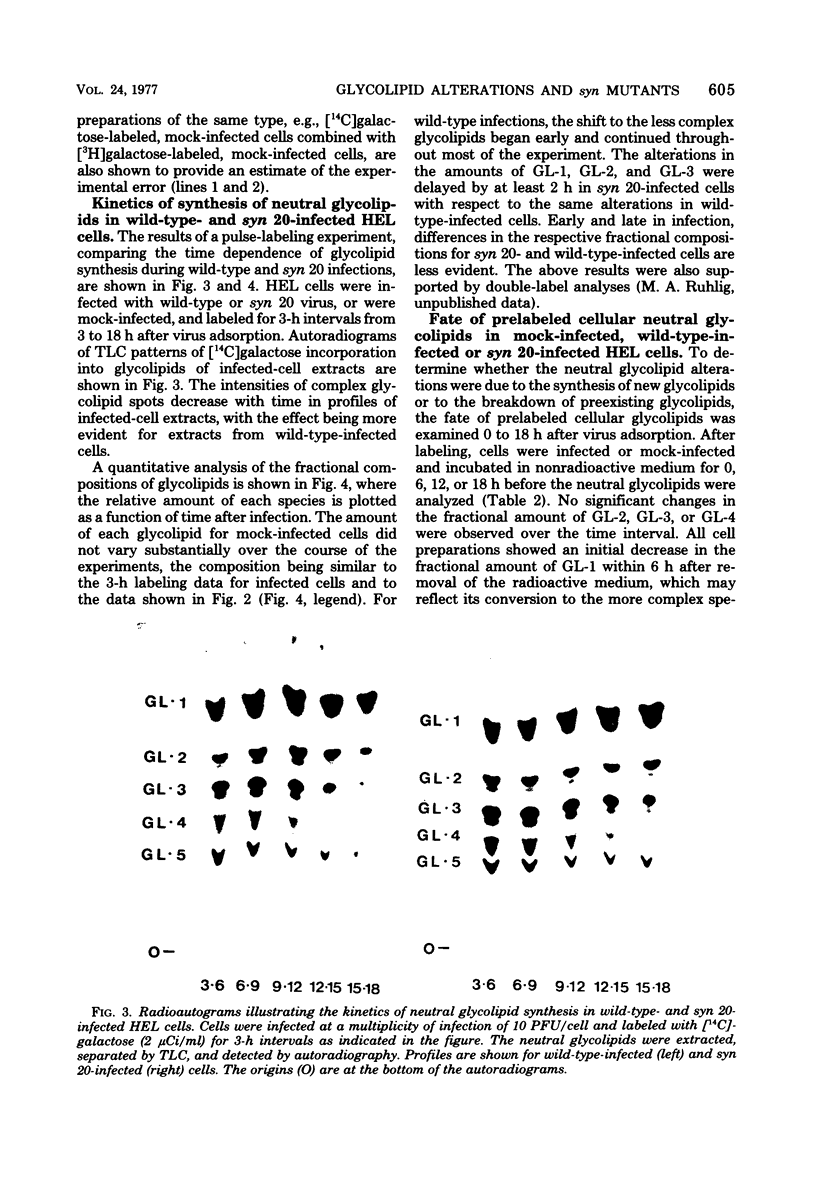
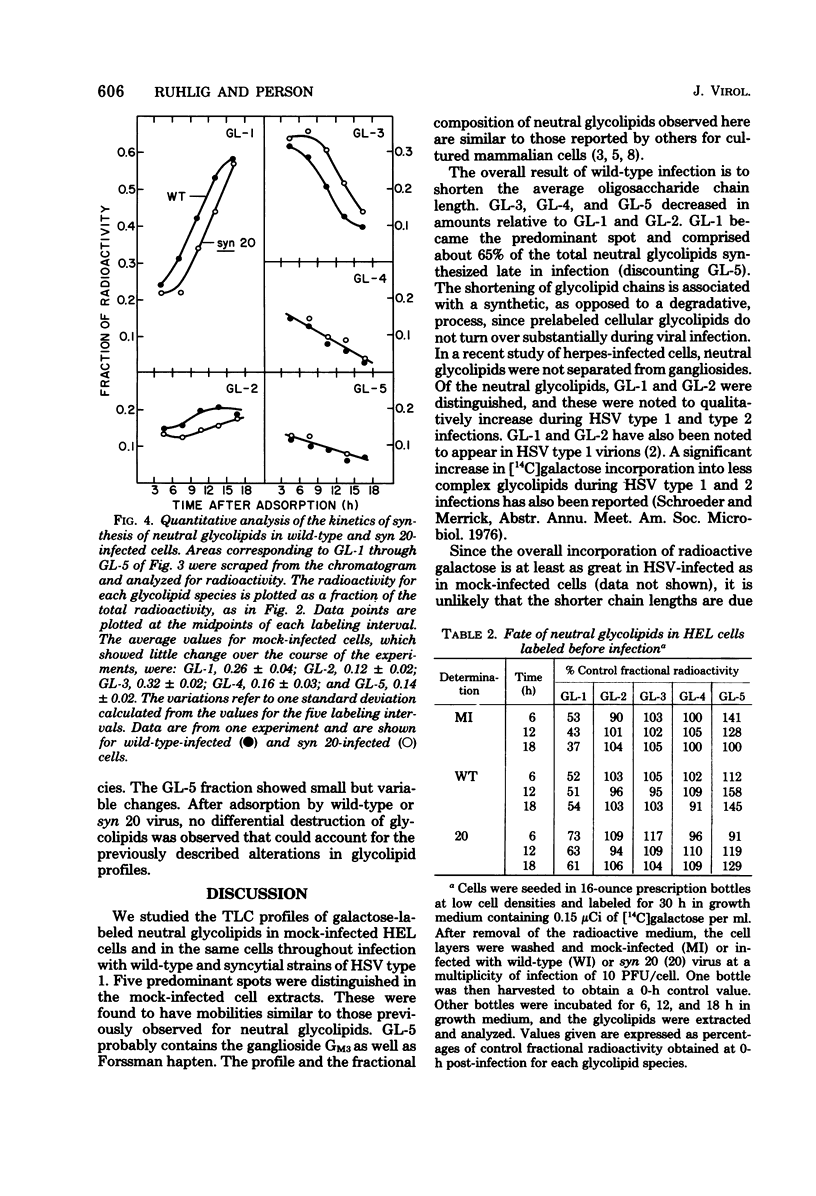
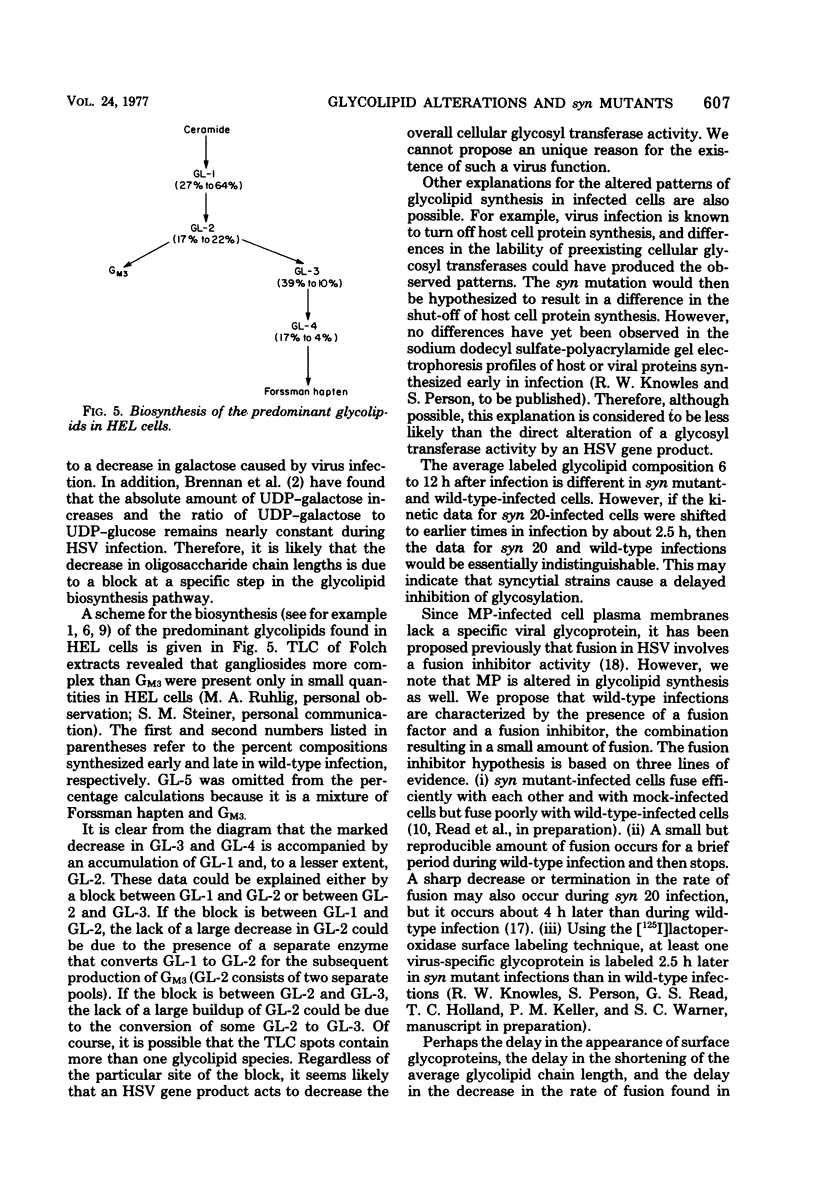
The isolation of syncytium-producing mutants of herpes simplex virus type 1 (KOS strain), which cause extensive cell fusion during otherwise normal infections, has been reported previously (S. Person, R. W. Knowles, G. S. Read, S. C. Warner, and V. C. Bond, J. Virol. 17:183-190, 1976). Seven of these mutants, plus two syncytial strains obtained elsewhere, were used to compare the incorporation of labeled galactose into neutral glycolipids of mock-infected, wild-type-infected, and syncytially infected human embryonic lung cells. Five predominant cellular glycolipid species were observed, denoted GL-1 through GL-5 in order of increasing oligosaccharide chain length; for example, GL-1 and GL-2 correspond to glycolipids that contain mono- and disaccharide units, respectively. Wild-type virus infection caused an increase in galactose incorporation into GL-1 and GL-2 relative to GL-3 through GL-5. For a single labeling interval from 4 to 10 h after adsorption, syncytial infections generally resulted in a relatively greater incorporation into more complex glycolipids than did wild-type infections. One mutant, syn 20, was compared with wild-type virus throughout infection by using a series of shorter labeling pulses and appeared to delay by at least 2 h the alterations observed during wild-type infections. These alterations are apparently due to defects in synthesis, since prelabeled cellular glycolipids were not differentially degraded during mock or virus infection.
Full text
PDF
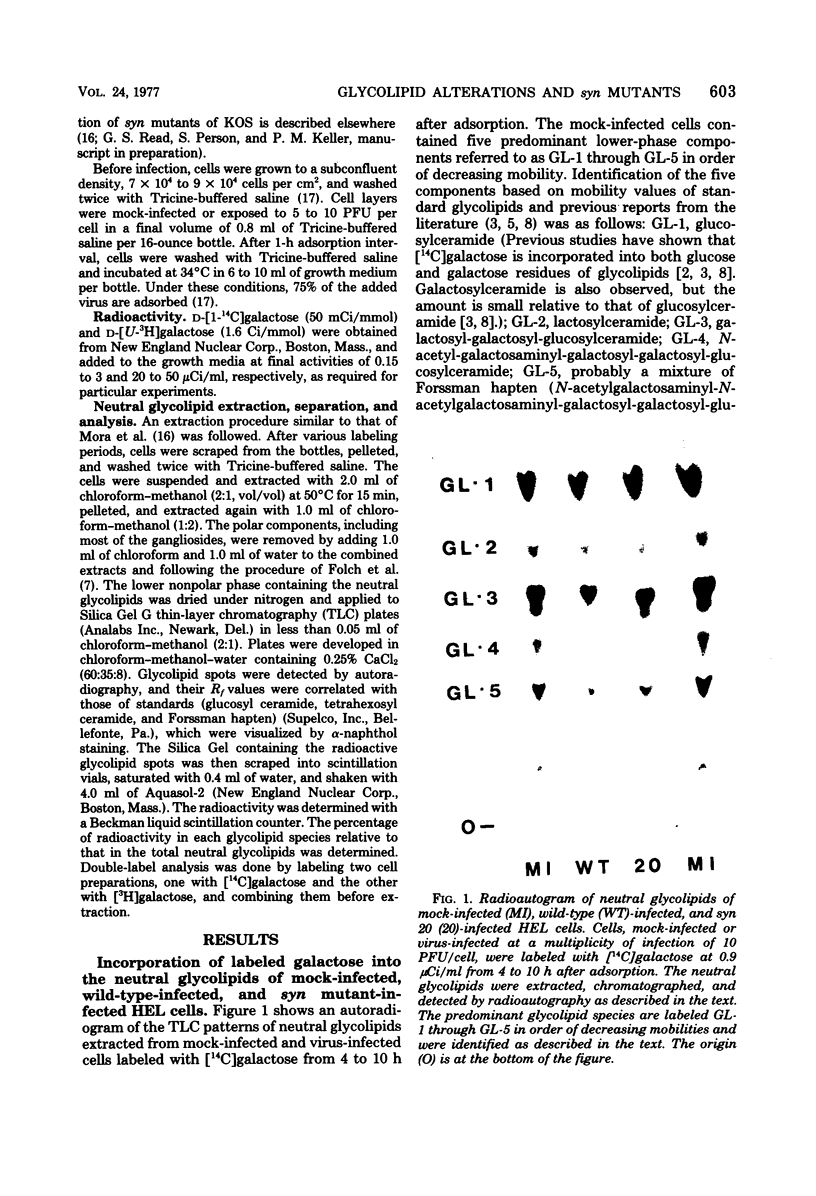
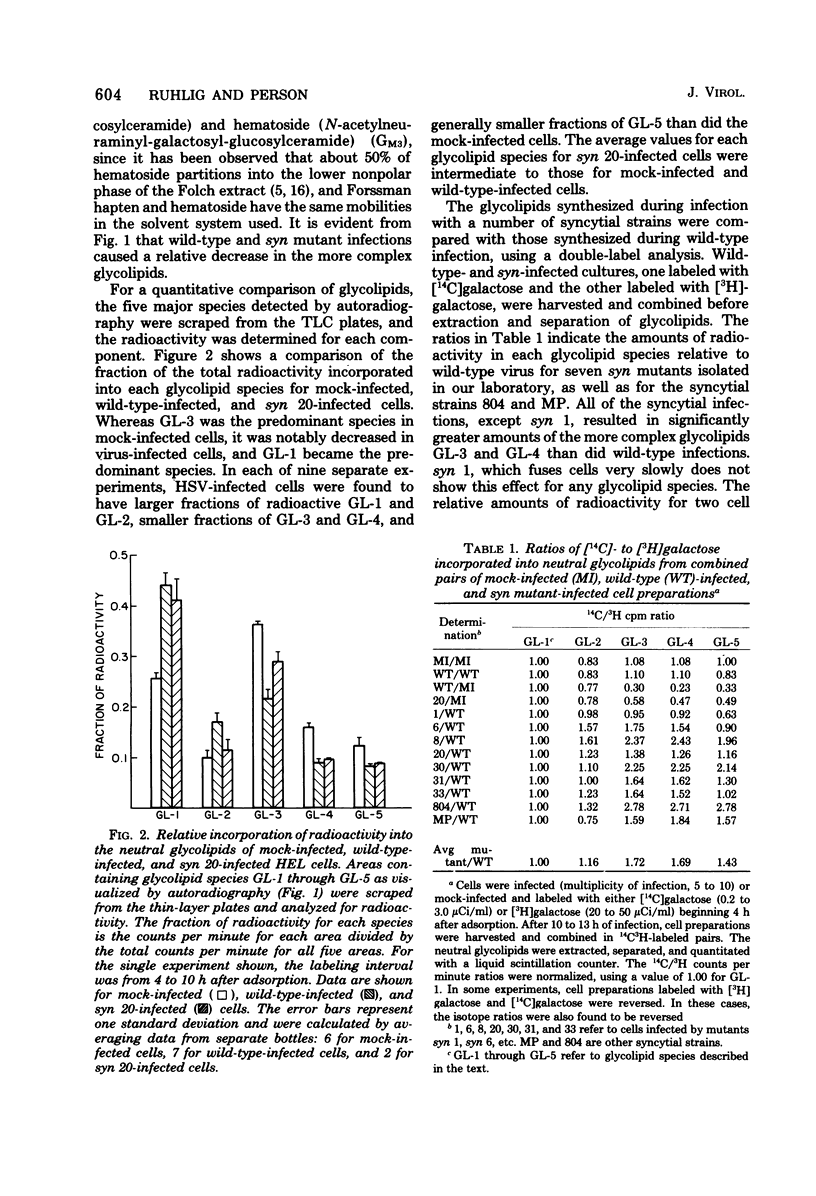

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady R. O., Fishman P. H. Biosynthesis of glycolipids in virus-transformed cells. Biochim Biophys Acta. 1974 Sep 9;355(2):121–148. doi: 10.1016/0304-419x(74)90001-8. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Steiner S. M., Courtney R. J., Skelly J. Metabolism of galactose in herpes simplex virus-infected cells. Virology. 1976 Jan;69(1):216–228. doi: 10.1016/0042-6822(76)90208-7. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Sweeley C. C., Velicer L. F. Glycosphingolipids of human KB cells grown in monolayer, suspension, and synchronized cultures. J Biol Chem. 1975 Jan 10;250(1):61–66. [PubMed] [Google Scholar]
- Dawson G., Matalon R., Dorfman A. Glycosphingolipids in cultured human skin fibroblasts. I. Characterization and metabolism in normal fibroblasts. J Biol Chem. 1972 Sep 25;247(18):5944–5950. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. Surface carbohydrates of hamster fibroblasts. I. Chemical characterization of surface-labeled glycosphingolipids and aspecific ceramide tetrasaccharide for transformants. J Biol Chem. 1975 Apr 10;250(7):2438–2446. [PubMed] [Google Scholar]
- Hakomori S. Structures and organization of cell surface glycolipids dependency on cell growth and malignant transformation. Biochim Biophys Acta. 1975 Mar 20;417(1):55–89. doi: 10.1016/0304-419x(75)90008-6. [DOI] [PubMed] [Google Scholar]
- Keller J. M., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M. The expression of the syn- gene of herpes simplex virus type 1. I. Morphology of infected cells. Virology. 1976 Feb;69(2):490–499. doi: 10.1016/0042-6822(76)90479-7. [DOI] [PubMed] [Google Scholar]
- Keller J. M. The expression of the syn- gene of herpes simplex virus type 1. II. Requirements for macromolecular synthesis. Virology. 1976 Jul 15;72(2):402–409. doi: 10.1016/0042-6822(76)90169-0. [DOI] [PubMed] [Google Scholar]
- Knowles R. W., Person S. Effects of 2-deoxyglucose, glucosamine, and mannose on cell fusion and the glycoproteins of herpes simplex virus. J Virol. 1976 May;18(2):644–651. doi: 10.1128/jvi.18.2.644-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D. B., Blough H. A. Preliminary biochemical characterization of the factors(s) responsible for herpesvirus-induced exogenous fusion. J Virol. 1976 Jun;18(3):1081–1087. doi: 10.1128/jvi.18.3.1081-1087.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H., Becht H., Rott R. Inhibition of herpes virus-induced cell fusion by concanavalin A, antisera, and 2-deoxy-D-glucose. J Virol. 1974 Aug;14(2):307–314. doi: 10.1128/jvi.14.2.307-314.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora P. T., Brady R. O., Bradley R. M., McFarland V. W. Gangliosides in DNA virus-transformed and spontaneously transformed tumorigenic mouse cell lines. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1290–1296. doi: 10.1073/pnas.63.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person S., Knowles R. W., Read G. S., Warner S. C., Bond V. C. Kinetics of cell fusion induced by a syncytia-producing mutant of herpes simplex virus type I. J Virol. 1975 Jan;17(1):183–190. doi: 10.1128/jvi.17.1.183-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S., Dales S. Biogenesis of poxviruses: genetically controlled modifications of structural and functional components of the plasma membrane. Virology. 1974 Jul;60(1):96–127. doi: 10.1016/0042-6822(74)90369-9. [DOI] [PubMed] [Google Scholar]