Abstract
Leishmaniases are parasitic diseases caused by protozoa of the genus Leishmania. The parasites, which infect various wild and domestic mammals, including humans, are transmitted by the bite of phlebotomine sand flies belonging to the Phlebotomus genus in the Old World and to several genera (including Lutzomyia, Psychodopygus and Nyssomyia) in the New World. In this paper, we consider the genus Sergentomyia as divided into seven subgenera, mainly based on spermathecal morphology: Sergentomyia, Sintonius, Parrotomyia, Rondanomyia, Capensomyia, Vattieromyia and Trouilletomyia. We also include the groups Grassomyia and Demeillonius but exclude the genera Spelaeomyia and Parvidens. The possible role of Sergentomyia in the circulation of mammalian leishmaniases in the Old World has been considered as Leishmania DNA and/or parasites have been identified in several species. However, several criteria must be fulfilled to incriminate an arthropod as a biological vector of leishmaniasis, namely: it must be attracted to and willing to feed on humans and any reservoir host, and be present in the same environment; several unambiguously identified wild female flies not containing blood meals have to be found infected (through isolation and/or typing of parasites) with the same strain of Leishmania as occurs in humans or any reservoir host; the presence of infective forms of Leishmania on naturally infected females and/or on colonized sand flies infected experimentally should be observed; and finally, the vector has to be able to transmit parasites as a result of blood-feeding on a susceptible mammal.
Keywords: Leishmania spp., leishmaniases, Sergentomyia sp., vector role
Abstract
Les leishmanioses sont des maladies causées par des protozoaires du genre Leishmania. Ces parasites infestent divers mammifères sauvages et domestiques incluant l’Homme. Ils sont transmis par la piqûre de phlébotomes appartenant au genre Phlebotomus dans l’Ancien Monde et à divers genres (Lutzomyia, Psychodopygus, Nyssomyia) dans le Nouveau Monde. Dans le cadre de ce travail, nous considérons par convention que le genre Sergentomyia contient sept sous-genres principalement définis par la structure des spermathèques : Sergentomyia, Sintonius, Parrotomyia, Rondanomyia, Capensomyia, Vattieromyia et Trouilletomyia. Nous considérons également les Grassomyia et les Demeillonius mais nous excluons les genres Spelaeomyia et Parvidens. Le rôle possible de Sergentomyia dans la transmission de leishmanioses dans l’Ancien Monde est de plus en plus évoqué depuis que des promastigotes ont été observées et que de l’ADN leishmanien a été mis en évidence chez plusieurs espèces du genre. Cependant, plusieurs critères doivent être validés pour incriminer un invertébré comme vecteur de leishmaniose : il doit être attiré et capable de prendre ses repas sanguins sur l’Homme et sur les réservoirs, et être présent dans le même environnement ; plusieurs femelles non gorgées doivent avoir été trouvées infestées (par isolement puis typage des parasites) avec la même souche leishmanienne que celle isolées chez l’Homme et les réservoirs ; la présence de formes infestantes doit être observée chez des femelles sauvages ou infestées expérimentalement; enfin, le vecteur doit être capable de transmettre le parasite, par piqûre infestante, à un hôte susceptible.
Introduction
Leishmaniases are parasitic diseases caused by protozoa belonging to the family Trypanosomatidae, genus Leishmania (Ross, 1903), infecting several mammal species, including humans. Human leishmaniases have diverse clinical manifestations. Visceral leishmaniasis (VL), caused by parasites of Leishmania Donovani complex (L. donovani (Laveran & Mesnil, 1903) in the Old World and L. infantum (Nicolle, 1908) in both the Old and New Worlds), is a severe disease of humans and other mammals which leads to death if left untreated. A number of different species of Leishmania cause cutaneous leishmaniasis (CL) or mucocutaneous leishmaniasis, which are responsible for considerable morbidity in a vast number of people in endemic foci. Leishmaniases are endemic in 98 countries on 4 continents, with more than 350 million people at risk. Published figures indicate an estimated incidence of 0.2–0.4 million VL cases and 0.7–1.3 million CL cases [3, 42].
Parasites are transmitted by the bite of an insect vector, the phlebotomine sand fly (order Diptera, family Psychodidae; subfamily Phlebotominae) of the genus Phlebotomus (Rondani & Berté, in Rondani 1840) in the Old World and of several genera (including Lutzomyia (França, 1924), Psychodopygus (Mangabeira, 1941) and Nyssomyia (Barretto, 1962)) in the New World [reviewed by 19, 26, 42].
Members of the genus Sergentomyia (França & Parrot 1920) [10] are widely distributed throughout the Old World, namely in Palearctic, Afrotropical, Oriental and Australasian regions, and in the Indian subregion. They are dominant species in tropical areas where Phlebotomus species are scarce or absent [reviewed by 2]. The species of this genus share the following characters: a mesanepisternum without setae, abdominal tergites 2–6 all or most usually carrying recumbent hairs, an usual 1/III–XV antennal formula in the males and 2/III–XV in the females, a cibarium with an armature of teeth and/or denticles more developed in females than in males, a single paramere, and a style with four terminal spines (or often 2 terminal and 2 subterminal) and an accessory spine [40]. However, there are some exceptions related to most of these characters and the genus Sergentomyia is in fact not clearly delimited. It seems difficult to define any strong synapomorphy supporting this group, which is in fact a catchall group.
Materials and methods
The role of Sergentomyia in the circulation of pathogenic Leishmania to humans and animals is reviewed primarily based on a search of the scientific literature available in the PubMed database up to October 2016 by combining the following keywords: “Sergentomyia AND Leishmania OR Taxonomy”. Reference lists of the available articles were also searched for publications deemed as relevant to this review. In addition, the Sergentomyia genus was defined conventionally.
Background on the systematics of the Sergentomyia genus
Due to the lack of a large-scale phylogenetic study on the systematics of the Old World sand fly, there is no synapomorphy of the Sergentomyia genus. Consequently, it is difficult to define this genus with precision; it is more a convention than a scientific group. The presence of cibarial teeth in the females of this genus is one of the most used characters to include species in the Sergentomyia. This is, however, not true. Some non-Sergentomyia species like Ph. papatasi (Scopoli, 1786), Ph. argentipes (Annandale & Brunetti in Annandale, 1908), Ph. stantoni (Newstead, 1914), Ph. fertei (Depaquit, Léger & Robert, 2002), Ph. berentiensis (Léger & Rodhain, 1978), and Idiophlebotomus (Quate & Fairchild, 1961) spp., exhibit many teeth or denticles, whereas Se. anodontis or Se. bailyi campester do not exhibit teeth or denticles evident to observe. Currently, the Sergentomyia genus can be divided into seven subgenera, mainly based on spermathecal morphology: Sergentomyia with smooth, thin-walled and wide spermathecae; Sintonius (Nitzulescu, 1931) and Trouilletomyia (Depaquit & Léger, 2014) with annealed spermathecae; Parrotomyia (Theodor, 1958) with elliptical capsule, smooth, thin- or thick-walled spermathecae; Rondanomyia (Theodor, 1958) with smooth and wide spermathecae; Capensomyia (Davidson, 1979) with convoluted spermathecae; and Vattieromyia (Depaquit, Léger & Robert, 2008). Many Sergentomyia species do not fall into these subgenera and remain ungrouped. Moreover, some authors include the taxa Grassomyia (Theodor, 1958) with spherical and spiny spermathecae and Demeillonius (Davidson, 1980) with irregularly transversely striated, apically provided with a projecting stalk spermathecae [25, 38] whereas others, like us, consider them to be genera [1, 4, 11]. Moreover, many species remain unclassified at the subgeneric level (the “ungrouped Sergentomyia”). To our knowledge, no authors [11, 25, 38] follow the position adopted by Artemiev [4] who proposed to create six subgenera: Perfilievia, Pharynxomyia, Longicoxia, Brevidentia, Luzonomyia and Irianomyia, including in these groups part of Neophlebotomus (França & Parrot, 1920), Parrotomyia and ungrouped species.
We consider the subgenus Rondanomyia as valid, and as not valid the subgenus Neophlebotomus according to the position adopted by Léger et al. [22]. Briefly, França & Parrot (1920) created the taxon Neophlebotomus to classify phlebotomine sand flies which are intermediate between Phlebotomus and Sergentomyia. Lewis [23] in a paper related to the phlebotomine sand flies of the oriental region, more probably misunderstanding than disagreeing with França & Parrot (1920), considered the subgenus Neophlebotomus as the senior synonym of Rondanomyia.
In fact, in this manuscript, pending a revision of this genus, we consider conventionally as Sergentomyia sensu lato the Old World species (Fig. 1) which do not belong to the genera Phlebotomus, Idiophlebotomus, Australophlebotomus (Theodor, 1948), Spelaeophlebotomus (Theodor, 1948), Chinius (Leng, 1987), Spelaeomyia (Theodor, 1948) and Parvidens (Theodor & Mesghali, 1964). The last ones (Fig. 2) cannot be considered as Sergentomyia s.l.
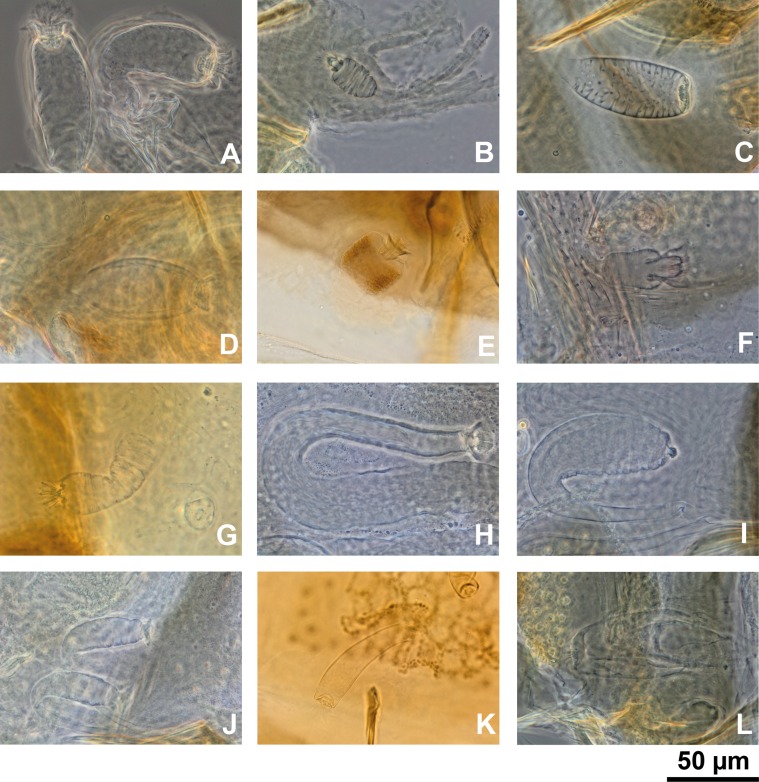
Figure 1.
Spermathecae of Sergentomyia spp. s. l.: Sergentomyia (Sergentomyia) minuta (A); Sergentomyia (Sintonius) clydei (B); Sergentomyia (Rondanomyia) goodmani (C); Sergentomyia (Parrotomyia) magna (D); Grassomyia sp. from Madagascar (E); Sergentomyia (Vattieromyia) anka (F); Demeillonius transvaalensis (G), and the following ungrouped species: Sergentomyia anodontis (H); Sergentomyia quatei (I); Sergentomyi asylvatica (J); Sergentomyia majungaensis (K); and Sergentomyia bailyi (L).
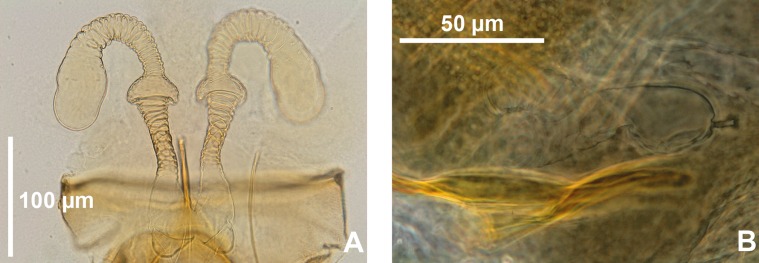
Figure 2.
Spermathecae of Spelaeomyia mirabilis (A) and Parvidens heischi (B).
Sergentomyia as possible vectors of Leishmania spp. pathogenic to humans and animals
As most Sergentomyia species feed preferentially on cold-blooded vertebrates, being proven vectors of reptile Leishmania species, it is generally accepted that they cannot transmit either Leishmania or any other pathogens to humans. However, based upon literature reviews, consideration of the role of Sergentomyia in the circulation of Old World Leishmania species with medical and veterinarian importance has been raised, but to fully prove the vector status of this Phlebotomine sand fly genus, several criteria need to be fulfilled [18], namely: (i) the vector must be found repeatedly infected in nature with the same Leishmania species as occurs in humans and any reservoir host(s), and this must be confirmed by comparison of isolates using isoenzymes and/or DNA; (ii) it must feed on humans and, in the case of zoonotic transmission, it must bite the reservoir host(s) as well; (iii) a strong ecological association between the vector, humans and any reservoir host should be evident; (iv) the vector must support the complete development of the parasite after the infecting blood meal has been digested, and (v) it must be able to transmit the parasite by bite to a susceptible host while taking a blood meal. In addition, and according to Ready [33], two more criteria based on mathematical modelling must be considered. The first one focused on demonstrating that the vector is essential for maintaining the transmission of the parasite, and the second on demonstrating a significant link between the decrease of disease prevalence and the decrease in biting density of the vector.
Indicative data about the potential role of some Sergentomyia species as a vector of mammalian Leishmania were given, namely the isolation of Leishmania major (Yakimoff & Shokkor, 1914) parasites from Sergentomyia ingrami (Newstead, 1914) collected in an endemic focus of cutaneous leishmaniasis in Kenya [28], and by the recent isolation of L. infantum from Se. dubia (Parrot, Mornet & Cadenat, 1945) and Se. schwetzi (Adler, Theodor & Parrot, 1929) in an endemic focus of canine leishmaniasis in Senegal [39]. Leishmania promastigotes have also been microscopically observed in several species of Sergentomyia: Grassomyia affinis (Theodor, 1933), Grassomyia squamipleuris (Newstead, 1912), Se. africana (Newstead, 1912), Se. antennata (Newstead, 1912), Se. bedfordi (Newstead, 1914), Se. clydei (Sinton, 1928), Se. garnhami (Heisch, Guggisberg & Teesdale, 1956), Se. graingeri (Heisch, Guggisberg & Teesdale, 1956), Se. ingrami, Se. kirki (Parrot, 1948), and S. schwetzi, in Kenya (reviewed by Kaddu et al. [15]) and in Ethiopia [13] but none of the parasites were biochemically or genetically typed and therefore they could not be confirmed to be Leishmania parasites.
Additional support for the potential role of Sergentomyia as a vector was provided by the detection of Leishmania DNA in several Sergentomyia species: that of L. major has been detected in Se. clydei and Se. minuta (Rondani, 1843) in Tunisia [5, 14], in Se. minuta in Portugal [7] and in Se. sintoni in Iran [31]. Despite the title of the publication, and the detection of L. major DNA in Spelaeomyia darlingi (Lewis & Kirk, 1954) in Mali [6], it is not possible to consider this species as a member of the genus Sergentomyia. In addition, L. donovani DNA has been detected in Se. babu (Annandale, 1910) in India [27], L. infantum DNA in Se. dubia, Se. magna (Sinton, 1932) and Se. schwetzi in Senegal [39] while “Leishmania siamensis” (nomen nudum) DNA has been found in Se. barraudi (Sinton, 1929) and in Se. gemmea (Lewis & Jeffery, 1978) in Thailand [17]. This so-called species, which belongs to the Leishmania enrietti (Muniz & Medina, 1948) complex, has not been formally named and described, and therefore is not taxonomically valid [2]. Moreover, it was recently shown by molecular tools that the majority of the strains previously described as “L. siamensis” may actually be Leishmania martiniquensis [32], the main causative agent of cutaneous leishmaniasis in Martinique Island (French West Indies) [8], including the Leishmania DNA sequenced from phlebotomine sand flies. Interestingly, it was recently experimentally proven that parasites of this complex developed late-stage infections in the biting midge Culicoides sonorensis (Wirth & Jones, 1957) [37] reinforcing the notion that vectors other than phlebotomine sand flies should be considered as part of epidemiological studies on Leishmania infecting mammals.
The detection of Leishmania tropica (Wright, 1903) DNA has been achieved from Se. ingrami and Se. hamoni (Abonnenc, 1958) collected in Ghana [30]. In addition, Maia et al. [24] also detected Leishmania sp. DNA phylogenetically related to those considered pathogenic to humans and dogs in Se. minuta collected in the South of Portugal.
Nevertheless, it is essential to keep in mind that PCR positivity alone should not be used for incrimination of a sand fly (or any other hematophagous arthropod) as a Leishmania vector, as the detection of DNA does not give any information about the parasites’ viability, nor about the presence as virulent metacyclic promastigotes, as the early phase of Leishmania development in the vector is non-specific and promastigotes are able to develop in various bloodsucking arthropods [reviewed by 36]. In fact, the detection of DNA of Leishmania through PCR-based tools has led to speculate about the vector competence of several “alternative” or “new” vectors [reviewed by 36]. For instance, the incrimination of biting midges as vectors of L. enrietti complex causing cutaneous leishmaniasis in red kangaroos [9] was the basis of the experimental infection of Culicoides nubeculosus with L. infantum and L. major parasites [35]; the authors demonstrated that, although both parasites species were able to develop early phases of infections, they were eliminated with the bloodmeal remnants; however, the DNA of both Leishmania species was detected until seven days post-infection, despite no living parasites being observed at that time point by microscopic examination, reinforcing the conclusion that the detection of Leishmania DNA does not prove the vector competence of any blood-sucking arthropod.
A primary determinant of vector competence in phlebotomine sand flies is the ability of Leishmania to survive defecation and to attach to midgut epithelium [reviewed by 16]. The direct microscopic observation of Leishmania promastigotes and their localization in the digestive tract is crucial before reaching any conclusion about the vectorial competence of an arthropod [reviewed by 36]. In fact, another criterion that a Leishmania vector should fulfil is the presence of parasites in the anterior midgut, on the stomodeal valve and the presence of infective forms on naturally infected females and/or on experimentally infected sand flies. The presence of flagellated parasites in dissected wild caught Se. dubia and Se. schwetzi females, which were subsequently successfully cultivated and characterized as L. infantum, was recently reported [39]. These results are contradictory to those obtained by Sadlova et al. [34] after testing the susceptibility of laboratory colonized Ethiopian Se. schwetzi to three Leishmania species capable of infecting humans (i.e. L. donovani, L. infantum and L. major). During early phases of infection, infection rates of all tested Leishmania species were very high (>90%) and comparable with those reached in control vectors (i.e. Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912) infected with L. infantum and Phlebotomus (Phlebotomus) duboscqi (Neveu-Lemaire, 1906) infected with L. major, respectively); however, none of them were able to develop successfully into late-stage infections. According to the authors, the refractoriness of this particular Sergentomyia species to the tested Leishmania species was probably related to the short period between the breakdown of the peritrophic matrix and the defecation of the bloodmeal remnants, thus avoiding the attachment of parasites to Se. schwetzi midgut epithelium. Similarly, in the study performed by Kaddu et al. [15], Se. adleri, Se. ingrami and Se. schwetzi did not support late-stage infections of L. donovani. Experimental data obtained by Kaddu et al. [15], Lawyer et al. [21] and Sadlova et al. [34] (i.e. the refractoriness of different Sergentomyia species to different Leishmania species pathogenic to humans) do not support conclusions based on field findings, namely the isolation of L. major from Se. garnhami [28] and L. infantum from Se. dubia and Se. schwetzi [39], confirming that the accidental results obtained in the field should always be interpreted with caution, and that more extensive studies focusing on isolation and typing of parasites from several unambiguously identified wild female flies not containing blood meals must be performed. In any case, the lack of vector competence of some African sand flies obtained under experimental conditions should not be extended to the whole of the genus as the competence and permissiveness of the different Phlebotomus species to different Old World Leishmania (e.g. Phlebotomus papatasi (Scopoli, 1786) is a proven vector of L. major but it is refractory to infection by L. infantum) has also been observed [reviewed by 16, 41]. All these findings have been summarized in Figure 3.
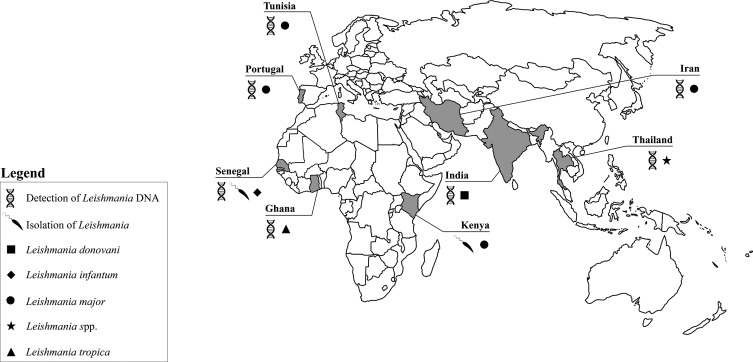
Figure 3.
Detection of Leishmania parasites and/or DNA in Sergentomyia species in the Old World.
The demonstration that the sand fly is attracted to humans and displays biting behaviour towards humans and any reservoir host is also essential for it to be considered a vector. Despite most Sergentomyia species being herpetophilic, some of them found infected with Leishmania pathogenic to humans (e.g. Sa. clydei, Sa. darlingi, Sa. minuta, Sa. schwetzi) have been reported to feed on mammals, including man [5, 6, 13, 24, 29, 39].
Another criterion is a strong ecological association between the phlebotomine sand fly, which should be abundant in the endemic areas of the disease, and man as well as any reservoir host. This association has been reported for Se. schwetzi, which seems to be the predominant phlebotomine sand fly species caught in some endemic foci of VL in Sudan [20] and Ethiopia [12], together with strong endophilic [20] and human-biting behaviours [13]. Similar results were recently obtained by Senghor et al. [39] in a focus of canine leishmaniasis caused by L. infantum where the presence of Se. dubia and Se. schwetzi was strongly associated with humans and dogs, and where the probability of infection was higher indoors and in peridomestic environments for Se. dubia sand flies and in peridomestic areas for Se. schwetzi. In addition, there was a significant correlation between the detection of L. infantum DNA in both Sergentomyia species with the presence of antibodies against the parasite in dogs and/or humans. Interestingly, in many foci where the role of Sergentomyia spp. is evoked, the classical Phlebotomus vectors are absent or scarce (West Africa, South-East Asia), whereas they are abundant and sympatric in some foci (Iran, Tunisia, Kenya, Portugal).
A fifth criterion is that the suspected vector will become infected by biting and feeding on the reservoir host or an equivalent laboratory model (xenodiagnosis). In the attempt made by Lawyer et al. [21] with laboratory reared Kenyan Se. schwetzi that fed on a lesion on the nose of a hamster infected with L. major, parasites multiplied slowly in the phlebotomine sand fly midgut, but did not migrate anteriorly nor survive beyond 90 h post-feeding. More studies evaluating the infectiousness of Leishmania-infected vertebrate hosts to laboratory reared Sergentomyia species are needed. Nevertheless, a major limitation to experimentally evaluate whether Sergentomyia spp. fulfils this criterion is the worldwide lack of Sergentomyia colonies other than Se. schwetzi, stressing that more efforts are needed to set up laboratory colonies of Sergentomyia species that have been incriminated in the transmission of mammal-infecting Leishmania.
Despite the importance of demonstrating that a potential vector is experimentally capable of transmitting parasites as a result of blood-feeding on mammals, this criterion is difficult to assess because phlebotomine sand flies must first be infected, then need to survive after blood digestion and must feed again on a non-infected susceptible host, a procedure that is quite difficult to accomplish [reviewed by 26]. This is probably the reason why the only attempt to partially demonstrate this criterion was performed by Mutinga et al. [28], who inoculated L. major parasites isolated from Se. garnhami in BALB/c mice and observed the development of the typical cutaneous lesions with the presence of numerous amastigotes. Despite the laboriousness of the experiments, determining the ability of Sergentomyia spp. to transmit Leishmania to a naïve mammal host is of crucial importance.
Conclusion
To sum up, some of the requirements needed for vectorial incrimination have been observed in Sergentomyia spp. such as: (i) epidemiological overlapping of the geographical distributions of Se. dubia and Se. schwetzi and canine/human Leishmania seroprevalence; (ii) evidence of anthropophilic behaviour (e.g. Se. minuta, Se. schwetzi); (iii) evidence that Se. dubia, Se. schwetzi and Se. ingrami support natural infections with promastigotes of the same Leishmania species as occurs in humans and reservoir hosts (i.e. L. infantum and L. major). Future work must be done to unravel whether and which Sergentomyia species accomplish all criteria needed to be incriminated as a vector of Old World Leishmania species pathogenic to mammals.
Acknowledgments
The authors would like to express their gratitude to Mr. André Pereira for his assistance with map design. C. Maia holds a FCT Investigator Starting Grant (IF/01302/2015) from Fundação para a Ciência e a Tecnologia, Ministério da Educação e Ciência, Portugal.
Cite this article as: Maia C & Depaquit J: Can Sergentomyia (Diptera, Psychodidae) play a role in the transmission of mammal-infecting Leishmania? Parasite, 2016, 23, 55.
References
- 1. Abonnenc E, Léger N. 1976. Sur une classification rationnelle des Diptères Phlebotomidae. Cahiers de l’O.R.S.T.O.M. Série Entomologie Médicale et Parasitologie, 14, 69–78. [Google Scholar]
- 2. Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, Sereno D. 2016. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Neglected Tropical Diseases, 10, e0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alvar J, Vélez I, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One, 7, e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Artemiev M. 1991. A classification of the subfamily Phlebotominae. Parassitologia, 33, 69–77. [PubMed] [Google Scholar]
- 5. Ayari C, Ben Othman S, Chemkhi J, Tabbabi A, Fisa R, Ben Salah A, BenAbderrazak S. 2016. First detection of Leishmania major DNA in Sergentomyia (Sintonius) clydei (Sinton, 1928, Psychodidae: Phlebotominae), from an outbreak area of cutaneous leishmaniasis in Tunisia. Infection, Genetics and Evolution, 39, 241–248. [DOI] [PubMed] [Google Scholar]
- 6. Berdjane-Brouk Z, Koné AK, Djimdé AA, Charrel RN, Ravel C, Delaunay P, del Giudice P, Diarra AZ, Doumbo S, Goita S, Thera MA, Depaquit J, Marty P, Doumbo OK, Izri A. 2012. First detection of Leishmania major DNA in Sergentomyia (Spelaeomyia) darlingi from cutaneous leishmaniasis foci in Mali. PLoS One, 7, e28266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campino L, Cortes S, Dionísio L, Neto L, Afonso MO, Maia C. 2013. The first detection of Leishmania major in naturally infected Sergentomyia minuta in Portugal. Memórias do Instituto Oswaldo Cruz, 108, 516–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desbois N, Pratlong F, Quist D, Dedet JP. 2014. Leishmania (Leishmania) martiniquensis n. sp. (Kinetoplastida: Trypanosomatidae), description of the parasite responsible for cutaneous leishmaniasis in Martinique Island (French West Indies). Parasite, 21, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dougall AM, Alexander B, Holt DC, Harris T, Sultan AH, Bates PA, Rose K, Walton SF. 2011. Evidence incriminating midges (Diptera: Ceratopogonidae) as potential vectors of Leishmania in Australia. International Journal for Parasitology, 41, 571–579. [DOI] [PubMed] [Google Scholar]
- 10. França C, Parrot L. 1920. Introduction à l’étude systématique des Diptères du genre Phlebotomus. Bulletin de la Société de Pathologie Exotique, 13, 695–708. [Google Scholar]
- 11. Galati E. 2016. Phlebotominae (Diptera, Psychodidae) Classificação, Morfologia, Terminologia e Identificação de Adultos. Apostila. Bioecologia e Identificação de Phlebotominae. Volume 1 Universidade de São Paulo: São Paulo, Brasil: p. 131. [Google Scholar]
- 12. Gebre-Michael T, Lane RP. 1996. The roles of Phlebotomus martini and P. celiae (Diptera: Phlebotominae) as vectors of visceral leishmaniasis in the Aba Roba focus, southern Ethiopia. Medical and Veterinary Entomology, 10, 53–62. [DOI] [PubMed] [Google Scholar]
- 13. Hailu A, Balkew M, Berhe N, Meredith S, Gemetchu T. 1995. Is Phlebotomus (Larroussius) orientalis a vector of visceral leishmaniasis in south-west Ethiopia? Acta Tropica, 60, 15–20. [DOI] [PubMed] [Google Scholar]
- 14. Jaouadi K, Ghawar W, Salem S, Gharbi M, Bettaieb J, Yazidi R, Harrabi M, Hamarsheh O, Ben Salah A. 2015. First report of naturally infected Sergentomyia minuta with Leishmania major in Tunisia. Parasite & Vectors, 8, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaddu JB, Mutinga MJ, Nyamori MP. 1986. Leishmania in Kenyan phlebotomine sandflies. 4. Artificial feeding and attempts to infect 6 species of laboratory-reared sandflies with Leishmaniadonovani. Insect Science and its Application, 7, 731–735. [Google Scholar]
- 16. Kamhawi S. 2006. Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends in Parasitology, 22, 439–445. [DOI] [PubMed] [Google Scholar]
- 17. Kanjanopas K, Siripattanapipong S, Ninsaeng U, Hitakarun A, Jitkaew S, Kaewtaphaya P, Tan-ariya P, Mungthin M, Charoenwong C, Leelayoova S. 2013. Sergentomyia (Neophlebotomus) gemmea, a potential vector of Leishmania siamensis in southern Thailand. BMC Infectious Diseases, 13, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Killick-Kendrick R. 1990. Phlebotomine vectors of the leishmaniasis: a review. Medical and Veterinary Entomology, 4, 1–24. [DOI] [PubMed] [Google Scholar]
- 19. Killick-Kendrick R. 1999. The biology and control of phlebotomine sand flies. Clinics in Dermatology, 17, 279–289. [DOI] [PubMed] [Google Scholar]
- 20. Lambert M, Dereure J, El-Safi SH, Bucheton B, Dessein A, Boni M, Feugier E, Dedet JP. 2002. The sandfly fauna in the visceral-leishmaniasis focus of Gedaref, in the Atbara-River area of eastern Sudan. Annals of Tropical Medicine and Parasitology, 96, 631–636. [DOI] [PubMed] [Google Scholar]
- 21. Lawyer PG, Ngumbi PM, Anjili CO, Odongo SO, Mebrahtu YB, Githure JI, Koech DK, Roberts CR. 1990. Development of Leishmania major in Phlebotomus duboscqi and Sergentomyia schwetzi (Diptera: Psychodidae). American Journal of Tropical Medicine and Hygiene, 43, 31–43. [DOI] [PubMed] [Google Scholar]
- 22. Léger N, Depaquit J, Robert V. 2005. Les phlébotomes de Madagascar (Diptera: Psychodidae). IV. Description de Sergentomyia (Rondanomyia) goodmani n. sp. Rétablissement du sous-genre Rondanomyia Theodor. Parasite, 12(1), 51–57. [DOI] [PubMed] [Google Scholar]
- 23. Lewis DJ. 1978. The phlebotomine sandflies (Diptera: Psychodidae) of the Oriental Region. Bulletin of the British Museum (Natural History), Entomology Series, 37(6), 217–343. [Google Scholar]
- 24. Maia C, Parreira R, Cristóvão JM, Freitas FB, Afonso MO, Campino L. 2015. Molecular detection of Leishmania DNA and identification of blood meals in wild caught phlebotomine sand flies (Diptera: Psychodidae) from southern Portugal. Parasites & Vectors, 8, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marcondes CB. 2007. A proposal of generic and subgeneric abbreviations for Phlebotomine sandflies (Diptera: Psychodidae: Phlebotominae) of the World. Entomological News, 118, 351–356. [Google Scholar]
- 26. Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. 2013. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Medical and Veterinary Entomology, 27, 123–147. [DOI] [PubMed] [Google Scholar]
- 27. Mukherjee S, Hassan MQ, Ghosh A, Ghosh KN, Bhattacharya A, Adhya S. 1997. Leishmania DNA in Phlebotomus and Sergentomyia species during a kala-azar epidemic. American Journal of Tropical Medicine and Hygiene, 57, 423–425. [DOI] [PubMed] [Google Scholar]
- 28. Mutinga MJ, Massamba NN, Basimike M, Kamau CC, Amimo FA, Onyido AE, Omogo DM, Kyai FM, Wachira DW. 1994. Cutaneous leishmaniasis in Kenya: Sergentomyia garnhami (Diptera Psychodidae), a possible vector of Leishmania major in Kitui District: a new focus of the disease. East African Medical Journal, 71, 424–428. [PubMed] [Google Scholar]
- 29. Ngumbi PM, Lawyer PG, Johnson RN, Kiilu G, Asiago C. 1992. Identification of phlebotomine sandfly bloodmeals from Baringo District, Kenya, by direct enzyme-linked immunosorbent assay (ELISA). Medical and Veterinary Entomology, 6, 385–388. [DOI] [PubMed] [Google Scholar]
- 30. Nzelu CO, Kato H, Puplampu N, Desewu K, Odoom S, Wilson MD, Sakurai T, Katakura K, Boakye DA. 2014. First detection of Leishmania tropica DNA and Trypanosoma species in Sergentomyia sand flies (Diptera: Psychodidae) from an outbreak area of cutaneous leishmaniasis in Ghana. PLoS Neglected Tropical Diseases, 8, e2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parvizi P, Amirkhani A. 2008. Mitochondrial DNA characterization of Sergentomyia sintoni populations and finding mammalian Leishmania infections in this sandfly by using ITS-rDNA gene. Iranian Journal of Veterinary Research, 9, 9–18. [Google Scholar]
- 32. Pothirat T, Tantiworawit A, Chaiwarith R, Jariyapan N, Wannasan A, Siriyasatien P, Supparatpinyo K, Bates MD, Kwakye-Nuako G, Bates PA. 2014. First isolation of Leishmania from Northern Thailand: case report, identification as Leishmania martiniquensis and phylogenetic position within the Leishmania enriettii complex. PLoS Neglected Tropical Diseases, 8, e3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ready P. 2013. Biology of Phlebotomine sand flies as vectors of disease agents. Annual Review of Entomology, 58, 227–250. [DOI] [PubMed] [Google Scholar]
- 34. Sadlova J, Dvorak V, Seblova V, Warburg A, Votypka J, Volf P. 2013. Sergentomyia schwetzi is not a competent vector for Leishmania donovani and other Leishmania species pathogenic to humans. Parasites & Vectors, 6, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seblova V, Sadlova J, Carpenter S, Volf P. 2012. Development of Leishmania parasites in Culicoides nubeculosus (Diptera: Ceratopogonidae) and implications for screening vector competence. Journal of Medical Entomology, 49, 967–970. [DOI] [PubMed] [Google Scholar]
- 36. Seblova V, Sadlova J, Carpenter S, Volf P. 2014. Speculations on biting midges and other bloodsucking arthropods as alternative vectors of Leishmania. Parasites & Vectors, 7, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seblova V, Sadlova J, Vojtkova B, Votypka J, Carpenter S, Bates PA, Volf P. 2015. The biting midge Culicoides sonorensis (Diptera: Ceratopogonidae) is capable of developing late stage infections of Leishmania enriettii. PLoS Neglected Tropical Diseases, 9, e0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seccombe AK, Ready PD, Huddleston LM. 1993. A catalogue of Old World phlebotomine sandflies (Diptera: Psychodidae, Phlebotominae). Occasional Papers on Systematic Entomology, 8, 1–57. [Google Scholar]
- 39. Senghor M, Niang A, Depaquit J, Faye M, Ferté H, Elguero E, Gaye O, Alten B, Perktas U, Cassan C, Faye B, Bañuls AL. 2016. Transmission of Leishmania infantum in the canine leishmaniasis focus of Mont-Rolland, Senegal: ecological, parasitological and molecular evidence for a possible role of Sergentomyia sand flies. PLoS Neglected Tropical Diseases, 10(11), e0004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Theodor O. 1948. Classification of the Old World species of the subfamily Phlebotominae (Diptera, Psychodidae). Bulletin of Entomological Research, 39, 85–115. [DOI] [PubMed] [Google Scholar]
- 41. Volf P, Myskova J. 2007. Sand flies and Leishmania: specific versus permissive vectors. Trends in Parasitology, 23, 91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. WHO. 2010. Control of the Leishmaniasis: Report of the Who Expert Committee on the Control of Leishmaniases. World Health Organization. WHO Technical Report Series, No. 949 pp. 186 http://www.who.int/neglected_diseases/2010report/NTD_2010report_web.pdf