Abstract
DAX-1 (NR0B1) and SF-1 (NR5A1) are two nuclear receptor transcription factors that play a key role in human adrenal and reproductive development. Loss of DAX-1 function is classically associated with X-linked adrenal hypoplasia congenita. This condition typically affects boys and presents as primary adrenal insufficiency in early infancy or childhood, hypogonadotropic hypogonadism at puberty and impaired spermatogenesis. Late onset forms of this condition and variant phenotypes are increasingly recognized. In contrast, disruption of SF-1 only rarely causes adrenal insufficiency, usually in combination with testicular dysgenesis. Variants in SF-1/NR5A1 more commonly cause a spectrum of reproductive phenotypes ranging from 46,XY DSD (partial testicular dysgenesis or reduced androgen production) and hypospadias to male factor infertility or primary ovarian insufficiency. Making a specific diagnosis of DAX-1 or SF-1 associated conditions is important for long-term monitoring of endocrine and reproductive function, appropriate genetic counselling for family members, and for providing appropriate informed support for young people.
Keywords: DAX-1; SF-1; X-linked adrenal hypoplasia congenita; hypogonadotropic hypogonadism; primary adrenal insufficiency; Addison disease; 46,XY disorders of sex development; hypospadias; infertility; primary ovarian insufficiency
Introduction
DAX-1 (Dosage-sensitive sex reversal - Adrenal hypoplasia congenita critical region on the X chromosome 1, officially known as NR0B1) and steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) are two transcription factors that belong to the nuclear receptor superfamily. Both DAX-1 and SF-1 are expressed in the adrenal gland and reproductive axis during fetal development and in adult life, and variations in genes encoding these factors are associated with an ever expanding range of adrenal and reproductive conditions.
In this review, we will provide a brief overview of the history and basic biology of DAX-1 and SF-1 and describe the range of clinical conditions associated with them. We will also provide some guidance on key issues related to management and support for families and children with DAX-1 and SF-1-associated conditions.
DAX-1 (NR0B1)
History of DAX-1
Adrenal hypoplasia is a life-threatening condition that results from underdevelopment (hypoplasia) or lack (agenesis) of the adrenal gland. This condition was first described by the pathologist Sikl in 1948 in a boy with “coal-black pigmentation” who died of a salt-losing adrenal crisis in the first weeks of life [1]. In the 1960s, an X-linked inheritance pattern became clear and, as boys survived following the use of steroid replacement treatment, it emerged that hypogonadotropic hypogonadism (HH) was an integral part of this condition. However, it was the association of X-linked adrenal hypoplasia congenita (AHC) with glycerol kinase deficiency (GKD) and Duchenne muscular dystrophy (DMD) that helped to localise the gene to the short arm of the X-chromosome (Xp21) and specific changes (mutations) or deletions of DAX-1 (NR0B1) as the cause of X-linked AHC were first described in 1994 [2]. Of note, duplication of this region was associated with 46,XY DSD/testicular dysgenesis, suggesting that over-expression of DAX-1 might suppress male sex development pathways and that DAX-1 could act as an “anti-testis” gene [3].
Biological role of DAX-1
DAX-1 is termed an “orphan” nuclear receptor, as no specific ligand has been identified. DAX-1 has an unusual structure; the carboxyl-terminal region of the protein contains twelve helices typical of other nuclear receptors, whereas the amino-terminal region contains 3.5 repeats of approximately 66–67 amino acids containing LXXLL motifs (Fig. 1) [4]. Early studies of DAX-1 function suggested it could interact with other nuclear receptors such as SF-1, and this has been confirmed following crystallization of DAX-1 with LRH-1 (NR5A2) [5]. Paradoxically, however, most functional studies have shown that DAX-1 is a repressor of gene transcription, which is unexpected given its positive role in adrenal and reproductive development, and the fact that loss of DAX-1 is associated with adrenal and reproduction dysfunction [6].
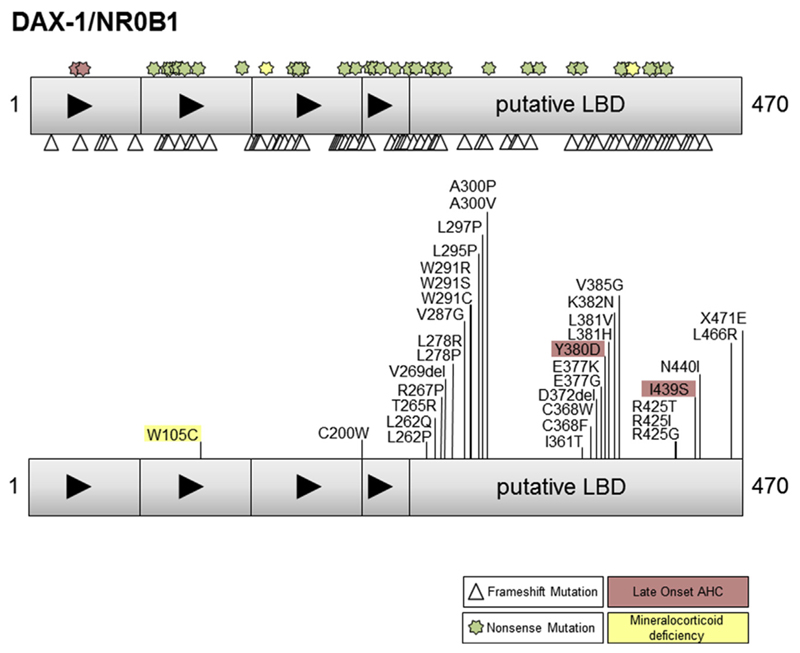
Fig. 1.
Cartoon of DAX-1 structure and a selection of the nonsense (stars), frameshift (triangles) and missense changes reported. Those changes associated with a milder phenotype (red) or isolated mineralcorticoid deficiency (yellow) are indicated. LBD = ligand binding domain. (Modified with permission from Lin et al., J Clin Endocrinol Metab 2006; 91: 3048–3054. Copyright © 2006 by The Endocrine Society).
The exact biological role of DAX-1 remains unclear. Targeted deletion of exon 2 of Dax1 (Nr0b1, also known as Ahch) using a cre-loxP strategy in mice resulted in animals that had disrupted spermatogenesis, but apparently normal adrenal function [7]. Breeding these mice onto different backgrounds disrupted testis development, as did overexpression of Dax1/Nr0b1 on a mouse background with weaker Sry expression. These studies supported a dosage sensitive role for Dax1 in testis development, but shed little light onto the lack of adrenal phenotype.
More recently, detailed analysis of adrenal function in aging Dax1/Nr0b1 deleted mice has shown that adrenal insufficiency does develop with time, after an initial period of enhanced hormonal sensitivity [8]. In fact, it is emerging that DAX-1 may play a key role in regulating stem cell development. DAX-1 is expressed in a population of progenitor stem cells where it represses differentiation so that expansion of the cell pool can occur. In the absence of DAX-1 (and together with tropic signals such as adrenocorticotropic hormone [ACTH]), the progenitor cells may differentiate into steroidogenic cells prematurely before expansion of cell number occurs [8,9]. This phenomenon may provide one model to account for the early postnatal hyperfunction of the gland followed by decline in function as the pool of cells available for regeneration is depleted.
In addition to the stem cell model, several studies have shown a positive activating role for DAX-1 in gene transcription rather than repressor role. Studies of the minimal promoter of CYP11B2 showed that DAX-1 can activate transcription, and augmentation of SF-1 activation was also shown on the PBX1 promoter in adrenal cells [10]. Furthermore, DAX-1 co-activation with the steroid receptor RNA activator (SRA) has been described [11]. These reports increase the range of potential actions of DAX-1 in different biological systems.
Clinical conditions associated with alterations in DAX-1
Classic X-linked adrenal hypoplasia congenita
Classic X-linked AHC includes the triad of: a) primary adrenal insufficiency (PAI), b) hypogonadotropic hypogonadism (HH), and c) impaired fertility.
The adrenal insufficiency presents during the first two months of life in approximately 40% of affected boys, or else more insidiously throughout childhood [12]. Typically, boys present with a salt-losing adrenal crisis due to mineralocorticoid (aldosterone) insufficiency, together with glucocorticoid (cortisol) insufficiency. A basal cortisol value within the normal range does not exclude the diagnosis; however, the response to ACTH-analogue stimulation will usually be impaired.
Boys with X-linked AHC typically have absent pubertal development [13]. This feature is consistent with HH, although puberty may start in some boys and usually arrests around Prader stage 3. Detailed studies of gonadotropin release and the response to pulsed gonadotropin-releasing hormone (GnRH) stimulation have suggested that a combined hypothalamic and pituitary defect occurs, which is responsible for impaired luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release. In general, studies using pulsatile GnRH delivered through pump systems have not been very successful in inducing puberty.
Following studies in the mouse, it has become apparent that disruption of DAX-1/NR0B1 may also cause a primary defect in spermatogenesis. The Dax1/Nr0b1 deleted mouse has altered testicular architecture and spermatogenesis [7]. Attempts to induce spermatogenesis in patients using FSH and human chorionic gonadotropin (hCG) have generally been disappointing [14]. However, data from humans about the exact mechanism and natural history of impaired spermatogenesis are currently limited (see management).
Other presentations in childhood
Although most boys present with PAI and classic features of X-linked AHC, alternative presenting phenotypes have emerged. These may include a predominant mineralocorticoid insufficiency and salt-loss, resembling other conditions such as aldosterone synthase deficiency or pseudohypoaldosteronism [15,16]. Rarely, patients may present with low cortisol, and are thought to have familial glucocorticoid deficiency. Therefore, an open mind needs to be kept in all boys with adrenal insufficiency if a specific molecular diagnosis has not been reached, especially if other features such as HH emerge or there is a family history consistent with X-linked inheritance.
In general, the adrenal features of X-linked AHC precede the reproductive features and it is rare for young adults to present with impaired puberty without some history or undiagnosed features of PAI. Indeed, a study of more than 100 subjects with HH or familial extreme delayed puberty did not reveal any pathogenic DAX-1/NR0B1 variants [17]. In contrast, a subgroup of boys with X-liked AHC may present with signs of increased hypothalamo-gonadotrope activation or early puberty. For example, boys may have an enlarged penis (macrophallia) at birth, or a degree of premature sexual maturation (precocious puberty) in early childhood [16,18]. Signs of puberty may occur before or after the diagnosis of adrenal insufficiency, and it is not entirely clear whether this is gonadotropin dependent or independent. Usually the sexual development arrests relatively early on.
Another presenting feature of X-liked AHC can be the metabolic or phenotypic features of GKD or neuromuscular effects of DMD, although most boys with a contiguous gene deletion develop adrenal dysfunction early.
Late-onset X-linked AHC
Occasionally, X-linked AHC is first diagnosed in young adulthood. Affected men typically have a history or features of a progressive or mild adrenal insufficiency and partial HH is diagnosed on further examination [19,20]. Rarely, a young man who presents with impaired puberty or arrested sexual development is found to have clinical or biochemical evidence of PAI, leading to the diagnosis of X-linked AHC [21]. Therefore, any history of fatigue, weight loss, exaggerated illness or hyperpigmentation should not be ignored. Brothers or maternal uncles can be affected, so a careful family history and investigations may be needed [22,23]. To date, a diagnosis of X-linked AHC has never been made in men being investigated for male factor infertility alone [24].
Presentation in females
Although X-linked AHC predominantly affects boys, expression of X-linked conditions can sometimes occur in girls or women due to skewed X-inactivation. This mechanism has been proposed for the delayed puberty seen in female family members in a kindred where boys have classic X-linked adrenal hyperplasia and for the mild DMD and adrenal insufficiency in a girl caused by a contiguous gene deletion of Xp21 [13,25].
Presymptomatic diagnosis
Once a diagnosis of X-linked AHC is made it is important to consider who else in the family might be at risk of developing adrenal insufficiency or who might carry the genetic alteration and pass it on. Analysis of sibling (brother) pairs with X-linked AHC has shown that the second child tends to be diagnosed at a younger age, probably due to increased awareness of the signs of adrenal insufficiency [26]. Making a presymptomatic diagnosis is an important aim as it prevents a life-threatening salt-losing crisis and associated risks, and allows issues to be managed prospectively rather than as an emergency [26]. Sisters and maternal aunts should be offered genetic testing as they are potential heterozygous carriers and can have affected sons [22,23].
The genetic basis of X-linked AHC
The gene encoding DAX-1 is officially termed NR0B1.
The gene consists of just two exons with a potential alternatively spliced exon 2, although the significance of this remains unclear.
As highlighted above, X-linked AHC was originally characterized as part of a contiguous gene deletion syndrome together with GKD and DMD (centromeric) or developmental delay (IL1RAPL1, telomeric). In a review of X-linked AHC in 2006, contiguous gene deletions accounted for 71 of 190 (38%) published cases at the time, probably reflecting a reporting bias [27]. Since then, isolated deletions of NR0B1 and specific point changes (mutations) within DAX-1/NR0B1 itself seem much more prevalent (Fig. 1) [27]. Very rarely, X-linked AHC can arise from rearrangements of the genetic locus for NR0B1 or from disruption of upstream regulatory regions.
Specific disease-causing variants in DAX-1/NR0B1 include nonsense mutations, frameshift mutations and point (missense) mutations (Fig. 1). Nonsense mutations are located throughout the gene/protein and loss of the very carboxyl-terminal region of the protein that contributes to the AF-2 domain is associated with a severe phenotype and loss of function in cell based transcription assays [6,28]. Of note, nonsense changes in the amino-terminal region can be found in patients with late-onset AHC, especially affecting codons 37 and 39 in the first repeat region of DAX-1 [20,22,23]. It has been suggested that alternative in-frame translation from a methionine at codon 83 generates a protein lacking an amino-terminal motif but with sufficient function to partially “rescue” the phenotype, resulting in delayed onset of symptoms [20].
Frameshift mutations in DAX-1/NR0B1 are generally highly disruptive and are located throughout the gene and protein. In contrast, missense changes (disease causing single nucleotide variants) are clustered within certain key regions of the putative ligand binding region of DAX-1, with several amino acid “hotspots” showing multiple changes (e.g. p.L262, p.W291, p.A300, p.E377, p.L381, p.R425) [28,29] (Fig. 1, lower panel). These amino acids often associate with the hydrophobic core of the protein and may play a role in protein stability as well as nuclear localization. Several specific changes in these regions have been associated with a milder or late-onset phenotype (e.g., p.I439S, p.Y380D) [19,21]. These changes have partial function in different gene transcription assays, in keeping with the milder clinical phenotype. Variants within the amino-terminal repeat regions of DAX-1 are very rare (e.g. p.C200W) and the significance of these in individuals with X-linked AHC is less clear [30].
Management of X-linked adrenal hypoplasia
Infants and children who present with acute adrenal insufficiency require appropriate management with resuscitation, fluid replacement and intravenous hydrocortisone treatment accordingly to standard guidelines. Careful attention to blood pressure, hypoglycaemia, electrolyte disturbances (hyponatraemia, hyperkalaemia) and any precipitating factors (e.g. sepsis) is needed. Replacement doses of hydrocortisone and fludrocortisone can be introduced once the child is stable, and salt supplements are needed for around the first 12–18 months of life in those boys who present in early infancy. Education and monitoring are needed with an awareness of when to increase steroid doses during illness or stress and when to administer emergency hydrocortisone injections. Medical alert systems should be worn and schools and emergency services should be familiar about dealing with acute problems. Easy access to hospital should be available. Alternative steroid replacement regimen may be tried in later life but it is best to avoid long acting steroids such as dexamethasone. Newer slow release preparations of hydrocortisone are becoming available.
Early investigations are needed at the time of presentation to confirm PAI and to establish a diagnosis of X-linked AHC in contrast to other causes of adrenal insufficiency. The differential diagnosis can vary with age. Typically, an inappropriately low cortisol (for the clinical situation), elevated ACTH, hyponatraemia, hyperkalaemia, low aldosterone and increased plasma renin activity (for age-adjusted normal ranges) should point to a diagnosis of PAI. Impaired cortisol response to synacthen stimulation is a useful assessment of adrenal reserve, though it is not always easy to perform this test if there is an acute presentation. Alternative diagnoses should be excluded such as congenital adrenal hyperplasia (most commonly 21-hydroxylase deficiency) and metabolic causes in the newborn period and autoimmune, metabolic (e.g. X-linked adrenoleukodystrophy) and other causes in childhood. Additional tests such as urine steroid profiling, measurement of 17-hydroxyprogesterone (17-OHP), very-long chain fatty acids (VLCFAs) and autoantibodies can be useful, and a detailed consideration of the many causes of PAI is needed. A family history of adrenal insufficiency in boys (or unexplained death) can be useful, although around 40% of male infants with salt-losing adrenal insufficiency and no family history or clear cause have X-linked AHC due to mutations in DAX-1/NR0B1 [27]. Genetic testing is invaluable to identify specific mutations in DAX-1/NR0B1 and to confirm the diagnosis of X-linked AHC. Measurement of creatine kinase and urinary glycerol is needed where a contiguous gene deletion syndrome is possible.
Most boys with X-linked AHC do not progress through puberty and need sex hormone replacement. Several attempts have been made to induce puberty using GnRH pumps but these have been generally unsuccessful suggesting a combined pituitary as well as hypothalamic defect [13]. Repeated injections of hCG often stimulate testosterone production but in practice it is generally easier to use standard preparations of testosterone to induce puberty and for maintenance sex-hormone replacement throughout adulthood.
Exogenous FSH together with hCG has been tried in an attempt to induce spermatogenesis in young men with X-linked AHC. Generally these approaches have been unsuccessful alone. However, the use of testicular sperm extraction (TESE) coupled with intracytoplasmic sperm injection (ISCI) has been reported to result in a successful pregnancy and live birth in a man with classic early onset X-linked AHC, who had received repeated courses of FSH/hCG stimulation previously [31]. This case brings hope to men with his condition, although more data are needed to know how successful this approach will be.
Information and support
Children and families with adrenal insufficiency need education and support from their local hospital and training in emergency measures and dealing with sickness (an example is at http://www.gosh.nhs.uk/medical-information/medicines-information/emergency-pack-children-cortisol-deficiency). Adrenal insufficiency support groups (sometimes termed “Addison's”) can be very helpful (http://www.addisons.org.uk; http://www.nadf.us) although often they are more focussed on adults. Psychological support may be needed to help the boys deal with puberty and fertility issues. Information about X-linked AHC is available at GeneReviews (http://www.ncbi.nlm.nih.gov/books/NBK1431/).
Steroidogenic factor-1 (SF-1, NR5A1, Ad4BP)
History of SF-1
Steroidogenic factor-1 (SF-1, officially termed NR5A1) was first identified in the early 1990s following the work of Keith Parker (USA) and Ken-ichirou Morohashi (Japan). Both groups postulated that a common master regulator of steroidogenesis exists [32,33]. Using different experimental approaches they identified a nuclear receptor that could regulate the promoters of steroidogenic genes in vitro. The key role for SF-1 in adrenal and reproductive development was confirmed following the generation of the Sf-1 knockout mouse model [34].
Biological role of SF-1
SF-1 is a member of the nuclear receptor superfamily. The amino-terminal region of SF-1 contains a classic two zinc finger DNA-binding domain, whereas the carboxyl-terminal region of SF-1 has a 12 helix structure similar to that found in nuclear receptors. SF-1 belongs to a small group of nuclear receptors that are believed to bind to DNA as a monomer, recognizing variations on a PyCA AGGTCA binding motif [35]. The “A” box region of SF-1 is thought to play a role in stabilizing binding, through interactions with other transcription factor partners may also be important.
SF-1 has an unusual “hinge” region between the main domains and may undergo various forms of post-translational modification such as phosphorylation (activation) and sumoylation [36,37]. SF-1 may also interact with DAX-1 directly to regulate gene transcription. SF-1 was originally termed an “orphan” nuclear receptor as no high affinity ligand was known. Following the crystallization of the SF-1 ligand binding region it was suggested that phospholipids can bind to SF-1 and regulate its function, possibly mediating the downstream effects of certain signalling pathways or interfering with DAX-1 repression [38].
SF-1 was originally shown to activate the promoters of many key genes involved in steroidogenesis, adrenal development and reproduction in relatively simple in vitro assays. However, strong in vivo evidence for the role of SF-1 in these systems came following the generation of the Sf-1 (Ftz-F1, Nr5a1) mouse model [34]. Targeted deletion of Nr5a1 caused adrenal agenesis, gonadal (testicular) dysgenesis with a subsequent female phenotype and persistence of Müllerian structures (uterus) in XY animals, altered structure of the ventromedial hypothalamus (VMH), variable degrees of HH, and hyposplenism [34,39]. Late-onset obesity due to altered central regulation of energy expenditure may be a long-term feature [40]. Haploinsufficient animals have a disordered adrenal architecture and reduced gonad weight, whereas altered ovarian reserve is seen in tissue-specific granulosa cell deletion of Nr5a1 in females [41,42]. These biological models are providing new insights into the in vivo effects of Sf-1 in different systems, including the brain.
Other approaches have been used to try to identify novel SF-1 targets, using hypothesis generating approaches rather than focussing on the promoters of likely candidate genes. Using a ChIP-on-CHIP approach, we have identified a potential role for SF-1 in regulating angiogenesis in the developing adrenal gland through remodelling by angiopoietin-2 (ANGPT2). In addition, bidirectional manipulation of SF-1 in adrenal cells followed by microarray analysis was used to show that SF-1 can positively regulate sterol-O-acyl transferase (SOAT1, ACAT1), an important adrenal mediator of cholesterol biosynthesis and possible target for treatment of adrenal cancer [43,44]. Other data following ChIP-Seq approaches to identify SF-1 targets are emerging [45,46].
Clinical conditions associated with alterations in SF-1
Gonadal (testicular) dysgenesis and adrenal insufficiency
Initial attempts to find disease causing variations (mutations) in SF-1/NR5A1 focussed on individuals with a phenotype similar to the mouse model of complete gonadal dysgenesis with persistent Müllerian structures and primary adrenal failure. This is a rare phenotype in humans, but a girl (46,XY), who had these features, was found to have a de novo heterozygous (dominant) mutation in SF-1/NR5A1 in 1999 [47]. The alteration (p.G35E) affects a key amino acid in the “P”-box of the first zinc finger of SF-1, a region crucial for recognizing DNA-binding motives of target genes (Fig. 2) [35]. This change was seen to be competitive in most assays but may have some dominant negative activity with certain binding complexes.
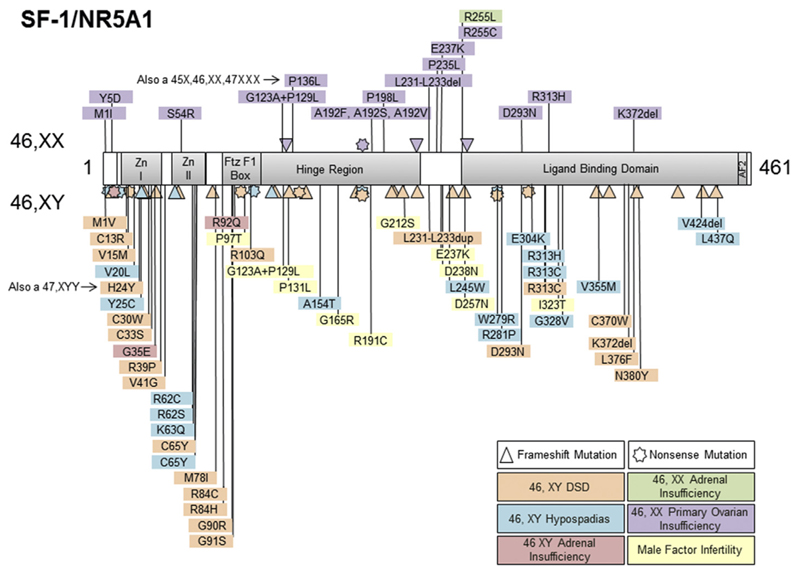
Fig. 2.
Cartoon of SF-1 structure and a selection of the changes reported in patients with adrenal insufficiency and/or reproductive phenotypes. Nonsense (stars), frameshift (triangles) and missense changes are indicated. Those associated with 46,XX primary ovarian insufficiency are shown in the upper panel and those in 46,XY phenotypes are shown in the lower panel. (Modified with permission from Lin et al., J Clin Endocrinol Metab 2006; 91: 3048–3054. Copyright © 2006 by The Endocrine Society).
A second child with a similar phenotype was reported in 2002 [48]. This child came from a consanguineous family and had a homozygous (recessive) mutation (p.R92Q) affecting a key amino acid in the “A”-box (within the FtzF1 box) of SF-1 that is involved in supporting monomeric binding (Fig. 2). This variant caused partial loss of function of SF-1, consistent with the need for the change to be present in a homozygous state and suggesting the functional gene dosage effects of SF-1 are important [35,48]. Although a heterozygous variant in SF-1/NR5A1 has been found in a girl (46,XX) with adrenal insufficiency, alterations in SF-1/NR5A1 in patients with an adrenal phenotype are rare [27,49].
46,XY DSD
In contrast to the effect of SF-1 on the adrenal gland, it has emerged in the past decade that heterozygous changes in SF-1/NR5A1 are a remarkably frequent (10–20%) cause of 46,XY DSD [50–53]. The most common phenotype is a baby with atypical (ambiguous) genitalia, with or more commonly without Müllerian structures, and with variable degrees of testicular dysgenesis and impaired androgen production. Often the testes are relatively well formed and the primary defect appears to be in testosterone synthesis. In other situations there can be adequate testosterone production in the newborn period and a baby is misdiagnosed as having partial androgen insensitivity syndrome [54,55]. Furthermore, several girls with SF-1/NR5A1 mutations have first presented with progressive androgen production and virilisation in adolescence, mimicking 5 alpha-reductase deficiency or 17 beta-hydroxysteroid dehydrogenase deficiency [56,57]. Hyposplenism has been described in one child with 46,XY DSD [58].
Hypospadias and undescended testes
Studies of boys with hypospadias have revealed SF-1/NR5A1 mutations in around 5% of those with penoscrotal hypospadias, a small phallus and at least one undescended testis [59]. SF-1/NR5A1 changes in boys with less severe forms of hypospadias are less common.
Bilateral anorchia
Analysis of a cohort of boys with bilateral anorchia (vanishing testis syndrome) found a heterozygous SF-1/NR5A1 mutation in one out of 24 children [60]. Although anorchia is often believed to reflect neonatal torsion or a vascular event, a small subgroup of bilateral anorchia may represent progressive late onset testicular dysgenesis, occurring after the external genitalia have formed.
Male factor infertility
At the mildest end of the spectrum, variations in SF-1/NR5A1 may be responsible for a subset of male factor infertility [61]. This likely represents 1–3.7% of infertility where chromosomal anomalies and Y chromosome microdeletions have been excluded [61–63]. Data are limited but some of these men may be at risk of a progressive decline in sperm function over time [61]. Furthermore, testosterone production may be reduced in men with SF-1 associated infertility, so endocrine function needs to be monitored in these situations.
Primary ovarian insufficiency (POI)
Although most focus has been on SF-1 in the testis, ovarian development and function is also influenced by SF-1 and POI has been reported in sisters or mothers of children with 46,XY DSD who were found to carry heterozygous SF-1/NR5A1 changes [64,65]. SF-1 likely has multiple effects on the ovary, including on stromal development, endocrine function and follicle recruitment and reserve. The clinical phenotype is very variable; complete early ovarian insufficiency and absence of puberty is seen in rare situations, whereas early menopause and decreased ovarian reserve is more common. Other women may harbour SF-1/NR5A1 mutations without any clinical effect on ovarian function, suggesting that SF-1 effects have variable penetrance. However, SF-1/NR5A1 mutations are relatively rare (1.4–1.6%) in women with sporadic POI of unknown aetiology [66,67].
Overactivity of SF-1
Overexpression or overactivity of SF-1 may be clinically relevant in certain conditions. Somatic duplication of the genetic locus encoding SF-1 has been reported in a high proportion of paediatric adrenal carcinomas, and overexpression of SF-1/NR5A1 has been shown to be present in many adult adrenal tumours and to be an important predictor of poor outcome [68,69]. Overactivity of SF-1 has been proposed to be associated with other conditions, such as hyperandrogenic polycystic ovarian syndrome (PCOS) and endometriosis [70].
The genetic basis of SF-1-associated conditions
The gene encoding SF-1 (NR5A1) is located on the long-arm of chromosome 9 (9q33.3) and consists of 7 exons (6 coding exons). Variations in SF-1/NR5A1 associated with specific disease phenotypes are shown in Fig. 2.
As described above, changes found in individuals with testicular dysgenesis and adrenal insufficiency are rare and tend to be located in critical amino acids for DNA binding, either in heterozygous or homozygous states. Changes associated with 46,XY DSD or hypospadias can be missense (point), nonsense or frameshift mutations throughout the gene, with point mutations often affecting the DNA-binding regions. Infertility-associated variants in SF-1/NR5A1 tend to be found more in the hinge region. A common polymorphism in SF-1/NR5A1 (p.G146A) has been reported to be higher in some small cohorts with hypospadias or cryptorchidism but this is a common variant in different ancestral populations and its influence as a modifier is not known. Haploinsufficiency of SF-1/NR5A1 has been detected by techniques such as MLPA in a small number of children with 46,XY DSD and a contiguous gene deletion syndrome including genitopatellar syndrome has also been described [71,72].
Most disease-associated variants in SF-1/NR5A1 are heterozygous, with rare homozygous partial loss of function changes described [48,58,64]. Often these occur de novo and are associated with infertility so cannot be passed on. However, around 30% of children with 46,XY DSD inherit a heterozygous change from their mother in a sex-limited dominant manner [51]. In these kindred there is a 50% chance of a 46,XY sibling being affected or 46,XX sibling being a carrier, and extended family members of the mother's side may have 46,XY DSD, hypospadias or infertility. This inheritance pattern can mimic an X-linked disorder and many of these children have been thought to have partial androgen insensitivity syndrome [54]. Female carriers are at risk of developing POI and need to be counselled accordingly. To date, no complete loss of function or deletions of SF-1/NR5A1 have been described.
Management of SF-1 associated conditions
As the clinical effects of SF-1 are wide and sometimes variable, management plans need to be tailored to individuals and their families. Those rare children presenting with adrenal insufficiency need resuscitation and adrenal steroid replacement as outlined earlier in the section on X-linked AHC. Children with various forms of 46,XY DSD should be assessed by a specialist multidisciplinary team (MDT) and parents informed and supported to make decisions regarding sex assignment and management, with the child's long-term interests as the focus. Key areas to consider are: likely gender identity; endocrine, urological and sexual function; fertility potential; and tumour risk. A small number of children with SF-1/NR5A1 mutations have been initially raised female but then changed to male, though data are very limited. Long term endocrine function and fertility are unpredictable and need to be monitored. The risk of germ cell tumours is unknown although intratubular germ cell neoplasia has been seen, so retained testes need careful surveillance [56]. Women and girls at risk of POI need careful monitoring and, if requested, endocrine function and ovarian reserve can be assessed. It is not known whether ovarian cryopreservation will be useful in maintaining integrity of the ovary for later retransplantation but this is being considered in several centres. The long term risks of developing adrenal insufficiency or other metabolic effects are unknown. Adrenal insufficiency is not common in those children with being followed up with 46,XY DSD but long-term studies are needed.
Information and support
Families and children with adrenal insufficiency need education and support from the local hospital as outlined for X-linked AHC. Children with 46,XY DSD need evaluation and support from an experienced MDT. Other family support organisations such as dsdfamilies (www.dsdfamilies.org) can be very helpful and specific resources for SF-1 are being developed (www.steroidogenicfactor1.info).
Summary
DAX-1 and SF-1 have important roles in human endocrine disorders. Although DAX-1 was identified as a cause of X-linked AHC more than 20 years ago, the range of clinical presenting features has expanded and late-onset forms of the condition are increasingly recognized. X-linked AHC should be considered in the differential diagnosis of all forms of adrenal insufficiency in boys and men, and in rare situations in girls too. Careful attention to adrenal replacement, puberty development and fertility is needed. Some hope has emerged that TESE-ISCI might provide an option for assisted reproduction, but more data are required. The first description of SF-1 as a cause of human adrenal and testicular dysgenesis was published more than 15 years ago, and the spectrum of SF-1 associated conditions has also expanded rapidly. Adrenal insufficiency is only very rarely associated with disruption of SF-1 whereas a range of reproductive phenotypes are more commonly seen, including male factor infertility and POI. Long-term outcome studies for all these conditions are needed to optimize management in the future. Support and education for young people and their families is an important priority.
Practice Points.
X-linked AHC due to DAX-1 (NR0B1) mutations is an important cause of adrenal insufficiency in boys and specific long-term focus on puberty and fertility is needed
Late-onset X-linked AHC is being increasingly recognised in the adult clinic
Genetic analysis can help to identify family members at risk of adrenal insufficiency and female carriers
Variations in SF-1/NR5A1 are a common cause of 46,XY DSD and can result in a spectrum of phenotypes
Inheritance patterns are variable and genetic analysis can help to identify women or girls who might be at risk of POI
Education and support for young people and families is important for both DAX-1 and SF-1-associated conditions
Research Agenda.
A better understanding of DAX-1 and SF-1 in development and tumorigenesis is needed, especially in relation to stem cell biology
The mechanisms of infertility associated with DAX-1 mutations are poorly understood and more insight into fertility options are needed
Long-term outcome studies are needed to understand how alterations in SF-1 influence gender, endocrine function, fertility and tumour risk
The natural history of POI associated with SF-1 is not well understood and it is not known if approaches such as ovarian cryopreservation will be useful
It is not known whether variations in SF-1/NR5A1 and DAX-1/NR0B1 may effect population health
Acknowledgements
JCA is a Wellcome Trust Senior Research Fellow in Clinical Science (098513).
References
- [1].Sikl H. Addison's disease due to congenital adrenal hypoplasia of the adrenals in an infant aged 33 days. J Pathol Bacteriol. 1948;60:323–6. doi: 10.1002/path.1700600220. [DOI] [PubMed] [Google Scholar]
- *[2].Muscatelli F, Strom TM, Walker AP, et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372:672–6. doi: 10.1038/372672a0. [DOI] [PubMed] [Google Scholar]
- [3].Bardoni B, Zanaria E, Guioli S, et al. A dosage sensitive locus at chromosome Xp21 is involved in male to female sex reversal. Nat Genet. 1994;7(4):497–501. doi: 10.1038/ng0894-497. [DOI] [PubMed] [Google Scholar]
- [4].Zanaria E, Muscatelli F, Bardoni B, et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372:635–41. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- [5].Sablin EP, Woods A, Krylova IN, et al. The structure of corepressor Dax-1 bound to its target nuclear receptor LRH-1. Proc Natl Acad Sci USA. 2008;105:18390–5. doi: 10.1073/pnas.0808936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ito M, Yu R, Jameson JL. DAX-1 inhibits SF-1 mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol. 1997;17:1476–83. doi: 10.1128/mcb.17.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yu RN, Ito M, Saunders TL, et al. Role of Ahch in gonadal development and gametogenesis. Nat Genet. 1998 Dec;20:353–7. doi: 10.1038/3822. [DOI] [PubMed] [Google Scholar]
- [8].Scheys JO, Heaton JH, Hammer GD. Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology. 2011;152:3430–9. doi: 10.1210/en.2010-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lalli E, Sassone-Corsi P. DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol Endocrinol. 2003;17:1445–53. doi: 10.1210/me.2003-0159. [DOI] [PubMed] [Google Scholar]
- [10].Ferraz-de-Souza B, Martin F, Mallet D, et al. CBP/p300-interacting transactivator, with Glu/Asp-rich C-terminal domain, 2, and pre-B-cell leukemia transcription factor 1 in human adrenal development and disease. J Clin Endocrinol Metab. 2009;94:678–83. doi: 10.1210/jc.2008-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu B, Yang WH, Gerin I, et al. Dax-1 and steroid receptor RNA activator (SRA) function as transcriptional coactivators for steroidogenic factor 1 in steroidogenesis. Mol Cell Biol. 2009;29:1719–34. doi: 10.1128/MCB.01010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reutens AT, Achermann JC, Ito M, et al. Clinical and functional effects of mutations in the DAX-1 gene in patients with adrenal hypoplasia congenita. J Clin Endocrinol Metab. 1999;84:504–11. doi: 10.1210/jcem.84.2.5468. [DOI] [PubMed] [Google Scholar]
- [13].Seminara SB, Achermann JC, Genel M, et al. X-linked adrenal hypoplasia congenita: a mutation in DAX1 expands the phenotypic spectrum in males and females. J Clin Endocrinol Metab. 1999;84:4501–9. doi: 10.1210/jcem.84.12.6172. [DOI] [PubMed] [Google Scholar]
- [14].Mantovani G, De Menis E, Borretta G, et al. DAX1 and X-linked adrenal hypoplasia congenita: clinical and molecular analysis in five patients. Eur J Endocrinol. 2006;154:685–9. doi: 10.1530/eje.1.02132. [DOI] [PubMed] [Google Scholar]
- [15].Wiltshire E, Couper J, Rodda C, et al. Variable presentation of X-linked adrenal hypoplasia congenita. J Pediatr Endocrinol Metab. 2001;14:1093–6. doi: 10.1515/jpem-2001-0804. [DOI] [PubMed] [Google Scholar]
- *[16].Landau Z, Hanukoglu A, Sack J, et al. Clinical and genetic heterogeneity of congenital adrenal hypoplasia due to NR0B1 gene mutations. Clin Endocrinol (Oxf) 2010;72:448–54. doi: 10.1111/j.1365-2265.2009.03652.x. [DOI] [PubMed] [Google Scholar]
- [17].Achermann JC, Gu WX, Kotlar TJ, et al. Mutational analysis of DAX1 in patients with hypogonadotropic hypogonadism or pubertal delay. J Clin Endocrinol Metab. 1999 Dec;84:4497–500. doi: 10.1210/jcem.84.12.6269. [DOI] [PubMed] [Google Scholar]
- [18].Domenice S, Latronico AC, Brito VN, et al. Adrenocorticotropin-dependent precocious puberty of testicular origin in a boy with X-linked adrenal hypoplasia congenita due to a novel mutation in the DAX1 gene. J Clin Endocrinol Metab. 2001;86:4068–71. doi: 10.1210/jcem.86.9.7816. [DOI] [PubMed] [Google Scholar]
- *[19].Tabarin A, Achermann JC, Recan D, et al. A novel mutation in DAX1 causes delayed-onset adrenal insufficiency and incomplete hypogonadotropic hypogonadism. J Clin Invest. 2000;105:321–8. doi: 10.1172/JCI7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ozisik G, Mantovani G, Achermann JC, et al. An alternate translation initiation site circumvents an amino-terminal DAX1 nonsense mutation leading to a mild form of X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 2003;88:417–23. doi: 10.1210/jc.2002-021034. [DOI] [PubMed] [Google Scholar]
- [21].Mantovani G, Ozisik G, Achermann JC, et al. Hypogonadotropic hypogonadism as a presenting feature of late-onset X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 2002;87:44–8. doi: 10.1210/jcem.87.1.8163. [DOI] [PubMed] [Google Scholar]
- [22].Guclu M, Lin L, Erturk E, et al. Puberty, stress and sudden death. Lancet. 2010;376:1512. doi: 10.1016/S0140-6736(10)61153-1. [DOI] [PubMed] [Google Scholar]
- [23].Raffin-Sanson ML, Oudet B, Salenave S, et al. A man with a DAX1/NR0B1 mutation, normal puberty, and an intact hypothalamic-pituitary-gonadal axis but deteriorating oligospermia during long-term follow-up. Eur J Endocrinol. 2013;168:K45–50. doi: 10.1530/EJE-12-1055. [DOI] [PubMed] [Google Scholar]
- [24].Mantovani G, Mancini M, Gazzano G, et al. Somatic mutational analysis of DAX1 in testes from men with idiopathic azoospermia. Fertil Steril. 2005;84:1542–4. doi: 10.1016/j.fertnstert.2005.05.037. [DOI] [PubMed] [Google Scholar]
- [25].Shaikh MG, Boyes L, Kingston H, et al. Skewed X inactivation is associated with phenotype in a female with adrenal hypoplasia congenita. J Med Genet. 2008;45:e1. doi: 10.1136/jmg.2007.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Achermann JC, Silverman BL, Habiby RL, et al. Presymptomatic diagnosis of X-linked adrenal hypoplasia congenita by analysis of DAX1. J Pediatr. 2000;137:878–81. doi: 10.1067/mpd.2000.108567. [DOI] [PubMed] [Google Scholar]
- *[27].Lin L, Gu WX, Ozisik G, et al. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years' experience. J Clin Endocrinol Metab. 2006;91:3048–54. doi: 10.1210/jc.2006-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Achermann JC, Ito M, Silverman BL, et al. Missense mutations cluster within the carboxyl-terminal region of DAX-1 and impair transcriptional repression. J Clin Endocrinol Metab. 2001;86:3171–5. doi: 10.1210/jcem.86.7.7660. [DOI] [PubMed] [Google Scholar]
- [29].Zhang YH, Guo W, Wagner RL, et al. DAX1 mutations map to putative structural domains in a deduced three-dimensional model. Am J Hum Genet. 1998;62:855–64. doi: 10.1086/301782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bernard P, Ludbrook L, Queipo G, et al. A familial missense mutation in the hinge region of DAX1 associated with late-onset AHC in a prepubertal female. Mol Genet Metab. 2006;88:272–9. doi: 10.1016/j.ymgme.2005.12.004. [DOI] [PubMed] [Google Scholar]
- *[31].Frapsauce C, Ravel C, Legendre M, et al. Birth after TESE-ICSI in a man with hypogonadotropic hypogonadism and congenital adrenal hypoplasia linked to a DAX-1 (NR0B1) mutation. Hum Reprod. 2001;26:724–8. doi: 10.1093/humrep/deq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rice DA, Mouw AR, Bogerd AM, et al. A shared promoter element regulates the expression of three steroidogenic enzymes. Mol Endocrinol. 1991;5:1552–61. doi: 10.1210/mend-5-10-1552. [DOI] [PubMed] [Google Scholar]
- [33].Morohashi K, Honda S, Inomata Y, et al. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1991;267:17913–9. [PubMed] [Google Scholar]
- [34].Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–90. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- [35].Ito M, Achermann JC, Jameson JL. A naturally occurring steroidogenic factor-1 mutation exhibits differential binding and activation of target genes. J Biol Chem. 2000;275:31708–14. doi: 10.1074/jbc.M002892200. [DOI] [PubMed] [Google Scholar]
- [36].Hammer GD, Krylova I, Zhang Y, et al. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell. 1999;3:521–6. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- [37].Lee FY, Faivre EJ, Suzawa M, et al. Eliminating SF-1 (NR5A1) sumoylation in vivo results in ectopic hedgehog signaling and disruption of endocrine development. Dev Cell. 2011;21:315–27. doi: 10.1016/j.devcel.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Blind RD, Sablin EP, Kuchenbecker KM, et al. The signaling phospholipid PIP3 creates a new interaction surface on the nuclear receptor SF-1. Proc Natl Acad Sci USA. 2014;111:15054–9. doi: 10.1073/pnas.1416740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shinoda K, Lei H, Yoshii H, et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn. 1995;204:22–9. doi: 10.1002/aja.1002040104. [DOI] [PubMed] [Google Scholar]
- [40].Majdic G, Young M, Gomez-Sanchez E, et al. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–14. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- [41].Bland ML, Jamieson CA, Akana SF, et al. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA. 2000;97:14488–93. doi: 10.1073/pnas.97.26.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pelusi C, Ikeda Y, Zubair M, et al. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod. 2008;79:1074–83. doi: 10.1095/biolreprod.108.069435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ferraz-de-Souza B, Lin L, Shah S, et al. ChIP-on-chip analysis reveals angiopoietin 2 (Ang2, ANGPT2) as a novel target of steroidogenic factor-1 (SF-1, NR5A1) in the human adrenal gland. FASEB J. 2001;25:1166–75. doi: 10.1096/fj.10-170522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ferraz-de-Souza B, Hudson-Davies RE, Lin L, et al. Sterol O-acyltransferase 1 (SOAT1, ACAT) is a novel target of steroidogenic factor-1 (SF-1, NR5A1, Ad4BP) in the human adrenal. J Clin Endocrinol Metab. 2011;96:E663–8. doi: 10.1210/jc.2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Baba T, Otake H, Sato T, et al. Glycolytic genes are targets of the nuclear receptor Ad4BP/SF-1. Nat Commun. 2014;5:3634. doi: 10.1038/ncomms4634. [DOI] [PubMed] [Google Scholar]
- [46].Lalli E, Doghman M, Latre de Late P, et al. Beyond steroidogenesis: novel target genes for SF-1 discovered by genomics. Mol Cell Endocrinol. 2013;371:154–9. doi: 10.1016/j.mce.2012.11.005. [DOI] [PubMed] [Google Scholar]
- *[47].Achermann JC, Ito M, Ito M, et al. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22:125–6. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- *[48].Achermann JC, Ozisik G, Ito M, et al. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab. 2002;87:1829–33. doi: 10.1210/jcem.87.4.8376. [DOI] [PubMed] [Google Scholar]
- [49].Biason-Lauber A, Schoenle EJ. Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet. 2000;67:1563–8. doi: 10.1086/316893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Correa RV, Domenice S, Bingham NC, et al. A microdeletion in the ligand binding domain of human steroidogenic factor 1 causes XY sex reversal without adrenal insufficiency. J Clin Endocrinol Metab. 2004;89:1767–72. doi: 10.1210/jc.2003-031240. [DOI] [PubMed] [Google Scholar]
- *[51].Lin L, Philibert P, Ferraz-de-Souza B. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab. 2007;92:991–9. doi: 10.1210/jc.2006-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kohler B, Lin L, Ferraz-de-Souza B, et al. Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum Mutat. 2008;29:59–64. doi: 10.1002/humu.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Allali S, Muller J-B, Brauner R, et al. Mutation analysis of NR5A1 encoding steroidogenic factor 1 in 77 patients with 46,XY disorders of sex development (DSD) including hypospadias. PLoS ONE. 2011;6:e24117. doi: 10.1371/journal.pone.0024117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Coutant R, Mallet D, Lahlou N, et al. Heterozygous mutation of steroidogenic factor-1 in 46,XY subjects may mimic partial androgen insensitivity syndrome. J Clin Endocrinol Metab. 2007;92:2868–73. doi: 10.1210/jc.2007-0024. [DOI] [PubMed] [Google Scholar]
- [55].Wu JY, McGown IN, Lin L, et al. A novel NR5A1 variant in an infant with elevated testosterone from an Australasian cohort of 46,XY patients with disorders of sex development. Clin Endocrinol (Oxf) 2013;78:545–50. doi: 10.1111/cen.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cools M, Hoebeke P, Wolffenbuttel KP, et al. Pubertal androgenization and gonadal histology in two 46,XY adolescents with SF-1 mutations and predominantly female phenotype at birth. Eur J Endocrinol. 2012;166:341–6. doi: 10.1530/EJE-11-0392. [DOI] [PubMed] [Google Scholar]
- [57].Tantawy S, Lin L, Akkurt I, et al. Testosterone production during puberty in two 46,XY patients with disorders of sex development and novel NR5A1 (SF-1) mutations. Eur J Endocrinol. 2012;167:125–30. doi: 10.1530/EJE-11-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zangen D, Kaufman Y, Banne E, et al. Testicular differentiation factor SF-1 is required for human spleen development. J Clin Invest. 2014;124:2071–5. doi: 10.1172/JCI73186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kohler B, Lin L, Mazen I, et al. The spectrum of phenotypes associated with mutations in steroidogenic factor 1 (SF-1, NR5A1, Ad4BP) includes severe penoscrotal hypospadias in 46,XY males without adrenal insufficiency. Eur J Endocrinol. 2009;161:237–42. doi: 10.1530/EJE-09-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Philibert P, Zenaty D, Lin L, et al. Mutational analysis of steroidogenic factor 1 (NR5a1) in 24 boys with bilateral anorchia: a French collaborative study. Hum Reprod. 2007;22:3255–61. doi: 10.1093/humrep/dem278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[61].Bashamboo A, Ferraz-de-Souza B, Lourenco D, et al. Human male infertility associated with mutations in NR5A1 encoding steroidogenic factor 1. Am J Hum Genet. 2010;87:505–12. doi: 10.1016/j.ajhg.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Röpke A, Tewes AC, Gromoll J, et al. Comprehensive sequence analysis of the NR5A1 gene encoding steroidogenic factor 1 in a large group of infertile males. Eur J Hum Genet. 2013;21:1012–5. doi: 10.1038/ejhg.2012.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ferlin A, Rocca MS, Vinanzi C, et al. Mutational screening of NR5A1 gene encoding steroidogenic factor 1 (SF-1) in cryptorchidism and male factor infertility and functional analysis of seven undescribed mutations. Fertil Steril. 2015;104:163–9. doi: 10.1016/j.fertnstert.2015.04.017. [DOI] [PubMed] [Google Scholar]
- *[64].Lourenco D, Brauner R, Lin L, et al. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med. 2009;360:1200–10. doi: 10.1056/NEJMoa0806228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Camats N, Pandey AV, Fernández-Cancio M, et al. Ten novel mutations in the NR5A1 gene cause disordered sex development in 46,XY and ovarian insufficiency in 46,XX individuals. J Clin Endocrinol Metab. 2012;97:E1294–306. doi: 10.1210/jc.2011-3169. [DOI] [PubMed] [Google Scholar]
- [66].Janse F, de With LM, Duran KJ, et al. Limited contribution of NR5A1 (SF-1) mutations in women with primary ovarian insufficiency (POI) Fertil Steril. 2012;97:141–6. doi: 10.1016/j.fertnstert.2011.10.032. [DOI] [PubMed] [Google Scholar]
- [67].Voican A, Bachelot A, Bouligand J, et al. NR5A1 (SF-1) mutations are not a major cause of primary ovarian insufficiency. J Clin Endocrinol Metab. 2013;98:E1017–21. doi: 10.1210/jc.2012-4111. [DOI] [PubMed] [Google Scholar]
- [68].Almeida MQ, Soares IC, Ribeiro TC, et al. Steroidogenic factor 1 overexpression and gene amplification are more frequent in adrenocortical tumors from children than from adults. J Clin Endocrinol Metab. 2010;95:1458–62. doi: 10.1210/jc.2009-2040. [DOI] [PubMed] [Google Scholar]
- [69].Sbiera S, Schmull S, Assie G, et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J Clin Endocrinol Metab. 2010;95:E161–71. doi: 10.1210/jc.2010-0653. [DOI] [PubMed] [Google Scholar]
- [70].Calvo RM, Asuncion M, Telleria D, et al. Screening for mutations in the steroidogenic acute regulatory protein and steroidogenic factor-1 genes, and in CYP11A and dosage-sensitive sex reversal-adrenal hypoplasia gene on the X chromosome, gene-1 (DAX-1), in hyperandrogenic hirsute women. J Clin Endocrinol Metab. 2001;86:1746–9. doi: 10.1210/jcem.86.4.7424. [DOI] [PubMed] [Google Scholar]
- [71].Barbaro M, Cools M, Looijenga LHJ, et al. Partial deletion of the NR5A1 (SF1) gene detected by synthetic probe MLPA in a patient with XY gonadal disorder of sex development. Sex Dev. 2011;5:181–7. doi: 10.1159/000328821. [DOI] [PubMed] [Google Scholar]
- [72].Schlaubitz S, Yatsenko SA, Smith LD, et al. Ovotestes and XY sex reversal in a female with an interstitial 9q33.3-q34.1 deletion encompassing NR5A1 and LMX1B causing features of Genitopatellar syndrome. Am J Med Genet A. 2007;143A:1071–81. doi: 10.1002/ajmg.a.31685. [DOI] [PubMed] [Google Scholar]