Abstract
Background
Although there are some animal models for biomarkers of contrast-induced acute kidney injury (CI-AKI), for cardiorenal syndrome (CRS) and for acute renal failure, the interplay between CI-AKI and CRS has yet to be evaluated. Insight into the pathogenesis of CRS is urgently needed from animal models in order to foster the discovery and implementation of novel biomarkers for this disease. Specially designed animal models for type 1 and 3 CRS, particularly CI-AKI, have not yet emerged.
Summary
We hypothesize that the aging male spontaneously hypertensive rat (SHR) is likely to be a suitable model. The SHR model is able to mimic risk factors for preclinical CRS that appears in the clinical setting, specifically hypertension, age, preexisting damage and dysfunction of the heart and kidney, endothelial dysfunction, increased level of reactive oxygen species, decreased level and bioavailability of nitric oxide (NO), impairment of the L-arginine-NO pathway, and insulin resistance. In the SHR, CI-AKI results in a different profile of AKI biomarkers than is seen with preexisting chronic kidney injury.
Key Messages
The SHR model can be used to evaluate the interaction between CI-AKI and CRS type 1 and 3 and to verify neutrophil gelatinase-associated lipocalin (NGAL) as a reliable CI-AKI biomarker for clinical application. Further research is warranted with a large number of aging male SHRs to prove NGAL as a sensitive, specific, highly predictive, early biomarker for CI-AKI.
Key Words: Acute kidney injury, Cardiorenal syndrome, Iodinated contrast media, Hypertension, Neutrophil gelatinase-associated lipocalin, Spontaneously hypertensive rat
Introduction
Cardiorenal syndrome (CRS) is characterized by different degrees of cardiac and renal dysfunction and the interactions of combined cardiac and renal damages. Five types of CRS have been recognized [1], including an acute group (type 1 CRS and type 3 renocardiac syndrome), a chronic group (type 2 and 4) as a counterpart of the acute group, and secondary syndrome (type 5) [1]. Type 1 CRS is defined as an acute worsening of heart function leading to acute kidney injury (AKI) [2,3,4]. In patients with acute myocardial infarction and acute heart failure who have left or right heart catheterization, the administration of contrast media can contribute to contrast-induced AKI (CI-AKI) [5]. Type 3 CRS is defined as acute worsening of kidney function leading to acute cardiac dysfunction [1,4]. Therefore, CI-AKI can potentially contribute to acute cardiac dysfunction in patients with stable cardiac function who undergo elective cardiac catheterization.
CI-AKI is typically defined as an increase in serum creatinine (Cr) of 0.5 mg/dl or a 25% increase from the baseline assessed at 48 h after the angiography procedure [5]. However, serum Cr is both an indirect and insensitive marker for the evaluation of AKI [5]. The discovery of novel biomarkers of AKI for the early diagnosis of CI-AKI based on a thorough understanding of the pathogenesis of CRS is urgently needed [5]. Although some animal models exist for CI-AKI biomarkers [6], for CRS [7,8], and for acute renal failure [9], the interaction between CRS and CI-AKI has not yet been evaluated. A new concept called ‘preclinical CRS’ has recently been introduced. Based on this concept, hypertension in elderly patients acts as a risk factor for subclinical cardiac and renal damage and predisposes these patients to developing CRS type 1 and 3 when they are exposed to contrast media [10].
The aging male spontaneously hypertensive rat (SHR) has been used as a model for evaluating contrast-induced cardiac and renal toxicity [11,12,13,14,15,16]. We, therefore, hypothesize that in the aging male SHR, preexisting chronic heart failure and hypertension can mimic preclinical CRS in the clinical setting. An interaction between acute cardiorenal and renocardiac syndromes may also exist in the SHR. In this review, we discuss how the aging male SHR can be a suitable animal model for the evaluation of the interaction between CI-AKI and CRS type 1 and 3.
Similar Risk Factors for CI-AKI Shared by the SHR and Humans with Preclinical CRS
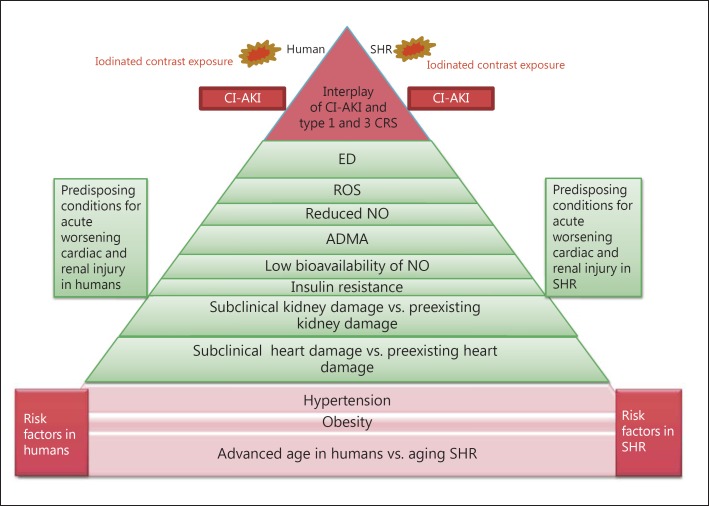
Table 1 and figure 1 show similar risk factors for CI-AKI, including hypertension, obesity, and age, shared by the SHR [17,18,19,20,21,22,23] and humans with preclinical CRS [10,18,23,24,25,26,27,28,29]. In humans with preclinical CRS, essential hypertension acts as a risk factor for subclinical cardiac and renal damage. Hypertension precedes overt cardiac and renal dysfunction [10] and accounts for 25% of all causes of chronic kidney disease (CKD) [18]. The SHR is a reliable model of a naturally developing pressure load akin to essential hypertension in humans; therefore, it is generally accepted that the SHR represents an analogue of human essential hypertension [17,30]. Severe hypertension occurs in 100% of SHRs with a systolic blood pressure of 180 mm Hg or higher. In the SHR, systolic blood pressure starts to rise at the age of 9 weeks (>150 mm Hg), continues to increase up to week 13, and remains elevated (on average 199.0 ± 8.1 mm Hg) until 65 weeks [31].
Table 1.
Risk factors, preexisting cardiac and kidney damage, organ interaction between heart and kidney, and predisposing conditions for worsening acute cardiac and renal injury and CI-AKI in SHRs which serve as a model of humans with preclinical CRS
| SHRs1 | Humans2 | |
|---|---|---|
| Risk factors | Hypertension | Hypertension |
| Obesity | Obesity | |
| Old age and male gender | Advanced age and male gender | |
| Preexisting damage | Preexisting cardiac damage | Subclinical cardiac damage |
| Preexisting renal damage | Subclinical renal damage | |
| Organ interaction | Cardiac hypertrophy and coronary circulation dysfunction leading to renal damage | LVH and coronary circulation dysfunction leading to renal damage |
| Renal glomerulosclerosis is associated with acute cardiac injury (myocyte necrosis and apoptosis) | Renal damage increases cardiovascular risk | |
| Predisposing conditions | ED ↑ | ED ↑ |
| ROS ↑ | ROS ↑ | |
| ADMA ↑ | ADMA ↑ | |
| NO ↓ | NO ↓ | |
| Bioavailability of NO ↓ | Bioavailability of NO ↓ | |
| L-arginine-NO pathway √ | L-arginine-NO pathway √ | |
| Insulin resistance ↑ | Insulin resistance ↑ | |
References: 14–20, 31.
References: 10, 24–30.
Fig. 1.
Comparison of risk factors and predisposing conditions for worsening acute cardiac and renal injury and CI-AKI in SHRs and humans with preclinical CRS.
Obese patients with hypertension are at greater risk for developing CKD. A direct link between obesity, hypertension, and the development of CKD has been identified, as evidenced by the development of insulin resistance, endothelial dysfunction (ED), increased levels of circulating proinflammatory cytokines, and renal damage [18]. Elevated levels of circulating free fatty acids and adipokines may contribute to the development of insulin resistance, type 2 diabetes, and renal injury [18]. Interestingly, when obese SHRs are fed a high-fat diet, they exhibit features similar to those of obese patients with hypertension, such as an increased level of insulin resistance, increased expression of proinflammatory cytokines (IL-1β, TNFα, IL-6, and MCP-1), and ED. Therefore, inflammation appears to play a major role in the pathogenesis of CKD in obese patients with hypertension [18].
Aging is one of the main risk factors for the development of CI-AKI in humans. In a prospective study, CI-AKI occurred in 5 of 13 (38.46%) elderly critically ill patients (≥65 years old, 77% men), while none of 13 younger patients (<65 years old, 100% men) developed CI-AKI. The mean serum Cr concentration increased 0.025 mg/dl/day in older patients for 5 days after contrast exposure, while serum Cr did not increase in younger patients [32]. In the aging male SHR model for CI-AKI, old male rats (10, 12, or 14 months old) were more susceptible to developing CI-AKI than younger males (5 and 8 months old) [12]. In 14-month-old SHRs, urinary albumin excretion was markedly increased as a result of dysfunction of the glomerular capillary filter, increased glomerular albumin permeability, and presumably an increased number of more unselective pores in the glomerular barrier [33].
Preexisting Cardiac and Renal Damage in the SHR Closely Resembles That in Humans with Preclinical CRS
In humans, subclinical cardiac damage is manifested in left ventricular hypertrophy (LVH), as determined by the left ventricular mass index (125 g/m2 for men and 110 g/m2 for women) [10], while subclinical renal damage is manifested in decreased levels of estimated glomerular filtration rate (eGFR), increased levels of albuminuria, and increased levels of serum Cr [10]. LVH is considered a predictor of adverse renal outcome in patients with high cardiovascular risk [24]. Augmented LV mass index and higher LVH rates are correlated with an increase in urinary albumin excretion [25]. Further, LVH is accompanied by a reduction of renal function in hypertensive patients [26].
In the SHR, LVH increases with age, especially at 30 weeks [31]. Interestingly, older SHRs have higher rates of myocardial infarction, cardiac scar formation and arteriolar nephrosclerosis, renal injury, and proteinuria [17]. In the SHR, the activity of the renin-angiotensin system is increased, as determined by the activity of the juxtaglomerular cell granules and as demonstrated by a histological finding of degranulation and atrophy in the juxtaglomerular apparatus [21]. Increases in urinary protein and albumin excretion are accompanied by glomerulosclerosis and arteriolar sclerotic changes in the juxtamedullary and deep cortical nephrons. These areas of the kidney are likely to be less protected in the SHR [31]. The interaction between preexisting cardiac and renal damage is also observed in SHRs. This interaction is evident in SHRs exposed to contrast. These SHRs show an acute worsening of cardiac damage (apoptosis and necrosis of cardiomyocytes) contributing to CI-AKI, as demonstrated by glomerular necrosis and sclerosis, tubular cell apoptosis and necrosis, interstitial lymphocytic infiltration, and apoptosis of endothelial cells and smooth muscle cells in small vessels of the heart and kidney [12,15].
Similar Pathogenesis of ED and Asymmetric Dimethylarginine in the SHR and Humans with Preclinical CRS
In the SHR, aging is a significant factor affecting vascular ED [19]. ED exists in aged SHRs (older than 25 weeks) but not in young SHRs [19]. ED precedes renal injury and exacerbates renal injury under certain conditions (e.g. high-fat diet) in which reactive oxygen species (ROS) are upregulated [18]. Furthermore, ED seems to be the consequence rather than a primary cause of high blood pressure [19,22]. ED is also associated with reduced endothelial nitric oxide synthase (eNOS) expression and NO production in the SHR [22]. Vasoconstrictor factors, such as endothelins, angiotensin II, prostaglandin H2, and thromboxane A2, are likely to play a role in the ‘vicious cycle’ leading to cardiac and renal damage [22]. In essential hypertension patients, vascular ED occurs during the aging process and is accompanied by a progressive reduction of NO bioavailability [34,35]. ROS-mediated cellular injury and renal parenchymal hypoxic-toxic injury are closely related to ED in patients with CI-AKI [36]. In hypertensive patients, endothelium-dependent vasodilation in the kidney is impaired by aging, and the L-arginine-NO pathway involving impaired arginine transport participates in the regulation of renal hemodynamics and renal excretory function [23]. In addition, ED has been reported to result in a subsequent decline of the eGFR in hypertensive patients [37].
Asymmetric dimethylarginine (ADMA) inhibits NOS production of NO by competing with its substrate, L-arginine. Plasma levels of ADMA are significantly higher in SHRs at 4 weeks (prehypertensive stage), 12 weeks (hypertensive stage), and 24 weeks (hypertensive end-organ damage stage) [34]. A significantly higher concentration of plasma ADMA also exists in aging (16-month-old) male SHRs [38]. These findings indicate that a reduced NO bioavailability is associated with hypertension and end-organ damage [36] and that ADMA is a risk factor for ED [38]. The information obtained from SHRs suggests a low bioavailability of NO and impairment of the L-arginine-NO pathway in humans [23].
If SHRs are fed a high-fat diet for 10 weeks, they develop insulin resistance [18]. High insulin levels can induce renal hemodynamic changes, glomerular hypertrophy, and mesangial cell proliferation [18]. Information obtained from the SHR model supports the idea that insulin resistance is increased in hypertensive patients [18]. In hypertensive patients, ADMA plasma levels and insulin resistance are significantly higher, and acetylcholine-stimulated forearm blood flow is significantly reduced, demonstrating the role of ADMA and insulin resistance in inducing ED [27]. Furthermore, plasma levels of ADMA are likely to be dynamically regulated and correlated with some measure of NO bioavailability [27].
Neutrophil Gelatinase-Associated Lipocalin for CI-AKI in the SHR Is Consistent with That in Patients with CI-AKI
Table 2 compares neutrophil gelatinase-associated lipocalin (NGAL) detected in SHRs and humans with CI-AKI. In SHRs with CI-AKI, the area under the receiver operating characteristic curve (AUROC) value of NGAL is 0.73, which is accepted as a value of failure [6]. In contrast, in humans with CI-AKI, 4 of 8 studies showed an excellent value (0.93–1.0) [39,40,41,42], 2 of 8 studies showed a good value of NGAL (0.83–0.84) [43,44], and 2 of 8 studies showed a poor value of NGAL (0.63–0.68) [29,45]. In CKD patients undergoing elective coronary angiography with contrast, postprocedural NGAL increases from baseline in each stage of CKD [46]. In patients undergoing open heart surgery with AKI, urine catalytic iron levels are highly correlated with urine NGAL levels [47]. The difference in AUROC values of NGAL between SHRs and humans may be due to the young age of SHRs (5 months old) in an animal model used by Rouse et al. [6]; therefore, the level of NGAL may not yet reflect severely worsening AKI. In contrast, older SHRs, at 14–16 months of age, may have higher levels of NGAL due to the loss of a renal functional reserve and contrast-induced acute deterioration of renal function. Therefore, we propose that older SHRs may be a better model for the evaluation of the interaction between CRS and CI-AKI. In addition, the appearance of NGAL occurs earlier in humans (2, 4, 6, 8, and 24 h after contrast administration) than in SHRs (72 h).
Table 2.
Comparison of the NGAL biomarker in SHRs and humans
| Study [ref.], year | SHRs or humans | AUROC1 | Note |
|---|---|---|---|
| Rouse et al. [6], 2014 | SHRs | 0.73 | 5 SHRs; urinary NGAL, 72 h after exposure to contrast medium iohexol |
| Liebetrau et al. [39], 2014 | Humans | 0.94 | 14 of 128 patients undergoing PCI; urinary NGAL, 1 day after PCI |
| Tasanarong et al. [43], 2013 | Humans | 0.84 | 16 of 130 CKD patients undergoing elective coronary procedures; urinary NGAL, at 6 h |
| Filiopoulos et al. [40], 2013 | Humans | 1.00 | 4 of 47 patients undergoing elective contrast-enhanced CT; plasma NGAL, 6 h after contrast administration |
| Lacquantiti et al. [41], 2013 | Humans | 0.99 | 23 of 60 patients receiving iomeprol; urinary NGAL (0.992), serum NGAL (0.995), 8 h after iomeprol administration |
| Alvelos et al. [42], 2011 | Humans | 0.93 | 14 of 119 acute HF patients with developing type 1 CRS within 48–72 h; plasma NGAL |
| Valette et al. [44], 2013 | Humans | 0.83 | 98 patients with sepsis or previous AKI, 30 developed CI-AKI; plasma NGAL 0.83 or 0.86, 6 or 24 h after contrast administration, respectively; however, the discriminative value of plasma NGAL to predict CI-AKI and mortality was poor |
| Okumura et al. [29], 2014 | Humans | 0.63 | 58 of 100 CKD patients undergoing angiographic procedures and with deterioration of eGFR; urinary NGAL, 2 h after the procedure |
| Liu et al. [47], 2012 | Humans | 0.66 | 39 of 311 patients undergoing diagnostic cardiac angiography or PCI; plasma NGAL, 4 h after the procedures |
CT = Computed tomography; HF = heart failure; PCI = percutaneous coronary interventions.
All values are indicated by the AUROC; a rough guide for classifying the accuracy of a diagnostic test is the traditional academic point system: AUROC 0.90–1.0 = excellent; 0.80–0.90 = good; 0.70–0.80 = fair; 0.60–0.70 = poor, and 0.50 – 0.60 = fail.
Interestingly, NGAL induces cardiomyocyte apoptosis via a pathway of elevated intracellular iron levels and Bax translocation [48]. This is consistent with findings in the SHR model with CI-AKI, which showed that cardiomyocyte apoptosis is associated with increased NGAL as well as increased Bax immunoreactivity [3,12]. Thus, NGAL is likely to act as a link between CI-AKI and acute heart failure in type 3 CRS. In addition, NGAL is actively upregulated and secreted in response to free catalytic iron that is both intracellular and extracellular as a result of cell damage and release from binding proteins in the electron transport chain, mitochondria, and structure proteins. Constitutive upregulation of NGAL production can mitigate chronic oxidative stress catalyzed by intra- and extracellular catalytic iron [49]. Interestingly, iron chelators like deferiprone were shown to protect kidneys against iron-induced oxidative damage in a clinical trial of CI-AKI [5].
The Concept of a Renal Functional Reserve: Insights from the Aging Male SHR Model
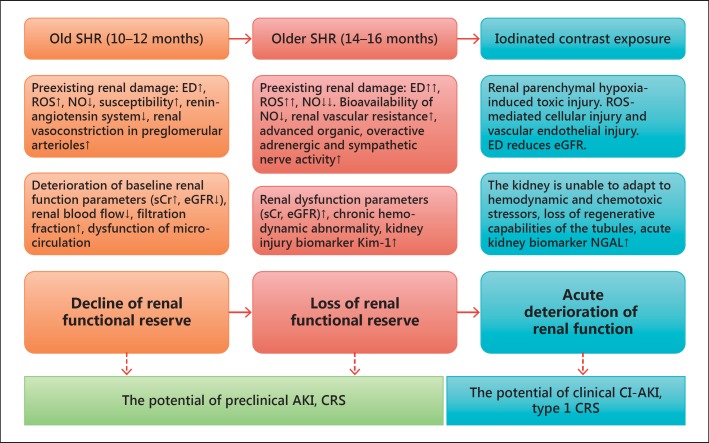
Figure 2 presents our proposed concept of a renal functional reserve in the SHR model based on current information [11,12,13,14,15,16,31,34,36,46,50,51,52]. In the proposed concept, we made 3 scenarios based on the gradual progress of preexisting renal damage: (1) decline of a renal functional reserve at the age of 10–12 months; (2) loss of a renal functional reserve at the age of 14–16 months, and (3) acute deterioration of renal function (acute renal failure) after iodinated contrast exposure. The first and second scenarios represent background kidney injury before contrast exposure. Mild contrast exposure in SHRs results in kidney injury but not total loss of renal function. Therefore, SHRs with mild contrast exposure can serve as a model for subclinical CI-AKI [46]. On the other hand, moderate or heavy contrast exposure in SHRs results in complete loss of renal function. Therefore, SHRs with moderate-to-heavy exposure can serve as an animal model for clinically overt CI-AKI.
Fig. 2.
Renal functional reserve and clinical relevance: insights from an aging male SHR model.
Can Characteristics of the SHR Translate to Patients with CRS Type 1 and 3?
Some may argue that characteristics of the SHR may not translate to patients with CRS type 1 and 3 in terms of sympathetic nervous system (SNS) activation and renin status. The incidence of hypertension is believed to increase with age and to be related to age-associated SNS changes [53]. In older hypertensive subjects, the plasma norepinephrine metabolic clearance rate is increased, and systemic SNS activity tends to be increased [54]. An increase in SNS activity has been reported in the initiation of hypertension in human essential hypertension [54,55]. Likewise, in male SHRs, increased SNS activity is related to the development of hypertension [56,57,58,59]. In the SHR, the activity of the renin-angiotensin system is also increased [21]. SHRs (13 and 35 weeks old) with established hypertension develop a persistent high-renin state which may be similar to that observed in 9–16% of essential hypertensive patients [60].
Conclusions
The aging male SHR develops spontaneous cardiac and renal damage that progresses with age, increasing the susceptibility to the cardiorenal damaging effects of contrast media. Thus, the aging male SHR provides a laboratory tool for the evaluation of the interaction between CI-AKI and CRS type 1 and 3 and the discovery of the novel biomarker NGAL for CI-AKI.
Statement of Ethics
This work did not involve human subjects or animals and was exempt from review by Baylor Institutional Review Board.
Disclosure Statement
There are no conflicts of interest to disclose.
Acknowledgments
The authors would like to acknowledge Wendy Hegefeld, PhD, at Baylor Scott and White Health for her help and guidance in manuscript preparation.
References
- 1.Ronco C, Lullo LD. Cardiorenal syndrome. Heart Failure Clin. 2014;10:251–280. doi: 10.1016/j.hfc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 3.Palazzuoli A, McCullough PA, Ronco C, Nuti R. Kidney disease in heart failure: the importance of novel biomarkers for type 1 cardio-renal syndrome detection. Intern Emerg Med. 2015;10:543–554. doi: 10.1007/s11739-015-1246-0. [DOI] [PubMed] [Google Scholar]
- 4.Clementi A, Virzi GM, Brocca A, de Cal M, Pastori S, Clementi M, Granata A, Vescovo G, Ronco C. Advances in the pathogenesis of cardiorenal syndrome type 3. Oxid Med Cell Longev. 2015 doi: 10.1155/2015/148082. DOI: 10.1155/2015/148082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough PA. Radiocontrast-induced acute kidney injury. Nephron Physiol. 2008;109:61–72. doi: 10.1159/000142938. [DOI] [PubMed] [Google Scholar]
- 6.Rouse R, Stewart SR, Thompson KL, Zhang J. Kidney injury biomarkers in hypertensive, diabetic, and nephropathy rat models with contrast medium. Toxicol Pathol. 2013;41:662–680. doi: 10.1177/0192623312464122. [DOI] [PubMed] [Google Scholar]
- 7.Bongartz LG, Braam B, Gaillard CA, Cramer MJ, Goldschmeding R, Verhaar MC, Doevendans PA, Joles JA. Target organ cross talk in cardiorenal syndrome: animal models. Am J Physiol Renal Physiol. 2012;303:F1253–F1263. doi: 10.1152/ajprenal.00392.2012. [DOI] [PubMed] [Google Scholar]
- 8.Szymanski MK, de Boer RA, Navis GJ, van Gilst WH, Hillege HL. Animal models of cardiorenal syndrome: a review. Heart Fail Rev. 2012;17:411–420. doi: 10.1007/s10741-011-9279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singn AP, Muthuraman A, Jaggi AS, Singh N, Grover K, Dhawan R. Animal models of acute renal failure. Pharmacol Rep. 2012;64:31–44. doi: 10.1016/s1734-1140(12)70728-4. [DOI] [PubMed] [Google Scholar]
- 10.Tsioufis C, Tsiachris D, Kasiakogias A, Dimitriadis K, Petras D, Goumenos D, Siamopoulos K, Stefanadis C. Preclinical cardiorenal syndrome interrelationships in essential hypertension. Cardiorenal Med. 2013;3:38–47. doi: 10.1159/000346817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duarte CG, Ellis S. Renal effects of a radiocontrast agent in aging spontaneously hypertensive rats. Contrib Nephrol. 1990;83:222–228. doi: 10.1159/000418803. [DOI] [PubMed] [Google Scholar]
- 12.Duarte CG, Zhang J, Ellis S. The SHR as a small animal model for radiocontrast renal failure. Relation of nephrotoxicity to animal's age, gender, strain, and dose of radiocontrast. Ren Fail. 1997;19:723–743. doi: 10.3109/08860229709037213. [DOI] [PubMed] [Google Scholar]
- 13.Duarte CG, Zhang J, Ellis S. Effects of radiocontrast and endothelin administration on systolic blood pressure and renal damage in male spontaneously hypertensive and Wistar Kyoto rats with phentolamine-induced adrenergic blockade. Invest Radiol. 1998;33:104–112. doi: 10.1097/00004424-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Duarte CG, Zhang J, Ellis S. Effects of radiocontrast, mannitol, and endothelin on blood pressure and renal damage in the aging male spontaneously hypertensive rat. Invest Radiol. 1999;34:455–462. doi: 10.1097/00004424-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Duarte CG, Ellis S. Contrast medium- and mannitol-induced apoptosis in heart and kidney of spontaneously hypertensive rats. Toxicol Pathol. 1999;27:427–435. doi: 10.1177/019262339902700406. [DOI] [PubMed] [Google Scholar]
- 16.Duarte CG, Zhang J, Ellis S. Review of studies establishing the aging male spontaneously hypertensive rat as a detector and quantifier of the kidney toxicity of radiocontrast media and other chemicals. Invest Radiol. 2001;36:56–63. doi: 10.1097/00004424-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto K, Tabei R, Yamori Y, Ooshima A. Spontaneously hypertensive rat as a useful model for hypertension research. Jikken Dobutsu. 1973;22(suppl):289–298. [PubMed] [Google Scholar]
- 18.Knight SF, Quigley JE, Yuan J, Roy S, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension. 2008;51:352–359. doi: 10.1161/HYPERTENSIONAHA.107.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernatova I, Conde MV, Kopincova J, González MC, Puzserova A, Arribas SM. Endothelial dysfunction in spontaneously hypertensive rats: focus on methodological aspects. J Hypertens. 2009;27(suppl 6):S27–S31. doi: 10.1097/01.hjh.0000358834.18311.fc. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto K, Abe M, Haneda T. Effect of regression of cardiac hypertrophy on ischemic myocardial damage in spontaneously hypertensive rats. Jpn Circ J. 1993;57:147–160. doi: 10.1253/jcj.57.147. [DOI] [PubMed] [Google Scholar]
- 21.Haga M, Sokabe H, Okamato K. Juxtaglomerular cell granules in the spontaneously hypertensive rat. Jpn Circ J. 1966;30:1479–1482. doi: 10.1253/jcj.30.1479. [DOI] [PubMed] [Google Scholar]
- 22.Vappaatalo H, Mervaala E, Nurminen ML. Role of endothelium and nitric oxide in experimental hypertension. Physiol Res. 2000;49:1–10. [PubMed] [Google Scholar]
- 23.Mattei P, Virdis A, Ghiadoni L, Taddei S, Salvetti A. Endothelial function in hypertension. J Nephrol. 1997;10:192–197. [PubMed] [Google Scholar]
- 24.Tsioufis C, Kokkinos P, Macmanus C, Thomopoulos C, Faselis C, Doumas M, Stefanadis C, Papademetriou V. Left ventricular hypertrophy as a determinant of renal outcome in patients with high cardiovascular risk. J Hypertens. 2010;28:2299–2308. doi: 10.1097/HJH.0b013e32833d95fe. [DOI] [PubMed] [Google Scholar]
- 25.Andrikou E, Tsioufis C, Dimitriadis K, Flessas D, Chatzistamatou V, Grassos C, Papavasilion M, Papadopoulos D, Stefanadis C. Parallel deterioration of albuminuria, arterial stiffness and left ventricular mass in essential hypertension: integrating target organ damage. Nephron Clin Pract. 2011;119:c27–c34. doi: 10.1159/000324215. [DOI] [PubMed] [Google Scholar]
- 26.Cerasola G, Nardi E, Mulè G, Palermo A, Cusimano P, Guarnet M, Arsena R, Giammarresi G, Carola Foraci A, Cottone S. Left ventricular mass in hypertensive patients with mild-moderate reduction of renal function. Nephrology (Carlton) 2010;15:203–210. doi: 10.1111/j.1440-1797.2009.01178.x. [DOI] [PubMed] [Google Scholar]
- 27.Perticone F, Sciacqua A, Maio R, Perticone M, Galiano Leone G, Bruni R, di Cello S, Pascale A, Talarico G, Greco L, Andrreozz F, Sesti G. Endothelial dysfunction, ADMA and insulin resistance in essential hypertension. Int J Cardiol. 2010;142:236–241. doi: 10.1016/j.ijcard.2008.12.131. [DOI] [PubMed] [Google Scholar]
- 28.Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 29.Okumura N, Hyashi M, Ishii H, Yoshikawa D, Yasuda Y, Goto M, Matsuo S, Oiso Y, Murohara T. Novel preprocedural and acute-phase postprocedural predictive factors for contrast-induced kidney injury in CKD patients. Int J Cardiol. 2014;172:e293–e296. doi: 10.1016/j.ijcard.2013.12.193. [DOI] [PubMed] [Google Scholar]
- 30.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 31.Feld LG, van Liew JB, Galaske RG, Boylan JW. Selectivity of renal injury and proteinuria in the spontaneously hypertensive rat. Kidney Int. 1977;12:332–343. doi: 10.1038/ki.1977.120. [DOI] [PubMed] [Google Scholar]
- 32.Palli E, Makris D, Papanikolaou J, Garoufalis G, Zakynthinos E. Contrast-induced nephropathy in aged critically ill patients. Oxid Med Cell Longev. 2014;2014:756469. doi: 10.1155/2014/756469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakoush O, Tencer J, Torffvit O, Tenstad O, Skogvall I, Rippe B. Increased glomerular albumin permeability in old spontaneously hypertensive rats. Nephrol Dial Transplant. 2004;19:1724–1731. doi: 10.1093/ndt/gfh276. [DOI] [PubMed] [Google Scholar]
- 34.Hsu CN, Huang LT, Lau CY, Tain YL. The combined ratios of L-arginine and asymmetric dimethylarginine as biomarkers in spontaneously hypertensive rats. Transl Res. 2012;159:90–98. doi: 10.1016/j.trsl.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Herrera MD, Mingorance C, Rodriguez-Rodriguez R, de Sotomayor MA. Endothelial dysfunction and aging: an update. Ageing Res Rev. 2010;9:142–152. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Perticone F, Mario R, Perticone M, Sciacqua A, Shehaj E, Naccarato P, Sesti G. Endothelial dysfunction and subsequent decline in glomerular filtration rate in hypertensive patients. Circulation. 2010;122:379–384. doi: 10.1161/CIRCULATIONAHA.110.940932. [DOI] [PubMed] [Google Scholar]
- 37.Pisani A, Riccio E, Andreucci MJ, Faga T, Ashour M, di Nuzzi A, Mancini A, Sabbatini M. Role of reactive oxygen species in pathogenesis of radiocontrast-induced nephropathy. Biomed Res Int. 2013;2013:868321. doi: 10.1155/2013/868321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raimondi L, Lodovici M, Visioli F, Sartiani L, Cioni L, Alfarano C, Banchelli G, Pirisino R, Cecchi E, Cerbai E, Muqelli A. N-3 polyunsaturated fatty acids supplementation decreases asymmetric dimethyl arginine and arachidonate accumulation in aging spontaneously hypertensive rats. Eur J Nutr. 2005;44:3237–3333. doi: 10.1007/s00394-004-0528-5. [DOI] [PubMed] [Google Scholar]
- 39.Liebetrau C, Gaede L, Blumenstein J, Rixe J, Teichert O, Willmer M, Weber M, Rolf A, Möllmann H, Hamm C, Nef H. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of contrast-induced nephropathy after percutaneous coronary intervention. Scand J Clin Lab Invest. 2014;74:81–88. doi: 10.3109/00365513.2013.860615. [DOI] [PubMed] [Google Scholar]
- 40.Filiopoulos V, Biblaki D, Lazarou D, Chrisis D, Fatourou M, Lafoyianni S, Vlassopoulos D. Plasma neutrophil gelatinase-associated lipocalin (NGAL) as an early predictive marker of contrast-induced nephropathy in hospitalized patients undergoing computed tomography. Clin Kidney J. 2013;6:578–583. doi: 10.1093/ckj/sft109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lacquantiti A, Buemi F, Lupica R, Giardina C, Murè G, Arena A, Visalli C, Baldari S, Aloisi C, Buemi M. Can neutrophil gelatinase-associated lipocalin help depict early contrast material-induced nephropathy? Radiology. 2013;267:86–93. doi: 10.1148/radiol.12120578. [DOI] [PubMed] [Google Scholar]
- 42.Alvelos M, Pimentel R, Pinho E, Gomes A, Lourenco P, Teles MJ, Almeida P, Guimarães JT, Bettencourt P. Neutrophil gelatinase-associated lipocalin in the diagnosis of type 1 cardio-renal syndrome in the general ward. Clin J Am Soc Nephrol. 2011;6:476–481. doi: 10.2215/CJN.06140710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tasanarong A, Hutayanon P, Piyaotai D. Urinary neutrophil gelatinase-associated lipocalin predicts the severity of contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. BMC Nephrol. 2013;14:270. doi: 10.1186/1471-2369-14-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valette X, Savary B, Nowoczyn M, Daaubin C, Pottier V, Terzi N, Sequin A, Fradin S, Charbonneau P, Hanouz JL, du Cheyron D. Accuracy of plasma neutrophil gelatinase-associated lipocalin in the early diagnosis of contrast-induced acute kidney injury in critical illness. Intensive Care Med. 2013;39:857–865. doi: 10.1007/s00134-013-2826-y. [DOI] [PubMed] [Google Scholar]
- 45.Liu XL, Wang ZJ, Yang Q, Yu M, Shen H, Nie B, Han HY, Gao F, Zhou YJ. Plasma neutrophil-gelatinase-associated lipocalin and cystatin C could early diagnose contrast-induced acute kidney injury in patients with renal insufficiency undergoing an elective percutaneous coronary intervention. Chin Med J (Engl) 2012;125:1051–1056. [PubMed] [Google Scholar]
- 46.Akrawinthawong K, Ricci J, Cannon L, Dixon S, Kupfer K, Stivers D, Alexander P, David S, McCullough PA. Subclinical and clinical contrast-induced acute kidney injury: data from a novel blood marker for determining the risk of developing contrast-induced nephropathy (ENCINO), a prospective study. Ren Fail. 2015;37:187–191. doi: 10.3109/0886022X.2014.991994. [DOI] [PubMed] [Google Scholar]
- 47.Akrawinthawong K, Shaw MK, Kachner J, Apostolov EO, Basnakian AG, Shah S, Tilak J, McCullough PA. Urine catalytic iron and neutrophil gelatinase-associated lipocalin as comparison early markers of acute kidney injury after cardiac surgery: a prospective pilot study. Cardiorenal Med. 2013;3:7–16. doi: 10.1159/000346815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu G, Ahn JH, Chang SY, Eguchi M, Ogier A, Han SJ, Park YS, Shim CY, Jang YS, Yang B, Xu A, Wang Y, Sweeney G. Lipocalin-2 induces cardiomyocyte apoptosis by increasing intracellular iron accumulation. J Biol Chem. 2012;287:4808–4817. doi: 10.1074/jbc.M111.275719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCullough PA, Williams FJ, Stivers DN, Cannon L, Dixon S, Alexander P, Runyan D, David S. Neutrophil gelatinase-associated lipocalin: a novel marker of contrast nephropathy risk. Am J Nephrol. 2012;35:509–514. doi: 10.1159/000339163. [DOI] [PubMed] [Google Scholar]
- 50.Trippodo NC, Frohlich ED. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ Res. 1981;48:309–319. doi: 10.1161/01.res.48.3.309. [DOI] [PubMed] [Google Scholar]
- 51.Heyman SN, Rosen S, Khamaisi M, Idèe J-M, Rosenberg C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010;45:188–195. doi: 10.1097/RLI.0b013e3181d2eed8. [DOI] [PubMed] [Google Scholar]
- 52.Scoditti E, Massaro M, Montinari MR. Endothelial safety of radiological contrast media: why being concerned. Vascul Pharmacol. 2013;58:48–53. doi: 10.1016/j.vph.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Tuck ML. The sympathetic nervous system in essential hypertension. Am Heart J. 1986;112:877–886. doi: 10.1016/0002-8703(86)90497-7. [DOI] [PubMed] [Google Scholar]
- 54.Supiano MA, Hogikyan RV, Sidani MA, Galecki AT, Krueger JL. Sympathetic nervous system activity and α-adrenergic responsiveness in older hypertensive humans. Am J Physiol. 1999;276:E519–E528. doi: 10.1152/ajpendo.1999.276.3.E519. [DOI] [PubMed] [Google Scholar]
- 55.Matsukawa T, Mano T, Gotoh E, Ishii M. Elevated sympathetic nerve activity in patients with accelerated essential hypertension. J Clin Invest. 1993;92:25–28. doi: 10.1172/JCI116558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gattone VH, Venan AP, Overhage JM, Severs WB. Developing renal innervations in spontaneously hypertensive rat: evidence for a role of the sympathetic nervous system in renal damage. J Hypertension. 1990;8:423–428. doi: 10.1097/00004872-199005000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Okamoto K, Nosaka S, Yamori Y, Matsumoto M. Participation of neural factor in the pathogenesis of hypertension in the spontaneously hypertensive rat. Jpn Heart J. 1967;8:168–180. doi: 10.1536/ihj.8.168. [DOI] [PubMed] [Google Scholar]
- 58.Wintermitz SR, Katholi RE, Oparil S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J Clin Invest. 1980;66:971–978. doi: 10.1172/JCI109966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Judy WV, Watanabe AM, Herry DR, et al. Sympathetic nerve activity: role in regulation of blood pressure in the spontaneously hypertensive rat. Circ Res. 1976;38(6 suppl 2):21–29. doi: 10.1161/01.res.38.6.21. [DOI] [PubMed] [Google Scholar]
- 60.Bagby SP, McDonald WJ, Mass RD. Serial renin-angiotensin studies in spontaneously hypertensive and Wistar-Kyoto normotensive rats. Transition from normal- to high-renin status during the established phase of spontaneous hypertension. Hypertension. 1979;1:347–354. doi: 10.1161/01.hyp.1.4.347. [DOI] [PubMed] [Google Scholar]