Abstract
The main challenge for gas storage and separation in nanoporous materials is that many molecules of interest adsorb too weakly to be effectively retained. Instead of synthetically modifying the internal surface structure of the entire bulk—as is typically done to enhance adsorption—here we show that post exposure of a prototypical porous metal-organic framework to ethylenediamine can effectively retain a variety of weakly adsorbing molecules (for example, CO, CO2, SO2, C2H4, NO) inside the materials by forming a monolayer-thick cap at the external surface of microcrystals. Furthermore, this capping mechanism, based on hydrogen bonding as explained by ab initio modelling, opens the door for potential selectivity. For example, water molecules are shown to disrupt the hydrogen-bonded amine network and diffuse through the cap without hindrance and fully displace/release the retained small molecules out of the metal-organic framework at room temperature. These findings may provide alternative strategies for gas storage, delivery and separation.
Metal-organic frameworks are extensively studied for gas storage applications, but one potential limitation is their relatively weak adsorption of gases. Here, the authors report that the exposure of metal-organic frameworks to ethylenediamine forms a monolayer thick cap which improves gas molecule retention.
Metal-organic framework (MOF) materials are crystalline nanoporous materials consisting of inorganic nodes (metal ions or clusters), also referred with secondary building units, and organic ligands as the connecting units1. Their high surface areas and micropore structure provide an ideal environment for adsorbing small molecules, which is the basis of many important applications such as energy storage and gas capture and separation2,3,4,5, and even biomedicine6. The main problem for gas storage is the relatively weak adsorption of many small gases in MOFs. The focus to enhance gas adsorption and separation has therefore been to develop metal centres that are more active (for example, exposed metal cations) and to functionalize the ligands by incorporating functional groups such as amine, hydroxyl and halide in the organic ligands to increase or tune the guest-host interaction2,4,7. These approaches tend to target specific molecules, for example, through the formation of Lewis acid–base pairs, and thus lack a wider applicability4. Furthermore, it requires novel and potentially complex synthesis procedures and often leads to a decrease of the internal surface area7,8,9.
An alternative approach is to find a way to cap the MOF microcrystals at the end of the loading process. While previous work has considered high molecular weight compounds such as polydimethysiloxane10 or copolymer Pluronic P-123 (ref. 11) to coat MOFs external surfaces by high temperature vapour-phase deposition or liquid immersion, these methods have not been used for and are not compatible with gas storage and release. Ethylenediamine (EDA) molecules, on the other hand, have low-molecular weight, relatively high vapour pressure (∼10 Torr, 20 °C) at ambient condition and contain terminal amine groups in EDA molecules, which are known to interact more strongly with a variety of MOFs, particularly those with open or unsaturated metal sites (for example, found in MOF-74) by forming metal-amine complexes12,13,14. Moreover, previous studies have shown that EDA molecules cannot easily penetrate into MOFs due to their size and strong interaction with the framework, requiring refluxing in solution (for example, anhydrous toluene)12,15,16. For instance, in Mg-MOF-74, that is, Mg2(dobdc) with dobdc=2,5-dihydroxybenzene dicarboxylic acid, the best attempts only lead to ∼0.13 EDA per Mg2+ metal centre, which is an order of magnitude less than theoretically possible12,16. The difficulty to fully load EDA in MOF-74 highlighted by these pioneering studies suggests that, without extensive refluxing, EDA molecules should only adsorb on the surface of MOF crystals. They are therefore attractive to coat external surfaces of MOFs, particularly MOF-74 that has a three-dimensional honeycomb lattice with one-dimensional (1D) channels (diameter ∼14 Å, Supplementary Fig. 1). In addition, MOF-74 contains a high density of coordinatively unsaturated metal sites, which are the highest binding energy sites for small molecules such as CO2, NO, SO2, CH4 and H2 (refs 17, 18, 19, 20, 21). Capping the end of the 1D channels, which constitute the only diffusion pathway for these small molecules22, could therefore be effective for storage of many such small molecules.
In this work, we demonstrate, using in situ infrared spectroscopy23,24,25 that is well-suited to determine absolute gas loading25, that post exposure of MOF-74 crystals to vapours of a ‘sticky' molecule such as EDA is very effective in trapping weakly bound small gas molecules (CO, CO2, SO2, C2H4) within the material, or to prevent their loading into an EDA-capped empty MOF. A combination of X-ray photoelectron spectroscopy with gas cluster ion sputtering (GCIS) and low-energy ion spectroscopy measurements establish that EDA is only adsorbed as a monolayer on the exterior surface of MOF crystals (<1 nm thick), that is, within the outermost pores of the microcrystals, thereby acting as capping molecules. Ab initio modelling provides an explanation for this observation and proposes a structure that accounts for the observed properties. Interestingly, this EDA barrier is transparent to water molecules that readily diffuse through it and remove pre-adsorbed molecules (for example, CO). Ab initio modelling attributes such a ‘gate opening' to the disruption of the H-bonded amine groups of EDA by water molecules.
Results
Characterization of EDA capping with CO molecules
We have initially focused on CO adsorption in Ni-MOF-74 because CO is a good probe of Lewis acid adsorption sites and sensitive to the local cationic environment26,27. Furthermore, the stretch frequencies (ν(CO)) of adsorbed and gas-phase species are easily distinguishable. Moreover, the CO-binding energy is higher in Ni-MOF-74 (∼52.7 kJ mol−1 determined by isotherm measurement28) than in other isostructural frameworks with M=Mg, Mn, Fe, Co and Zn (ref. 28). In all frameworks, the isotherms are fully reversible at room temperature, consistent with weak binding with uncoordinated metal sites through electrostatic, σ and π orbital interactions28.
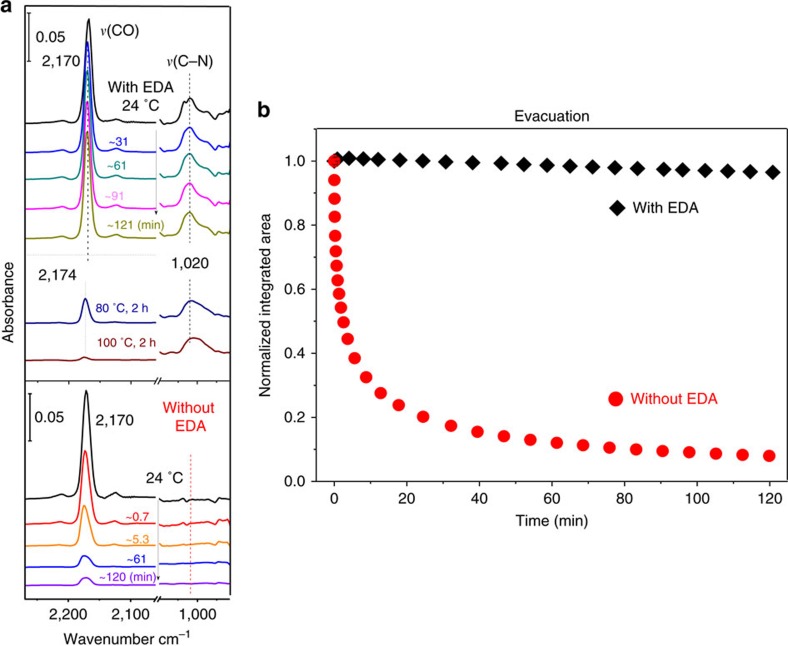
After activation and CO loading (∼40 Torr), the ν(CO) band is first observed at 2,174 cm−1, then shifts to 2,170 cm−1 as saturation is reached (∼30 min)28,29; in both cases it remains clearly distinct from the gas-phase band centred at 2,143 cm−1 (Supplementary Fig. 2). The main shift is attributed to the formation of a Ni2+…CO adduct within the open metal site29. The coverage-dependent shift (∼−4 cm−1) is attributed to additional CO–CO lateral interaction and/or potential slight structural rearrangement of the metal-adduct as the loading increases21,24,29. The occupation reaches ∼0.7 molecules per metal site at ∼40 Torr28. Upon evacuation (pressure <20 mTorr), CO is removed within ∼30 min as shown in the red curve in Fig. 1.
Figure 1. Time evolution of the ν(CO) band at 2,170 cm−1 upon evacuation.
(a) Infrared spectra of adsorbed CO and EDA in pristine (bottom) and EDA post-loaded (top) Ni-MOF-74 sample upon evacuation (<20 mtorr). All spectra are collected at 24 °C and referenced to the pristine activated MOF in vacuum. The annealing sequence includes 2 h at 80 °C, cooling back to room temperature for data collection, and an additional 2 h at 100 °C, cooling back to room temperature for data collection. (b) Normalized integrated area of ν(CO) band at 2,170 cm−1 upon evacuation for pristine (red circles) and EDA post-loaded (black diamonds) Ni-MOF-74 sample. The integrated areas are normalized to their maximum value obtained at t=0 (top spectrum). The error bars are calculated from the variations in the measured (normalized) integrated areas and do not exceed 0.02.
If immediately upon evacuation (<∼3 s) a CO/EDA gas mixture (∼40 Torr/∼4 Torr) is introduced into the cell (when>∼95% CO is still trapped) and kept for ∼10 min, the intensity of the CO band remains constant (Supplementary Fig. 2). Furthermore, when the system is evacuated (pressure<20 mTorr), the CO band decreases by <3% after a 2-h evacuation, as shown in the black diamond of Fig. 1. These data unambiguously show that CO can be trapped by introduction of EDA vapour, without hindering the total MOF capacity for CO adsorption.
Detailed information about the nature of the EDA is obtained in the infrared absorption spectrum (Supplementary Note 1 and Supplementary Fig. 2): on one hand, the two strong νas,s(–CH2) vibrational peaks at 2,936 and 2,860 cm−1 indicate that gas-phase EDA is clearly present in the CO/EDA mixture, although they quickly disappear as EDA molecules are adsorbed onto the MOFs and the cell internal walls. On the other hand, evidence for adsorbed EDA on or into MOF-74 is provided by a distinct absorption peak at 1,020 cm−1 (Fig. 1), corresponding to the ν(C–N) mode of the amine–metal complex30. This peak increases very slowly during ∼10 min as EDA adsorbs on the sample.
The stretch mode of initially adsorbed CO gas (2,170 cm−1) does not decrease or shift during and after EDA loading, as would typically occur during co-adsorption of gases inside the MOF (Supplementary Note 2 and Supplementary Fig. 3 for the case of NH3 co-adsorption), indicating that the CO molecules not only remain trapped, but also do not interact with the newly added EDA molecules. This observation supports our hypothesis that no EDA molecules penetrate inside the MOF. If EDA interacted with CO inside MOF-74, the CO stretch frequency would be shifted either due to displacement to a secondary binding site or to interaction with EDA. To quantify this statement ab initio calculations were performed (Supplementary Fig. 4 and Supplementary Table 1) and show that, if EDA penetrated inside the MOF, the CO-binding energy would be changed only by ∼3 kJ mol−1 and its frequency would be shifted by 5–9 cm−1, which is not observed.
The above observations and analyses lead us to conclude that pre-adsorbed CO and post-loaded EDA molecules are spatially separated, with EDA residing on the periphery of the MOF microcrystals (after replacing CO molecules only in the outermost pores, since the EDA Ebinding=125 kJ mol−1>>CO Ebinding=52.7 kJ mol−1) and acting as a cap that confines pre-loaded CO molecules inside the MOF. However, a direct experimental confirmation of EDA localization is needed.
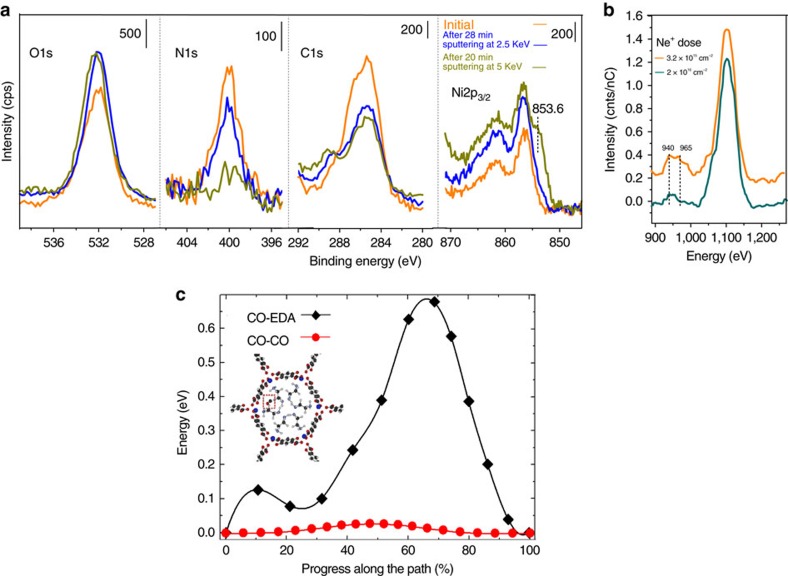
To test and quantify whether EDA is localized at the surface (that is, only the outmost pores) of the MOF crystallites, we have combined X-ray photoelectron spectroscopy (XPS), a surface sensitive technique, with argon GCIS that provides gentle removal of surface atoms (particularly appropriate for organic materials, see ‘Methods' section). Specifically, clusters of ∼2,500 Ar atoms can be generated and charged, then accelerated onto the surface (for example, with 2.5–5 keV). Upon reaching the surface, the cluster decomposes, dividing its kinetic energy among all the Ar atoms, that is, each atom carries ∼1–2 eV kinetic energy. Consequently, these atoms can only remove surface atoms and do not disturb underlying bulk atoms of the rather fragile MOF structure31. After each sputtering cycle, XPS data (Ni2p3/2, C1s, N1s and O1s peaks) are recorded on the sample post-loaded with EDA right after the gas exposure measurement (orange line in Fig. 2a) and after sputtering at 2.5 keV for 28 min (blue line in Fig. 2a), and 5 keV for 20 min (brown line in Fig. 2a). Since the MOF contains only O, Ni and C atoms, a comparison of the N1s core level (N is only contained in EDA) with O1s, Ni2p3/2, and C1s, provides information on the depth distribution of EDA. While sputtering at 2.5 keV (∼1 eV per Ar atom) for 28 min only partially removes EDA (N1s signal), sputtering with 5 keV (∼ 2 eV per Ar atom) for 20 min fully removes nitrogen. The remaining minor feature in the N1s spectral region is due to plasmon oscillations of K2s of the KBr substrate (Supplementary Fig. 5). The oxygen and Ni signals remain essentially unchanged. The initial decrease of the C signal is associated with the removal of adventitious hydrocarbons physisorbed on the MOF surface. Note that the intensities of N, O and Ni increase slightly after the initial sputtering as screening by adventitious carbon is removed. Thereafter, the C1s, O1s and Ni2p3/2 signals remain constant. The shoulder at 853.6 eV in the Ni2p3/2 peak after removal of EDA is tentatively attributed to surface reconstruction of the Ni corner atoms due to displacement (perturbation) of surface atoms. Importantly, all the above observations clearly point to the localization of EDA at the periphery (surface region) of the MOF microcrystals.
Figure 2. XPS and LEIS characterization of EDA monolayer layer and diffusion profiles of CO molecules.
(a) XPS spectra of Ni-MOF-74 with post-loaded EDA, before (orange curve) and after being sputtered at 2.5 keV for 28 min (blue curve) and 5 keV for 20 min (dark green curve). (b) LEIS spectra of Ni-MOF-74 with post-loaded EDA, after gentile sputtering with a dose of 3.2 × 1015 and 2.2 × 1016 cm−2 Ne+ ions. A slight red shift of the peaks after the second sputtering (10 eV corrected in the Figure), most likely resulting from sample charging due to the ion exposure and insulating nature of the MOFs, has been corrected. (c) Energy barrier for the diffusion of a CO molecule along the 1D channel of Ni-MOF-74. Red circles: all the metal centers are saturated with CO (Supplementary Fig. 7). Black diamonds: all the metal centers are saturated with EDA molecules. The inset in c shows the relaxed atomic position of a CO molecule at the middle of the Ni-MOF-74 channel, where all the adsorption metal sites have been saturated with EDA molecules. The dashed red box shows the CO molecule. Black, red, white, grey and blue spheres represent C, O, H, N and Ni atoms, respectively.
To further verify the localization of EDA at the periphery of the microcrystals, we performed low-energy ion scattering (LEIS) measurements of EDA-pretreated MOF powders. The ultra-shallow penetration depth of this technique (∼1 nm) makes it particularly sensitive to elements at the surface. The spectra are recorded with 3 keV He+ ions, and sputtering is performed with 5 keV Ne+ ions. Figure 2b shows that, after removing adventitious carbon with a dose of 3.2 × 1015 cm−2 Ne+ ions, there is a clear peak associated with N at ∼950 eV in addition to the O peak at 1,100 eV (Supplementary Fig. 6). The N peak has two components: a surface peak at 960 eV and a subsurface peak at 940 eV, the latter being attributed to EDA at grain boundaries or on tilted surfaces. The surface peak completely disappears after a dose of 2.2 × 1016 cm−2 Ne+ ions, confirming that it is located only at the surface well within 1 nm. Additional sputtering does not appreciably change the relative intensity of the N signature, confirming that it originates from EDA at grain boundaries or tilted surfaces. Together, the XPS and LEIS measurements indicate that EDA forms a monolayer (<1 nm thick) at the surface of the MOF microcrystals.
This knowledge makes it possible to model the EDA arrangement within the Ni-MOF-74 unit cell using ab initio calculations. We find that the structure shown in Supplementary Fig. 7a is the most stable and that the binding energy per EDA molecule increases from 125 kJ mol−1 for ∼0.17 EDA per Ni2+ (1 EDA per unit cell) to 141 kJ mol−1 for 1 EDA per Ni2+ (1 EDA per metal centre, that is, saturation). This stabilization of aggregated EDA molecules arises from H bonding of the head amine groups (that is, those pointing to the centre of the unit cell, not strongly bonded to the metal centres), as detailed in Supplementary Fig. 7a. These findings are consistent with previous ab initio calculations performed in Mg-MOF-74 in which the binding energy was found to increase monotonically with loading from 95 kJ mol−1 at ∼0.17 EDA per Mg2+ to 125 kJ mol−1 1 EDA per Mg2+ (ref. 12). There is thus a significant energy benefit to form a complete layer due to EDA clustering and we conclude that a full EDA layer is completed within the first unit cell of the MOF. Once the top surface (<1 nm) is sealed with a complete layer, further EDA diffusion is not possible due to severe steric constraints. Note that other alkyl amine molecules (for example, trimethylenediamine, n-propylamine, ethanolamine) are less effective than EDA molecules to retain small molecule CO (Supplementary Note 3 and Supplementary Fig. 8). This further indicates that the head NH2 groups are crucial to aggregate EDA into a complete layer. We further model the diffusion of the CO molecules through the longitudinal channels of Ni-MOF-74, as described in Supplementary Note 4 (Supplementary Fig. 9 and Supplementary Movies 1 and 2). The results (Fig. 2c) show that the CO diffusion barrier increases from 0.028 eV for a CO-loaded MOF to 0.68 eV for MOF with a monolayer of EDA, that is, a 24-times increase, which is consistent with our experimental observations.
While CO is clearly trapped at room temperature, the removal of CO can be completed by mild annealing up to 100 °C under vacuum (pressure<20 mTorr) and EDA remains mostly unperturbed (Fig. 1). The effect of EDA on CO re-adsorption can now be examined (Supplementary Note 5), using the same loading conditions (∼40 Torr). Supplementary Figure 10 shows that the CO uptake is dramatically reduced compared with the pristine activated MOF-74 (EDA-free), taking over 45 min to reach only ∼25% of the CO loading obtained in pristine MOF-74 loaded in ∼30 min.
Extension to other small molecules
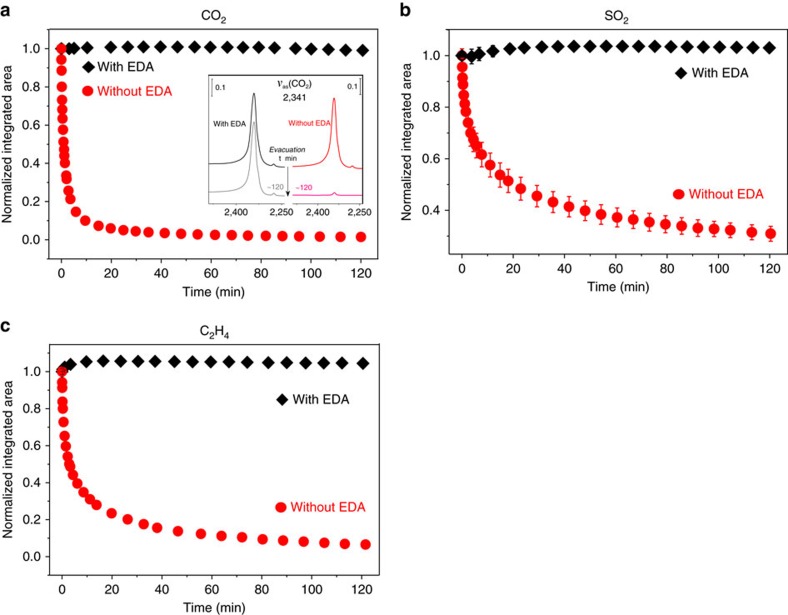
To test whether EDA acts as a cap in general, we have used this method with other small molecules (CO2, SO2 and C2H4, see Supplementary Note 6 and Supplementary Figs 11–14) that are also weakly bonded in MOF-74 and rapidly diffuse out at room temperature. As shown in Fig. 3 for Ni-MOF-74 and Supplementary Fig. 15 for Zn, Co-MOF-74, we find that EDA again provides an effective barrier to retain those molecules. Furthermore, the same method was successfully applied to other MOFs structure such as HKUST-1 (ref. 32; Supplementary Figs 16 and 17) to trap CO2 and NO, the latter being an active biological molecule6.
Figure 3. Time evolution of the vibrational bands νas(CO2) νas(SO2) and δ(CH2) upon evacuation.
(a) Normalized integrated area of CO2 asymmetric stretching mode νas upon evacuation (<20 mTorr) in pristine (red circles) and EDA post-loaded (black diamonds). The spectral evolution of νas(CO2) bands is shown in Supplementary Fig. 11. The error bar of normalized integrated area does not exceed ∼0.025 for the intensity determination of the νas(CO2) band. The inset of a shows the spectra of νas(CO2) band at t=0 and ∼120 min. (b) Normalized integrated areas of SO2 asymmetric stretching mode νas upon evacuation (<20 mTorr) in pristine (red circles) and EDA post-loaded (black diamonds), The spectral evolution of the νas(SO2) is shown in Supplementary Fig. 12. The larger error bar in b for the νas(SO2) band was due to interferences of the MOF phonon bands, leading to uncertainties in determining the baseline in the difference spectra (Supplementary Fig. 12c). (c) Normalized integrated areas of C2H4 wagering mode δ upon evacuation (<20 mTorr) in pristine (red circles) and EDA post-loaded (black diamonds). The spectral evolution of δ(CH2) bands is shown in Supplementary Fig. 13. The error bar of normalized integrated area does not exceed ∼0.025 for the intensity determination of the δ(CH2) bands. For the pristine sample, the initial point (that is, t=0) is chosen as the peak intensity after evacuation of gas phase (CO2, SO2, C2H4) for ∼10 s; for MOFs post-loaded with EDA, the starting point is still after gas removal at the end of EDA exposure (that is, t=0); the integrated areas are normalized to the maximum value obtained at t=0.
Exposure to water vapour and release of adsorbed molecules
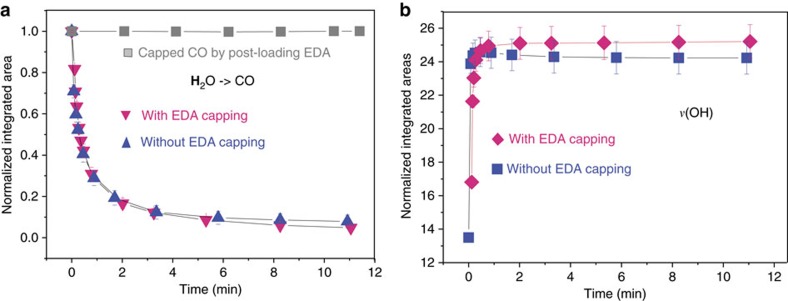
The most striking result was obtained with water molecules, chosen because they can form hydrogen bonds with amine groups: water was observed to pass through the EDA layer without any hindrance and was able to remove pre-adsorbed CO completely. The experiment was started by capping CO molecules in MOFs under 40 Torr by growing an EDA layer via vapour-phase deposition as shown in Fig. 1. After evacuation for ∼1.5 h (that is, CO still retained), 8 Torr vapour-phase H2O was introduced into the cell and infrared spectra recorded as a function of time. Figure 4a (and Supplementary Fig. 18) clearly shows that the adsorbed CO peak dramatically weakens while the water stretching band ν(OH) quickly strengthens. Clearly, water molecules diffuse into the MOF channel and force the pre-adsorbed CO molecules out through the EDA layer, still present as evidenced by its characteristics ν(C–N) band at 1,020 cm−1. To quantify the rate of water penetration, the same experiment was performed without EDA capping. CO molecules were loaded into Ni-MOF-74 at 40 Torr for ∼30 min. Followed by a quick evacuation (<3 s), 8 Torr H2O was introduced into the cell (Supplementary Fig. 18). Figure 4b shows that there is no measurable difference in the intensity decrease of ν(CO) and increase of ν(H2O) between pristine and EDA-capped MOF, as though the EDA layer did not exist. The dramatically different behaviour of water compared with other gases is tentatively attributed to the ability of water to interact with –NH2 through H bonding.
Figure 4. Time evolution of the intensities of the ν(CO) and ν(OH) bands.
(a) ν(CO) band decay after exposure to 8 Torr H2O vapour in pristine (blue triangle) and EDA-capped (pink triangle) Ni-MOF-74. The grey square curve represents the ν(CO) band evolution in EDA-capped Ni-MOF-74 under vacuum (<20 mTorr) The error bar of the sharp ν(CO) band does not exceed 0.04. (b) Water ν(OH) band increase in pristine (blue square) and EDA-capped (pink diamond) Ni-MOF-74 at ∼8 Torr vapour phase. The error bar of the ν(H2O) broad band is larger due to uncertainties in determining the baseline in the difference spectra (Supplementary Fig. 18).
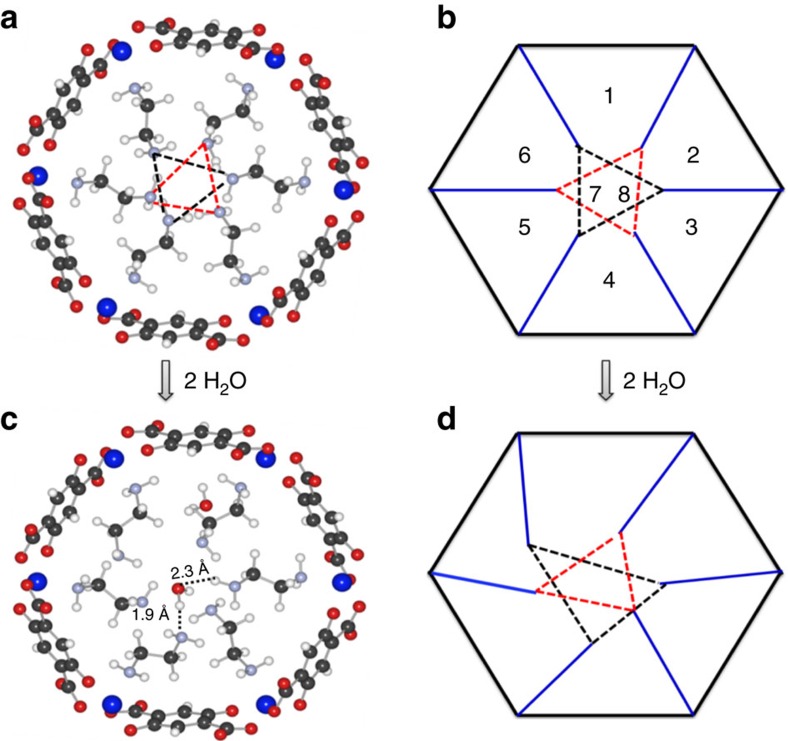
To examine this hypothesis, we have investigated the perturbation of the EDA adsorption geometry upon adding water molecules by ab initio calculations. When MOF channels are fully loaded with EDA molecules, the –NH2 head groups of the adsorbed EDA molecules point towards the centre of the MOF represented as blue lines in Fig. 5b. There are six –NH2 divided into two sets, each one of them making an imaginary triangle with N atoms at the apex in the middle of the channel, see the black and red triangles. These two triangles are located in planes parallel to the page, but not in the same plane. There are six adsorption sites for water near the linkers (1–6), and two in the middle of the channel (7 and 8). We observe in Fig. 5c,d that the addition of H2O molecules clearly enlarges the triangles. For instance, two water molecules placed at sites 1 and 7 increase the area of the triangle by tilting the –C–C– and –C–N– bond angles of several EDA molecules away from the centre of the channel (Fig. 5d and Supplementary Fig. 19). These water adsorption states are energetically favourable (Supplementary Fig. 20) since water molecules establish the hydrogen bonding with –NH2 group, evidenced by the short H…N or H…O distance (Fig. 5c). By continually adding water molecules up to 4 and 6, the area of these triangles in most cases becomes significantly larger (Supplementary Note 7 and Supplementary Figs 19 and 21), enabling water molecules more easily to enter through the channel. This ‘gate opening mechanism' also works for other MOF structures, leading for instance to the removal of NO molecules from within HKUST-1 by water exposure. (Supplementary Fig. 22).
Figure 5. Relaxed atomic position of EDA molecules inside Ni-MOF-74 channel.
Black, red, white, grey and blue spheres represent C, O, H, N and Ni atoms, respectively. (a) Configuration of 6 EDA molecules before loading 2 H2O molecules. (b) Structural scheme of the Ni-MOF-74 loaded with the 6 EDA molecules, which are represented as blue lines. The numbers in the figure represent eight possible adsorption sites for the H2O molecules. (c) Configuration of EDA molecules after adding 2 H2O molecules into site 1 and 8. (d) Perturbation of triangle areas (empty space) induced by loading 2 H2O molecules into site 1 and 7.
Discussion
We have demonstrated, combining in situ Fourier transform infrared (FTIR) and ab initio simulations, that small molecules such as CO, CO2, SO2 and C2H4 can be efficiently trapped inside the pores of the MOF-74 system simply by introducing EDA vapour at the end of the loading process. This method avoids the need to perform complex modification of the MOF to increase the internal binding energies for each gas molecule. The EDA molecules are able to block the release of several small molecules from the 1D channels of MOF-74 due to their propensity to agglomerate and organize themselves into a hydrogen-bonded network within the outermost unit cell. This generic approach is also applicable to other MOFs structures and overcomes the limitation of weak interactions between guest molecules and the porous materials. We have further shown that water molecules can easily penetrate this molecular EDA barrier layer and displace previously trapped gas, thus providing a novel method to release trapped gases at room temperature. The understanding of this selective EDA membrane, derived from combined in situ measurements and ab initio calculations, opens up new avenues for gas storage, delivery and separation, and suggests new applications for molecular membranes.
Methods
Synthesis of MOFs samples
The MOFs samples including Ni-MOF-74 Co-MOF-74, Zn-MOF-74 and HKUST-1 are synthesized by following the modified procedure (Supplementary Methods) from refs 32, 33. The crystal structures are confirmed by comparing XRD pattern (Supplementary Fig. 23) and Raman spectra (Supplementary Fig. 24 and Supplementary Note 8) with literature report. After thorough solvent exchange as described in Supplementary Methods, the surface areas reach 913, 1,077, 774 m2 g−1 for Ni-MOF-74, Co-MOF-74, Zn-MOF-74 (ref. 34), respectively, consistent with the values reported in the original literature33.
In situ infrared spectroscopy
All infrared spectroscopic data presented are taken by using a Nicolet 6700 FTIR spectrometer (purchased from Thermo Scientific Inc., USA) equipped with a liquid N2 -cooled mercury cadmium telluride MCT-A detector. A high-pressure cell, purchased from Specac Ltd., UK (product number P/N 5850c), is placed in the sample compartment of the infrared spectrometer with the sample at the focal point of the beam. The MOFs (powder, ∼2 to ∼5 mg) are gently pressed onto a KBr pellet (∼1 cm diameter, 1–2 mm thick) and placed in the high-pressure cell. The cell is connected to different gas lines (EDA vapour, NH3, CO, CO2, SO2, CH2CH2 and so on) for exposure and a vacuum line for evacuation. A pre-chamber is installed close to the cell to mix EDA vapour with other gases (see the diagram in Supplementary Fig. 25). The samples are then activated by evacuation (base pressure<20 mTorr) at 180 °C for at least 3 h and then cooled back to room temperature for gas exposure measurements. All spectra are recorded in transmission mode from 650 cm−1 (MCT-A) to 4,000 cm−1 (4 cm−1 spectral resolution).
X-ray photoelectron spectroscopy and gas cluster sputtering
X-ray photoelectron measurements were performed in conjunction with gas cluster ion beams, initially developed in the late 90's (ref. 35). The principle for sputtering with individual Ar atoms has been well described36. GCIS is particularly attractive to gently remove the top layers of fragile organic materials37,38,39,40. It has been used in conjunction with XPS to explore the depth distribution of atoms41. When standard Ar+ sputtering is used (∼1 keV per Ar+ ion), there is considerable perturbation of the MOF with substantial preferential removal of O and C relative to Ni (not shown), which makes it impossible to determine the location of EDA. Therefore, Ar GCIS is used in removing the surface EDA molecules on MOFs sample. A large cluster (∼2,500 Ar atoms) is generated and charged by removal of 1 electron, then accelerated by a 2.5 or 5 keV potential difference. Upon impact, the kinetic energy of the cluster is distributed among all Ar atoms (that is, ∼1 or 2 eV per atom), which is insufficient to penetrate into the MOF, limiting the sputtering to surface species only. The incidence angle of the cluster is 45°, the bombarded area is 1 × 1 mm2, and the sample is rotated at a rate of 0.2 r.p.m. for 5–15 min and then 0.5 r.p.m. for 2 min to achieve a uniform sputtering. All the data were recorded with charge compensation. For XPS measurements, the MOFs pellet used for infrared measurements is taped on the puck with double sided tape. A Al kα monochromated source is used with a beam size of 200 × 200 μm2. Spectra are recorded at a 45° takeoff angle with respect to the surface. The base pressure is typically below 4 × 10−8 Pa and the Ar pressure during the sputtering is 2 × 10−6 Torr.
Low-energy ion scattering
LEIS measurements are performed using a Qtac analyzer (IonTOF Gmbh, Münster, Germany) using 3 keV He+ and 5 keV Ne+ as the probe and sputtering ions, respectively. The He+ current used for the measurements is ∼4 nA, and the Ne+ sputtering current is ∼11 nA. The instrument employs a double-toroidal analyzer that collects all ions scattered within an angular range of 144–146° and images them according to their energy onto a position sensitive detector. Samples for LEIS are prepared by pressing the EDA-pretreated MOF powders into a tungsten mesh and mounting the mesh onto an SiO2/Si wafer. A 1.5 × 1.5 mm2 sample area is analyzed. Ne+ sputtering is performed using the LEIS ion gun, and thus the ions impinge at normal incidence on the sample, unlike the conventional 45° sputtering geometry.
Ab initio calculations
Ab initio calculations are performed at the density functional theory level, as implemented in Quantum Espresso42. To correctly capture the crucial van der Waals interaction between the MOF and the guest molecules, we use the non-local functional vdW-DF (refs 43, 44, 45, 46). Ultra-soft pseudopotentials are used with cutoffs of 544 and 5,440 eV for the wave functions and charge density, respectively. Due to the large dimensions of the unit cell, only the Γ-point is sampled. To model the diffussion process we use a transition-state search algorithm, that is, the climbing-image nudged-elastic band method47,48. This method is chosen because it finds the lowest-energy pathway between an initial and final state, which may well deviate from a straight line (that is, linear interpolation) between the two. Furthermore, this method allows us to obtain a clear picture of the interaction between the CO molecule and the EDA molecules blocking the pores, which cannot easily be obtained by other methods such as ab initio molecular dynamics. We start from the experimental rhombohedral structure of Ni-MOF-74 with 54 atoms in its primitive cell and space group 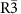 . The description through hexagonal axes is a=b=25.719 Å and c=6.741 Å (ref. 49), and α=β=90° and γ=120°. We optimized all atomic positions until the forces are <2.6 × 10−4 eV Å−1.
. The description through hexagonal axes is a=b=25.719 Å and c=6.741 Å (ref. 49), and α=β=90° and γ=120°. We optimized all atomic positions until the forces are <2.6 × 10−4 eV Å−1.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Additional information
How to cite this article: Tan, K. et al. Trapping gases in metal-organic frameworks with a selective surface molecular barrier layer. Nat. Commun. 7, 13871 doi: 10.1038/ncomms13871 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures, Supplementary Table, Supplementary Notes, Supplementary Methods and Supplementary References.
CO molecule diffusing through Ni-MOF-74 channel where the metal centers are saturated with other CO molecules
CO molecule diffusing through Ni-MOF-74 channel where the metal centers are saturated with ethylenediamine (EDA) molecules
Acknowledgments
The synthesis, spectroscopic characterization, and modelling performed at Rutgers, UT Dallas, and Wake Forest University were supported in full by the Department of Energy, Basic Energy Sciences, division of Materials Sciences and Engineering (DOE Grant No. DE-FG02-08ER46491). In addition, modelling used resources of the Oak Ridge Leadership Computing Facility at Oak Ridge National Laboratory, which is supported by the Office of Science of the Department of Energy under Contract DE–AC05–00OR22725.
Footnotes
Author contributions K.T. carried out all the infrared spectroscopic measurements. J.-F.V. performed XPS measurement. E.M took the LEIS measurement. H.W. prepared all the MOFs samples, guided by J.L. S.Z. carried out all the calculations. E.F. provided support for infrared data analysis. Y.J.C. and T.T. directed and supervised the characterization and modelling research, respectively.
References
- Ferey G. Hybrid porous solids: past, present future. Chem. Soc. Rev. 37, 191–214 (2008). [DOI] [PubMed] [Google Scholar]
- Suh M. P., Park H. J., Prasad T. K. & Lim D.-W. Hydrogen storage in metal–organic frameworks. Chem. Rev. 112, 782–835 (2011). [DOI] [PubMed] [Google Scholar]
- Li J.-R., Sculley J. & Zhou H.-C. Metal–organic frameworks for separations. Chem. Rev. 112, 869–932 (2011). [DOI] [PubMed] [Google Scholar]
- Sumida K. et al. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 112, 724–781 (2011). [DOI] [PubMed] [Google Scholar]
- Kuppler R. J. et al. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 253, 3042–3066 (2009). [Google Scholar]
- Horcajada P. et al. Metal–organic frameworks in biomedicine. Chem. Rev. 112, 1232–1268 (2011). [DOI] [PubMed] [Google Scholar]
- Cohen S. M. Postsynthetic methods for the functionalization of metal–organic frameworks. Chem. Rev. 112, 970–1000 (2012). [DOI] [PubMed] [Google Scholar]
- Mulfort K. L. & Hupp J. T. Alkali metal cation effects on hydrogen uptake and binding in metal-organic frameworks. Inorg. Chem. 47, 7936–7938 (2008). [DOI] [PubMed] [Google Scholar]
- Liu H. et al. Ligand functionalization and its effect on CO2 adsorption in microporous metal–organic frameworks. Chem. Asian J. 8, 778–785 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang W., Hu Y., Ge J., Jiang H.-L. & Yu S.-H. A facile and general coating approach to moisture/water-resistant metal–organic frameworks with intact porosity. J. Am. Chem. Soc. 136, 16978–16981 (2014). [DOI] [PubMed] [Google Scholar]
- Fernandez C. A. et al. Hydrophobic and moisture-stable metal-organic frameworks. Dalton Trans. 44, 13490–13497 (2015). [DOI] [PubMed] [Google Scholar]
- Choi S., Watanabe T., Bae T.-H., Sholl D. S. & Jones C. W. Modification of the Mg/DOBDC MOF with amines to enhance CO2 adsorption from ultradilute gases. J. Phys. Chem. Lett. 3, 1136–1141 (2012). [DOI] [PubMed] [Google Scholar]
- McDonald T. M. et al. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal–organic framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 134, 7056–7065 (2012). [DOI] [PubMed] [Google Scholar]
- Hwang Y. K. et al. Amine grafting on coordinatively unsaturated metal centers of MOFs: consequences for catalysis and metal encapsulation. Angew. Chem. Int. Ed. Engl. 47, 4144–4148 (2008). [DOI] [PubMed] [Google Scholar]
- Lee W. R. et al. Diamine-functionalized metal-organic framework: exceptionally high CO2 capacities from ambient air and flue gas, ultrafast CO2 uptake rate, and adsorption mechanism. Energy Environ. Sci. 7, 744–751 (2014). [Google Scholar]
- Andirova D., Lei Y., Zhao X. & Choi S. Functionalization of metal–organic frameworks for enhanced stability under humid carbon dioxide capture conditions. ChemSusChem. 8, 3405–3409 (2015). [DOI] [PubMed] [Google Scholar]
- Dietzel P. D. C. et al. Adsorption properties and structure of CO2 adsorbed on open coordination sites of metal-organic framework Ni2(dhtp) from gas adsorption, IR spectroscopy and X-ray diffraction. Chem. Commun. 5125–5127 (2008). [DOI] [PubMed] [Google Scholar]
- Wu H., Zhou W. & Yildirim T. High-capacity methane storage in metal−organic frameworks M2(dhtp): the important role of open metal sites. J. Am. Chem. Soc. 131, 4995–5000 (2009). [DOI] [PubMed] [Google Scholar]
- Liu Y., Kabbour H., Brown C. M., Neumann D. A. & Ahn C. C. Increasing the density of adsorbed hydrogen with coordinatively unsaturated metal centers in metal−organic frameworks. Langmuir 24, 4772–4777 (2008). [DOI] [PubMed] [Google Scholar]
- Tan K. et al. Competitive coadsorption of CO2 with H2O, NH3, SO2, NO, NO2, N2, O2, and CH4 in M-MOF-74 (M=Mg, Co, Ni): the role of hydrogen bonding. Chem. Mater. 27, 2203–2217 (2015). [Google Scholar]
- Bonino F. et al. Local structure of CPO-27-Ni metallorganic framework upon dehydration and coordination of NO. Chem. Mater. 20, 4957–4968 (2008). [Google Scholar]
- Canepa P., Nijem N., Chabal Y. J. & Thonhauser T. Diffusion of small molecules in metal organic framework materials. Phys. Rev. Lett. 110, 026102 (2013). [DOI] [PubMed] [Google Scholar]
- Nijem N. et al. Molecular hydrogen ‘pairing' interaction in a metal organic framework system with unsaturated metal centers (MOF-74). J. Am. Chem. Soc. 132, 14834–14848 (2010). [DOI] [PubMed] [Google Scholar]
- Nijem N. et al. Spectroscopic characterization of van der Waals interactions in a metal organic framework with unsaturated metal centers: MOF-74–Mg. J. Phys. Condens. Matter 24, 424203 (2012). [DOI] [PubMed] [Google Scholar]
- Garrone E. & Otero Arean C. Variable temperature infrared spectroscopy: a convenient tool for studying the thermodynamics of weak solid–gas interactions. Chem. Soc. Rev. 34, 846–857 (2005). [DOI] [PubMed] [Google Scholar]
- Vimont A. et al. Investigation of acid sites in a zeotypic giant pores chromium(iii) carboxylate. J. Am. Chem. Soc. 128, 3218–3227 (2006). [DOI] [PubMed] [Google Scholar]
- Hadjiivanov K. I. & Vayssilov G. N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 47, 307–511 (2002). [Google Scholar]
- Bloch E. D. et al. Reversible CO binding enables tunable CO/H2 and CO/N2 separations in metal–organic frameworks with exposed divalent metal cations. J. Am. Chem. Soc. 136, 10752–10761 (2014). [DOI] [PubMed] [Google Scholar]
- Chavan S. et al. Response of CPO-27-Ni towards CO, N2 and C2H4. Phys. Chem. Chem. Phys. 11, 9811–9822 (2009). [DOI] [PubMed] [Google Scholar]
- Chopra T. P. et al. Ethylenediamine grafting on oxide-free H-, 1/3 ML F-, and Cl-terminated Si(111) surfaces. Chem. Mater. 27, 6268–6281 (2015). [Google Scholar]
- Seah M. P., Havelund R., Shard A. G. & Gilmore I. S. Sputtering yields for mixtures of organic materials using argon gas cluster ions. J. Phys. Chem. B. 119, 13433–13439 (2015). [DOI] [PubMed] [Google Scholar]
- Chui, S. S. -Y. Lo, S. M. -F., Charmant J. P. H., Orpen A. G. & Williams I. D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 283, 1148–1150 (1999). [DOI] [PubMed] [Google Scholar]
- Caskey S. R., Wong-Foy A. G. & Matzger A. J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 130, 10870–10871 (2008). [DOI] [PubMed] [Google Scholar]
- Tan K. et al. Water reaction mechanism in metal organic frameworks with coordinatively unsaturated metal ions: MOF-74. Chem. Mater. 26, 6886–6895 (2014). [Google Scholar]
- Yamada I. Novel materials processing and applications. by gas cluster ion beams. Eur. Phys. J. D. 9, 55–61 (1999). [Google Scholar]
- Yamada I., Matsuo J., Toyoda N. & Kirkpatrick A. Materials processing by gas cluster ion beams. Mater. Sci. Eng. R-Rep. 34, 231–295 (2001). [Google Scholar]
- Seah M. P., Havelund R., Shard A. G. & Gilmore I. S. Sputtering yields for mixtures of organic materials using argon gas cluster ions. J. Phys. Chem. B 119, 13433–13439 (2015). [DOI] [PubMed] [Google Scholar]
- Seah M. P., Spencer S. J. & Shard A. G. Angle dependence of argon gas cluster sputtering yields for organic materials. J. Phys. Chem. B 119, 3297–3303 (2015). [DOI] [PubMed] [Google Scholar]
- Chernysh V. S., Ieshkin A. E. & Ermakov Y. A. The new mechanism of sputtering with cluster ion beams. Appl. Surf. Sci. 326, 285–288 (2015). [Google Scholar]
- Cristaudo V., Poleunis C., Czerwinski B. & Delcorte A. Ar cluster sputtering of polymers: effects of cluster size and molecular weights. Surf. Interface Anal. 46, 79–82 (2014). [Google Scholar]
- Yun D.-J. et al. Study on the molecular distribution of organic composite films by combining photoemission spectroscopy with argon gas cluster ion beam sputtering. J. Mater. Chem. C 3, 276–282 (2015). [Google Scholar]
- Paolo G. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009). [DOI] [PubMed] [Google Scholar]
- Thonhauser T. et al. Spin signature of nonlocal correlation binding in metal-organic frameworks. Phys. Rev. Lett. 115, 136402 (2015). [DOI] [PubMed] [Google Scholar]
- Langreth D. C. et al. A density functional for sparse matter. J. Phys. Condens. Matter 21, 084203 (2009). [DOI] [PubMed] [Google Scholar]
- Thonhauser T. et al. Van der Waals density functional: self-consistent potential and the nature of the van der Waals bond. Phys. Rev. B 76, 125112 (2007). [Google Scholar]
- Berland K. et al. van der Waals forces in density functional theory: a review of the vdW-DF method. Rep. Prog. Phys. 78, 066501 (2015). [DOI] [PubMed] [Google Scholar]
- Henkelman G. & Jónsson H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000). [Google Scholar]
- Henkelman G., Uberuaga B. P. & Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000). [Google Scholar]
- Zhou W., Wu H. & Yildirim T. Enhanced H2 adsorption in isostructural metal−organic frameworks with open metal sites: strong dependence of the binding strength on metal ions. J. Am. Chem. Soc. 130, 15268–15269 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures, Supplementary Table, Supplementary Notes, Supplementary Methods and Supplementary References.
CO molecule diffusing through Ni-MOF-74 channel where the metal centers are saturated with other CO molecules
CO molecule diffusing through Ni-MOF-74 channel where the metal centers are saturated with ethylenediamine (EDA) molecules
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.