Abstract
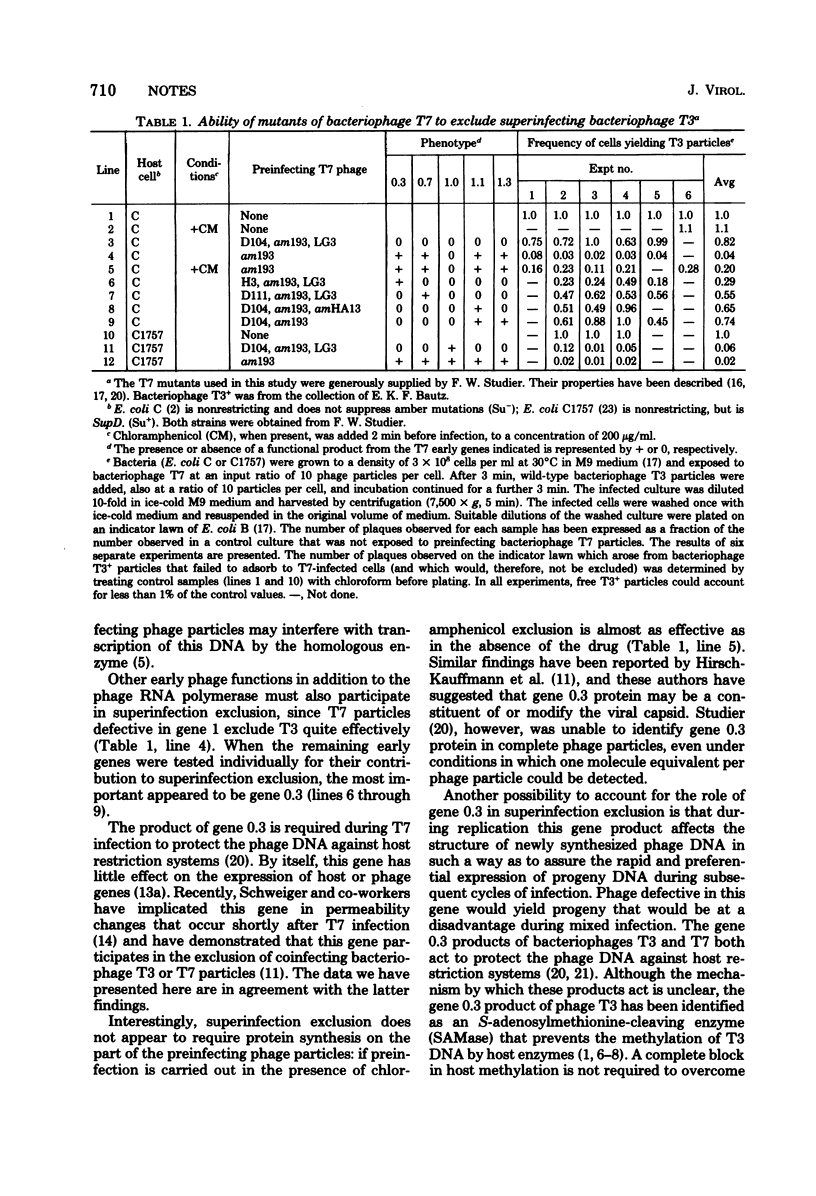
Only two of the early genes of bacteriophage T7 were found to play a significant role in exclusion of superinfecting bacteriophage T3 particles; genes 0.3 and 1. Protein synthesis by the preinfecting phage particle was not required for efficient exclusion. These findings are discussed with regard to the known functions of these genes during T7 development.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G., WEIGLE J. J. Host controlled variation in bacterial viruses. J Bacteriol. 1953 Feb;65(2):113–121. doi: 10.1128/jb.65.2.113-121.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Hausmann R. Genetic map of bacteriophage T3. J Virol. 1973 Aug;12(2):417–419. doi: 10.1128/jvi.12.2.417-419.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunovskis I., Summers W. C. The process of infection with coliphage 17. VI. A phage gene controlling shutoff of host RNA synthesis. Virology. 1972 Nov;50(2):322–327. doi: 10.1016/0042-6822(72)90383-2. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Bautz F. A., Bautz E. K. Different template specificities of phage T3 and T7 RNA polymerases. Nat New Biol. 1971 Mar 17;230(11):94–96. doi: 10.1038/newbio230094a0. [DOI] [PubMed] [Google Scholar]
- GOLD M., HAUSMANN R., MAITRA U., HURWITZ J. THE ENZYMATIC METHYLATION OF RNA AND DNA. 8. EFFECTS OF BACTERIOPHAGE INFECTION ON THE ACTIVITY OF THE METHYLATING ENZYMES. Proc Natl Acad Sci U S A. 1964 Aug;52:292–297. doi: 10.1073/pnas.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M., Hausmann R., Gold M., Hurwitz J. The enzymatic methylation of ribonucleic acid and deoxyribonucleic acid. X. Bacteriophage T3-induced S-adenosylmethionine cleavage. J Biol Chem. 1966 May 10;241(9):1995–2006. [PubMed] [Google Scholar]
- Hausmann R. Synthesis of an S-adenosylmethionine-cleaving enzyme in T3-infected Escherichia coli and its disturbance by co-infection with enzymatically incompetent bacteriophage. J Virol. 1967 Feb;1(1):57–63. doi: 10.1128/jvi.1.1.57-63.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Kauffmann M., Pfenning-Yeh M., Ponta H., Herrlich P., Schweiger M. A virus-specified mechanism for the prevention of multiple infection--T7- and T3-mutual and superinfection exclusion. Mol Gen Genet. 1976 Dec 22;149(3):243–249. doi: 10.1007/BF00268524. [DOI] [PubMed] [Google Scholar]
- Hyman R. W. Physical mapping of T7 messenger RNA. J Mol Biol. 1971 Oct 28;61(2):369–376. doi: 10.1016/0022-2836(71)90386-x. [DOI] [PubMed] [Google Scholar]
- Krueger D. H., Presber W., Hansen S., Rosenthal H. A. Biological functions of the bacteriophage T3 SAMase gene. J Virol. 1975 Aug;16(2):453–455. doi: 10.1128/jvi.16.2.453-455.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister W. T., Barrett C. L. Roles of the early genes of bacteriophage T7 in shutoff of host macromolecular synthesis. J Virol. 1977 Sep;23(3):543–553. doi: 10.1128/jvi.23.3.543-553.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H., Altendorf K. H., Schweiger M., Hirsch-Kaufmann M., Pfennig-Yeh M. L., Herrlich P. E. coli membranes become permeable to ions following T7-virus-infection. Mol Gen Genet. 1976 Dec 8;149(2):145–150. doi: 10.1007/BF00332882. [DOI] [PubMed] [Google Scholar]
- Simon M. N., Studier F. W. Physical mapping of the early region of bacteriophage T7 DNA. J Mol Biol. 1973 Sep 15;79(2):249–265. doi: 10.1016/0022-2836(73)90004-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic analysis of non-essential bacteriophage T7 genes. J Mol Biol. 1973 Sep 15;79(2):227–236. doi: 10.1016/0022-2836(73)90002-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Movva N. R. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J Virol. 1976 Jul;19(1):136–145. doi: 10.1128/jvi.19.1.136-145.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Siegel R. B. Transcription of late phage RNA by T7 RNA polymerase. Nature. 1970 Dec 19;228(5277):1160–1162. doi: 10.1038/2281160a0. [DOI] [PubMed] [Google Scholar]
- Sunshine M. G., Thorn M., Gibbs W., Calendar R., Kelly B. P2 phage amber mutants: characterization by use of a polarity suppressor. Virology. 1971 Dec;46(3):691–702. doi: 10.1016/0042-6822(71)90071-7. [DOI] [PubMed] [Google Scholar]



