Abstract
Homozygous frameshift variants in CNTNAP1 have recently been reported in patients with arthrogryposis and abnormal axon myelination. In two brothers with severe congenital hypotonia and foot deformities, we identified compound heterozygous variants in CNTNAP1, reporting the first causative missense variant, p.(Cys323Arg). Motor nerve conductions were markedly decreased. Nerve microscopical lesions confirmed a severe hypomyelinating process and showed loss of attachment sites of the myelin loops on the axons, which could be a characteristic of Caspr loss-of-function. We discuss the pathophysiology of the myelination process and we propose to consider this disorder as a congenital hypomyelinating neuropathy.
Introduction
Congenital hypotonia is a non-specific feature of a large range of genetic disorders. Given this strong pathophysiology heterogeneity, a molecular diagnosis is often lacking.1 In early and severe cases, fetal akinesia can manifest by arthrogryposis. Defects in motor neuron function, neuromuscular transmission or muscle contraction may be involved. In rare cases, constitutive defects in myelin constituents impair the myelination process.
A few congenital hypomyelinating neuropathies (CHN) have been reported so far (MIM#605253). Variants in EGR2 or MPZ genes (MIM159440, MIM129010) can lead to early onset of hypotonia, areflexia, distal muscle weakness, very slow nerve conduction velocities and hypomyelination process.2, 3 MPZ (Myelin Protein Zero) is the major structural protein of peripheral nerve myelin. The Egr2 transcription factor is critical for expression of myelin proteins and lipids.4 A homozygous deletion of an EGR2 enhancer was also reported in a patient with congenital amyelinating neuropathy.5 Recently, variants in GPR126 were reported in patients with lethal congenital contracture syndrome and lack of myelin basic protein.6
Homozygous frameshift variants in CNTNAP1 (Contactin-associated protein 1) have been identified in seven patients with arthrogryposis and abnormal axon myelination, considered as lethal congenital contracture syndrome (MIM#616286).7 CNTNAP1 encodes Caspr that is essential to the paranodal junctions.8 We report on two brothers affected with hypotonia and CHN caused by compound heterozygous CNTNAP1 variants.
Materials and methods
Clinical assessment
Patient 1 was the first male child of non-consanguineous French parents. He was born eutrophic at 34 WG (weeks of gestation) after a pregnancy complicated by polyhydramnios, treated by amniodrainage. A severe and generalized hypotonia was immediately reported and was associated with respiratory distress requiring intubation, absence of swallowing, foot varus deformity, low gesticulation, weak facial expression and poor eye contact. Brain MRI was normal. Auditory and visual evoked potentials were altered. Electroneuromyogram revealed a severe decrease of sensorimotor nerve conduction velocities at 1 week, suggestive of CHN. Death occurred at 2 months of age.
Patient 2 was the third male child to same couple. Polyhydramnios with decreased fetal movements was noticed at 22 WG, necessitating an amnioreduction. He was born eutrophic at 36 WG, showing no respiratory movements. He died a few minutes after birth, with palliative care procedure. Mild retrognathism and bilateral clubfeet were observed. Muscle biopsy revealed early muscle innervation disorder.
Electron microscopy
Sural nerve biopsy was performed for both children. Samples were fixed for 3 h in 2.5% glutaraldehyde in Sœrensen buffer and osmificated for 1 h in 1% OsO4. Afterwards, they were rinsed in Sœrensen buffer, dehydrated in graded acetone and embedded in Epon. Semi-thin sections (1 μm) were stained with toluidine blue. Ultra-thin sections were stained with uranyl acetate and lead citrate. The sections were observed through a JEOL electron microscope.
DNA analysis
Written informed parent consents were obtained for whole-exome sequencing (WES). DNA was extracted from muscle tissue according to standard procedure. WES was performed with the Clinical Research Exome kit (Agilent, Santa Clara, CA, USA) on Illumina HiSeq2500 (paired-end sequencing, 2 × 75 bp). Reads were aligned to the human reference genome sequence (NCBI build 37.3, hg19). Bioinformatics analyses were performed according to the best practices of GATK (v3.4). Variants were annotated using ANNOVAR and filtered with in-house scripts to remove synonymous variants, non-coding variants and variants with an allele frequency above 0.5% in ExAC (http://exac.broadinstitute.org/) and EVS (http://evs.gs.washington.edu/EVS/) and >1 in a local database of 120 exomes. The possible functional impact of amino-acid changes was predicted by SIFT (Sorting Intolerant from Tolerant), PolyPhen-2 hvar and CADD score (Combined Annotation Dependent Depletion). The Alamut software (Interactive biosoftware) was used to study retained variant sites among different species. Segregation analysis by direct sequencing was then performed in the family to confirm candidate variants. The variants were submitted in ClinVar database.
Results
Nerve biopsy
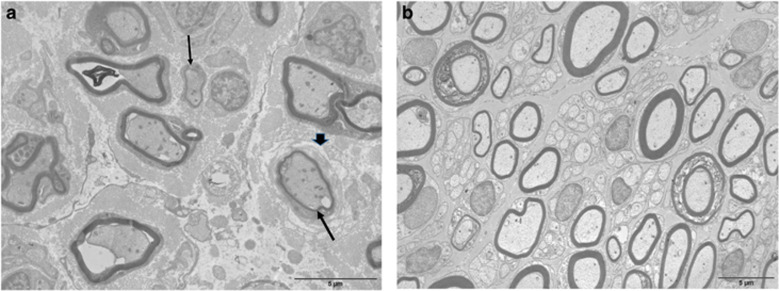
The same lesions were observed on both biopsies. On sural nerve biopsy semi-thin sections of patient 1, there was a significant loss of myelinated fibers, which was homogeneous between nerve fascicles. Most of the remaining myelinated fibers presented too thin myelin sheaths. Electron microscopic examination showed that a few axons had no myelin and several hypomyelinated fibers were surrounded by proliferations of basal membrane laminae, like onion bulbs (Figure 1). Myelin debris in the cytoplasms of some Schwann cells, in a few macrophages and in some endothelial cells were also seen. On longitudinal sections, frequent and marked widenings of the nodes of Ranvier were observed. Moreover, most of the paranodal regions were characterized by loss of attachment sites of the myelin loops, which seemed to be associated to Schwann cell processes penetrating between the axon and the loops.
Figure 1.
sural nerve (case 1) transverse section, electron microscopy. (a) Significant rarefaction of myelinated fibers; some are hypomyelinated (thin arrows). One of them is surrounded by a concentric proliferation of basal laminae (thick arrow). (b) Age-matched control of sural nerve.
DNA analysis
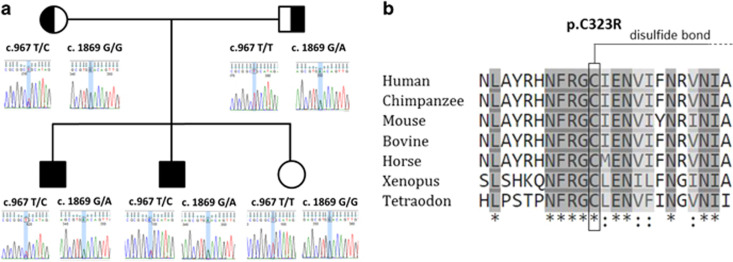
WES was performed in patient 2 with a 70 × mean coverage. After filtering, we identified two heterozygous variants in CNTNAP1 (NM_003632.2, exons are numbered like in NG_042091.1): c.[967T>C][1869G>A] (dbSNP rs768554986 with ID 237757 and dbSNP rs878853221 with ID 237756). Missense variant c.[967T>C], p.(Cys323Arg) was found only twice over 120 018 alleles in ExAC and was absent from EVS and 1000genome databases. It was predicted deleterious by SIFT, PolyPhen-2 hvar and CADD (phred score: 25.8). It substitutes a highly conserved cysteine predicted to be involved in a disulfide bond (Uniprot) in a laminin G domain, possibly resulting in protein misfolding. Nonsense variant c.[1869G>A], p.(Trp623*) in exon 13 likely results in the degradation of mRNA by nonsense mediated decay. Both variants were confirmed by Sanger sequencing in the proband (Figure 2). Segregation analysis showed that the variants were inherited from father and mother respectively, not found in healthy sister and both present in affected brother.
Figure 2.
(a) Segregation analyses for CNTNAP1 variants c.[967T>C][1869G>A], p.(Cys323Arg) and p.(Trp623*) in the two affected brothers, their healthy parents and their healthy sister. (b) Orthologous alignment for p.(Cys323Arg) substitution: cysteine in position 323 is highly conserved across species. Source: UniProt.
Discussion
We report two affected siblings with novel variants in CNTNAP1. Previously, seven patients with CNTNAP1 frameshift variants from four consanguineous families were reported.7 All nine patients shared common features namely polyhydramnios, fetal akinesia, severe neonatal hypotonia, facial diplegia, absence of swallowing and of spontaneous breathing and arthrogryposis. The most striking feature in our patients was generalized hypotonia. However, foot deformities were noticed and can be considered as a minor sign of arthrogryposis. Motor nerve conductions were markedly decreased in all patients. The nerve microscopical lesions of our patients showed a severe hypomyelinating process as reported by Laquérriere et al. We also observed unusual lesions of the paranodal regions characterized by loss of attachment sites of the myelin loops on the axons.
Frameshift CNTNAP1 variants previously reported are located in exons 18 and 19. Our patients carry a missense variant in exon 7, probably resulting in non-functional protein and a nonsense variant inducing a premature stop codon in exon 13. CNTNAP1 encodes a contactin-associated transmembrane receptor (Caspr), with high expression level in central and peripheral nervous system.9 Caspr is localized to the paranodal axoglial junction of myelinated axons.10 The specific organization of Ranvier nodes and paranodal regions is necessary to action potential saltatory conduction.11 These domains are the result of interactions between axons and myelinating glial cells such as Schwann cells in the peripheral nervous system.12 Caspr interacts with Contactin-1, which is a glycosyl phosphatidyl inositol-anchored neural cell adhesion molecule, to form an extracellular complex with neurofascin resulting to axoglial junction.8, 13 The missense variant identified in our family is located in a disulfide bond within a laminin G domain, in the extracellular region of Caspr, which seems to be involved in molecular interaction between Caspr and Contactin-1. This variant likely interferes with adhesion process at axoglial junction and explains the abnormal myelin organization. In ExAC database, although 635 missense variants were expected in CNTNAP1, 404 were observed with a z-score of 4.5, suggesting that, despite being responsible for a recessive condition, CNTNAP1 may be intolerant to missense variants.14
Mice lacking Caspr displayed growth failure with severe neurological defects, including cerebellar syndrome, generalized motor paresis and premature death.8 Absence of Caspr resulted in altered distribution of juxtaparanodal components, including Kv1 channels and contactin-1.15 Finally, dissociations of the myelin loops from the axons were described in optic nerves of Cntn1-KO mice.16 Given that similar lesions were observed in our patients in sural nerve biopsy, such unusual anomalies could be a hallmark of Caspr/Contactin-1 complex loss of function.
In conclusion, our data extend the phenotype associated with CNTNAP1 variants and confirm Caspr critical role in human myelination. Considering the suggestive electroneuromyogram pattern, nerve conduction velocities should be performed when severe congenital hypotonia remains unexplained, even in absence of complete arthrogryposis sequence. Hence, we propose to reclassify this phenotype into the congenital hypomyelinating neuropathy group. Finally, our report suggest that abnormal interaction between Caspr and Contactin-1 is the main pathophysiological hypothesis for this rare and severe disease.
Acknowledgments
We thank our colleagues for referring the patients and patients’ family for participating in this study.
Footnotes
The authors declare no conflict of interest.
References
- Hall JG: Arthrogryposis multiplex congenita: etiology, genetics, classification, diagnostic approach, and general aspects. J Pediatr Orthop Part B 1997; 6: 159–166. [PubMed] [Google Scholar]
- Warner LE, Mancias P, Butler IJ et al: Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat Genet 1998; 18: 382–384. [DOI] [PubMed] [Google Scholar]
- Szigeti K, Saifi GM, Armstrong D, Belmont JW, Miller G, Lupski JR: Disturbance of muscle fiber differentiation in congenital hypomyelinating neuropathy caused by a novel myelin protein zero mutation. Ann Neurol 2003; 54: 398–402. [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Svaren J, Le N, Araki T, Watson M, Milbrandt J: EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron 2001; 30: 355–368. [DOI] [PubMed] [Google Scholar]
- Funalot B, Topilko P, Arroyo MAR et al: Homozygous deletion of an EGR2 enhancer in congenital amyelinating neuropathy. Ann Neurol 2012; 71: 719–723. [DOI] [PubMed] [Google Scholar]
- Ravenscroft G, Nolent F, Rajagopalan S et al: Mutations of GPR126 Are Responsible for Severe Arthrogryposis Multiplex Congenita. Am J Hum Genet 2015; 96: 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laquérriere A, Maluenda J, Camus A et al: Mutations in CNTNAP1 and ADCY6 are responsible for severe arthrogryposis multiplex congenita with axoglial defects. Hum Mol Genet 2014; 23: 2279–2289. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y et al: Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 2001; 30: 369–383. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Lustig M et al: Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J 1997; 16: 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W et al: The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol 1997; 139: 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J: Multiple functions of the paranodal junction of myelinated nerve fibers. J Neurosci Res 2009; 87: 3250–3258. [DOI] [PubMed] [Google Scholar]
- Peles E, Salzer JL: Molecular domains of myelinated axons. Curr Opin Neurobiol 2000; 10: 558–565. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Devaux JJ: Neuro-glial interactions at the nodes of Ranvier: implication in health and diseases. Front Cell Neurosci 2013; 7: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV et al: Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016; 536: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A, Adamsky K, Vainshtein A et al: Caspr and caspr2 are required for both radial and longitudinal organization of myelinated axons. J Neurosci 2014; 34: 14820–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çolakoğlu G, Bergstrom-Tyrberg U, Berglund EO, Ranscht B: Contactin-1 regulates myelination and nodal/paranodal domain organization in the central nervous system. Proc Natl Acad Sci USA 2014; 111: E394–E403. [DOI] [PMC free article] [PubMed] [Google Scholar]