Abstract
Radioactivity released from disasters like Chernobyl and Fukushima is a global hazard and a threat to exposed biota. To minimize the deleterious effects of stressors organisms adopt various strategies. Plants, for example, may delay germination or stay dormant during stressful periods. However, an intense stress may halt germination or heavily affect various developmental stages and select for life history changes. Here, we test for the consequence of exposure to ionizing radiation on plant development. We conducted a common garden experiment in an uncontaminated greenhouse using 660 seeds originating from 33 wild carrots (Daucus carota) collected near the Chernobyl nuclear power plant. These maternal plants had been exposed to radiation levels that varied by three orders of magnitude. We found strong negative effects of elevated radiation on the timing and rates of seed germination. In addition, later stages of development and the timing of emergence of consecutive leaves were delayed by exposure to radiation. We hypothesize that low quality of resources stored in seeds, damaged DNA, or both, delayed development and halted germination of seeds from plants exposed to elevated levels of ionizing radiation. We propose that high levels of spatial heterogeneity in background radiation may hamper adaptive life history responses.
Classical life history theory predicts that organisms will allocate resources to different life history traits, characteristics that affect ontogeny, reproduction and survival, to optimize overall fitness1. Life history traits are shaped by an interaction between extrinsic ecological factors affecting fitness, and intrinsic genetic constraints2. Understanding how the environment affects growth, survival, reproduction, and hence fitness, allows for prediction of the evolution of life history strategies3. However, environmental heterogeneity, even on a very local scale, generates heterogeneous fitness optima and affects intra-population variation in life histories4. Thus environmental heterogeneity can maintain variation in characters and reveal trade-offs between life history traits5.
A life history trade-off occurs when an increase in fitness due to a change in one trait is opposed by a decrease in fitness caused by another, functionally linked trait6. While trade-offs in life history characteristics play crucial roles in shaping evolutionary trajectories, it is often unclear why trade-offs persist. A possible mechanism maintaining polymorphism in life histories is spatial heterogeneity that provides an opportunity for maintaining different optima in fitness-related traits with decreasing predictability of environmental variation5. Environmental heterogeneity can promote different combinations of life history traits and generate trade-offs even at spatially local scales4. Consequently, a single optimal life history strategy might be challenged7. A recent study suggests that even among close relatives the trade-off between growth and maintenance can be mediated by an increased level of oxidative stress8, the imbalance between production of oxygen species during normal physiological processes and the level of antioxidant defence8.
In plants trade-offs are often related to resource limitation that constrains expenditure on defence against herbivores and other stressors, or that can be allocated to growth and development9. This has potential consequences for adaptive and plastic responses. For example, contamination, such as found in Chernobyl and Fukushima10,11, can affect both growth rate and survival12, as exposure to even low, but chronic ionizing radiation can cause increased oxidative damage and decreases in antioxidant defences13. In the Chernobyl area, which is contaminated mainly by caesium-137, developmental instability was found in three plant species that showed a gradual increase in fluctuating asymmetry and frequency of phenodeviants with increasing radiation14. Moreover, oxidative stress and ionizing radiation has been linked to a variety of detrimental effects13, including elevated mutation rates15 and aberrations in the development of pollen16, and has been shown to affect gene expression and epigenetic regulation of responses to stress during plant ontogeny17. Experimental investigations of rice in the Fukushima region revealed strong transcriptomic effects through activation of genes involved in DNA repair and of defence/stress responses following exposure of seedlings to radiation in contaminated fields18,19. The activation of DNA repair and anti-stress mechanisms suggested that resources in seedlings were reallocated from development to defence pathways, although the authors did not provide any measures of developmental costs18. The hypothesis that plant development can indeed be constrained in contaminated areas due to radiation exposure20 comes from research showing that Scots pine Pinus sylvestris growth has decreased after the nuclear disaster in Chernobyl21. Intriguingly, the response in tree growth was also influenced by other environmental and phenotypic traits suggesting a complex interaction between life-history traits related to developmental and environmental stress caused by ionizing radiation21. Nonetheless, experimental tests using common garden approaches are required to verify that exposure to chronic ionizing radiation are indeed responsible for the effects observed in wild populations.
The Chernobyl nuclear accident (26 April, 1986) contaminated vast areas of Europe, but parts of Ukraine, Belarus and Russia were particularly hard hit11,22. Even within the Chernobyl Exclusion Zone, the limited-entry area around the power station, deposition of radionuclides was highly heterogeneous, resulting in levels of ionizing radiation that varied by five orders of magnitude within relatively short distances of a few hundred meters. Such heterogeneity in radiation levels can cause intra-population variability in selection, potentially preventing the evolution of adaptations to mitigate the elevated oxidative stress caused by ionizing radiation23 depending on the amounts of gene flow among metapopulaitons. Here, we conducted controlled common garden experiment under benign green house conditions for wild plants that originated from sites that differed in exposure to radioactive contamination by a factor of almost 400 (Table S2). We tested for the importance of exposure to radioactive contamination of maternal plants on development of the following generation, from germination of seeds until the development of several leaves in young plants. We investigated growth rate between several distinct developmental stages to test if the level of exposure to radioactive contamination, and stored resources accumulated in seeds, affected life history characteristics and potential trade-offs between them. In plants, the seedling stage is often a demographic bottleneck in a species’ life history being particularly vulnerable to resource limitations and herbivore attacks24. Embryos of wild carrots Daucus carota, subsp. carota, the model species studied here, develop slowly over several weeks25, thus being vulnerable to environmental stress early during the developmental period26. Carrot seedlings usually develop in autumn, and they are subsequently vernalized during winter to induce flowering the next summer27. Consequently, we hypothesized that seed germination would be particularly susceptible to the negative effects of radiation and that subsequent developmental stages would be affected by the previous ones. Additionally, we expected that measurable impacts of ionizing radiation would decrease at later developmental stages, partly because sensitive individuals would be purged from the population during the previous developmental periods (i.e. purifying selection), with older plants being less sensitive to radiation as a consequence of this selection.
Results
Radioactive exposure levels
Radiation levels measured with a hand-held dosimeter were strongly positively correlated with measures of exposure from soil samples with gamma dosimetry (r = 0.93, p < 0.0001). Also, gamma radiation measurements indicated marked accumulation of radionuclides in carrot roots and above ground parts (Table S1), and significantly correlated with results from a hand-held dosimeter (root: r = 0.63, p = 0.002; above ground plant parts: r = 0.56, p = 0.009; Figure S1). Gamma radiation measurements of soil, roots and plants were also strongly positively correlated (soil-root: r = 0.68, soil-plant: r = 0.63 and root-plant: r = 0.83, p < 0.002). Radiation levels at locations of seed sampling, as measured with a hand-held dosimeter, varied from 0.08 to 30.2 μGy/h with mean and median of 4.83 and 0.50 μGy/h, respectively (Table S2). Estimated exposure to ground contamination varied from 30.9 to 11648.6 kBq/m2 (with mean and median of 1862.9 and 192.9 kBq/m2) for Cs137, the main radionuclide in the area. Seed mass varied between 0.38 and 2.22 mg with mean and median of 1.04 and 1.06 mg, respectively. Seed mass was not affected by radiation level received by the maternal plant (Pearson partial correlation controlled for the identity of maternal plants, r = −0.04, p = 0.28; Table 1).
Table 1. Mixed model results of the effects of radioactive contamination (Radiation) and seed mass (and earlier stages of development) on various progressive developmental stages of wild carrots collected as seeds from the Chernobyl Exclusion Zone (see Table S3 for results for emergence time of leaves 1, 3–5 and 7).
| Dependent variables | Model Log-Likelihood | Effect | Estimate | SE | value | pLLRT# |
|---|---|---|---|---|---|---|
| Germination | −273.3 | Radiation | −1.2321 | 0.001 | −949.7 | 0.0019 |
| Germination time | −436.1 | Radiation | 0.0773 | 0.023 | 3.37 | 0.0011 |
| Seed mass | 0.2867 | 0.129 | 2.22 | 0.026 | ||
| 2nd leaf emergence | −917.3 | 1st leaf | 0.2173 | 0.418 | 0.52 | NA |
| Cotyledon | 1.5753 | 0.360 | 4.37 | NA | ||
| Radiation | 0.2711 | 0.052 | 5.17 | NA | ||
| Seed mass | 0.3576 | 0.270 | 1.32 | NA | ||
| 1st l. * Rad. | −0.2180 | 0.040 | −5.47 | <0.00001*** | ||
| 1st l. * Mass | −1.3547 | 0.398 | −3.40 | 0.0202 | ||
| Cot. * Mass | 1.2002 | 0.349 | 3.43 | 0.0139 | ||
| 6th leaf emergence | −802.0 | 1st leaf | −0.1867 | 0.066 | −2.82 | NA |
| 4th leaf | 0.3833 | 0.131 | 2.92 | 0.009 | ||
| 5th leaf | 1.7129 | 0.119 | 14.35 | <0.00001*** | ||
| Radiation | −0.3048 | 0.087 | −3.50 | NA | ||
| 1st l. * Rad. | 0.2355 | 0.069 | 3.44 | 0.0007** |
Values - “z” for Germination model assuming logit link function, “t” for the rest models assuming log link function. NA - part of higher-order interaction, LLRT skipping term. Terms significant after applying Bonferroni corrections for multiple separate tests for 7 leaves: ***<0.00001, **0.00001–0.01. #Interactions with pLLRT > 0.04 were progressively excluded from presented model.
Germination success and time
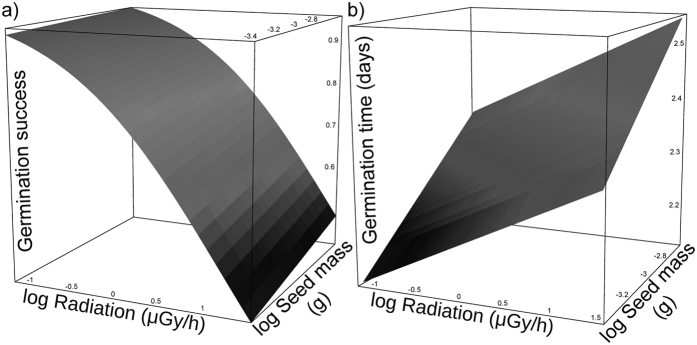
Of the 660 seeds collected from 33 maternal plants (20 seeds per plant), 503 (76.2%) germinated successfully. One plant germinated no seeds at all and five germinated the complete set of 20 seeds planted (Table S2). The probability of germination was only affected by the level of radioactive contamination at the location that the maternal plants were collected (z = −949.7, p = 0.0019), while it was not significantly affected by seed mass or its interaction with radiation (interaction was removed from final model, separate model indicated: z = 0.620, p = 0.8; Table 1, Fig. 1a; see also supplementary information: Table S3). Germination success was on average 39% higher (interquartile range) for seeds from the 1st than from the 3rd quartile of the radioactivity distribution (Pearson partial correlation between radiation and germination, controlling for common mother of seeds: r = −0.20, p < 0.0001). Both the level of radioactive contamination (z = 3.37, p = 0.0011) and seed mass (z = 2.22, p = 0.026) affected number of days required for germination (Table 1, Fig. 1b). Seeds originating from plants exposed to higher levels of radioactive contamination (r = 0.21, p < 0.0001), and heavier seeds (r = 0.10, p = 0.022), germinated later than seeds originating from less contaminated areas, and from lighter seeds. The interaction between seed mass and radiation was non-significant.
Figure 1.
Effects of radioactive contamination (Radiation) and seed mass on early stages of development: (a) germination probability and (b) germination time in carrots from the Chernobyl Exclusion Zone grown in an uncontaminated greenhouse, in common garden experiment.
Development of cotyledons
Of the 503 seedlings, 490 developed cotyledons (97.4%). The probability of cotyledon emergence was significantly related to germination time (z = 58.73, p < 0.0001; Table 1). Plants that germinated earlier (r = 0.85, p < 0.00001) also developed cotyledons faster. The level of radiation correlated with the time to cotyledon emergence (r = 0.19, p = 0.00002), but the effect of radiation did not significantly influence the time to cotyledon emergence or seed mass in a mixed model (z = 1.68, p = 0.09; Table S3).
Development of leaves
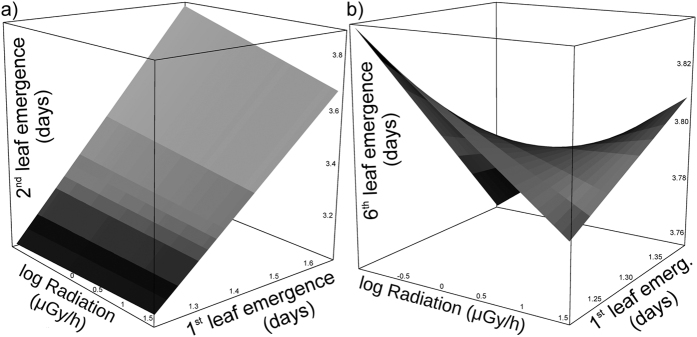
At the date the experiment was terminated (10 December 2015), the following number of plants developed the following number of leaves: 1st leaf was developed by 482 plants, 2nd by 481, 3rd by 472, 4th by 460, 5th by 444, 6th by 372, 7th by 199, 8th by 81, 9th by 39, 10th by 26, 11th by 12, 12th by 4 and 13th by 4. Seed mass did not significantly affect the emergence of leaves (p > 0.05), except for the 1st leaf, where it interacted with the germination time and cotyledon emergence time (Table 1 and S3). When including the level of development of previous stages in the models, the level of radioactive contamination always showed a significant effect (except for 1st and 3rd leaves), either as a main effect (for 5th leaf) or in interaction with previous developmental stages (for leaves 2, 4, 6 and 7; Table 1, Table S3, Fig. 2). Even when considering the non-independence of multiple tests for 7 developed adult leaves, Bonferroni-corrected tests were significant for radiation level for leaves 2, 4, 5 and 6. The significant correlation between radiation level and the emergence time of adult leaves progressively became weaker with developmental stage (1st leaf: r = 0.21, p < 0.00001, 2nd leaf: r = 0.17, p = 0.0002, 3rd leaf: r = 0.13, p = 0.004, 4th leaf: r = 0.11, p = 0.017) until reaching low and non-significant levels (5th leaf: r = 0.03, p = 0.51, 6th leaf: r = 0.01, p = 0.84, 7th leaf: r = −0.08, p = 0.49; Fig. 3). However, the direct effects of radiation were masked by higher-order interactions in most mixed models (Table 1, Fig. 2).
Figure 2.
Effects of interactions between radioactive contamination (Radiation) and earlier stage of development (time of development of the 1st leaf) on the time of development of 2nd (a) and 6th (b) leaves in carrots from the Chernobyl Exclusion Zone grown in an uncontaminated greenhouse, in common garden experiment.
Figure 3. Partial Pearson correlations (accounting for common mother affiliation of seeds) between developmental stages and radioactive contamination (Radiation), seed mass, early (Germination time) and previous stages (Previous stage) on subsequent developmental stages.
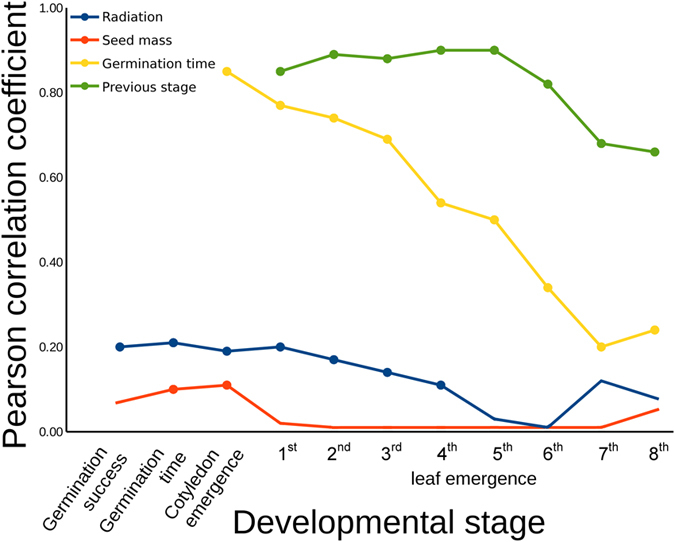
Dots indicate significant correlations. Correlations between Radiation and Germination are presented as absolute values (it was negative and indicated seeds from highly radioactive locations have low probability of germination).
Differences between developmental stages
Seeds from mothers exposed to, on average, lower levels of radiation germinated better (means for undeveloped and developed: xundeveloped ± SE = 7.65 ± 0.79, xdeveloped = 3.95 ± 0.38; t = −8.50, p < 0.0001), and developed better between 2nd and 3rd (xundeveloped = 12.84 ± 4.75, xdeveloped = 3.83 ± 0.38; t = −2.72, p = 0.007) and between 3rd and 4th leaf developmental stages (xundeveloped = 9.33 ± 3.82, xdeveloped = 3.68 ± 0.38; t = −1.97, p = 0.049). Significant differences between cumulative averages of plants that did not develop and those that developed persisted until the 7th leaf (t ≤ −2.85, p ≤ 0.0046). Also smaller seeds germinated better (xundeveloped = 0.00112 ± 0.00003, xdeveloped = 0.00102 ± 0.00001; t = −2.87, p = 0.004), and tended to have higher probability of developing cotyledons (xundeveloped = 0.00122 ± 0.00012, xdeveloped = 0.00102 ± 0.00001; t = −1.94, p = 0.052).
Discussion
We found that chronic exposure to ionizing radiation impacted early-stage development of wild plants by reducing the ability of seeds to germinate or delaying their development. Thus, maternal radiation exposure had statistically significant negative impacts on the probability of seed germination (Fig. 1a). Moreover, time of germination was also delayed as a consequence of elevated radiation exposures (Fig. 1b). We did not find a correlation between seed mass and radioactivity suggesting that pericarp (fruit wall) and endosperm developed normally in plants from contaminated locations, as these structures constitute almost the entire mass of the fruit. However, a relatively long embryonic development in carrots25 is a critical step in which exposure to stressful conditions is usually the most damaging and it may negatively impact complete plant development. Indeed, the results of our common garden experiment indicated that early stages of development of embryos (and their germination) are directly and negatively affected by the level of radioactive contamination at the site where maternal plants grew and developed seeds (Fig. 1; Table 1). As expected, such early development also had strong negative effects on later stages of ontogeny of adult leaves (Fig. 2), with likely consequences for overall performance of adult plants14,21. Moreover, strong negative effects of radioactive contamination early during development may result in selective mortality before later stages and adulthood28,29. Detecting strong effects of radiation on early stages of ontogeny may have important outcomes for studies aiming to test the consequence of ionizing radiation focused only on adult individuals21,30. These findings are particularly important as they suggest that previous studies conducted mostly on adults31 have likely underestimated radiation effects compared to those that include consequences for earlier stages of development32 and across generations.
Our results also suggest that radiation can impact life history trade-offs related to growth rate and ontogeny of distinct life stages33. We showed significant variability among individuals in vulnerability to radioactive contamination as some plants are able to develop faster and experience earlier emergence of particular ontogenetic stages, at least during early life, while others are not. However, individuals that develop slower and have later emergence of particular ontogenetic stages can either have slower or faster development of the next leaf, as a response to radioactivity by previous developmental stage interactions (Fig. 2a). Such an effect suggests the fast development might cause trade-offs in ontogeny under stressful conditions34, resulting in a complex distribution of leaf emergence times observed in our experiment (Fig. 2b). Therefore, we hypothesize that development-radiation interactions can hamper fitness optimization in the Chernobyl area. This is because ionizing radiation can cause spatially variable levels of oxidative stress, where protective mechanisms would compete for resources with other life history traits to a variable extent, depending on the level of local exposure12. Therefore, radiation in general decreases fitness, but the timing of ontogenetic stages are modified by the environment5 and are highly dependent on the timing of early ontogeny.
The observed response to radiation can be both genetically adaptive35,36 and phenotypically plastic37. The common garden design such as that employed here can be used to detect genetically based differences in phenotypes among populations. In our case, because early seed development occurs on the maternal plants, which were exposed to varying environmental conditions, we are unable to test for genetic differences. Such tests would require multi-generational experiments under common garden conditions38 overcoming across generation maternal effects, persistent even after environmental specific effects are removed39. Therefore, our findings relate to the direct effects of radiation on developing seeds as expressed by germination responses, and the effects of maternal genotype, phenotype and environment (e.g. radiation exposure) on developing seeds and their interaction effects on developing seedlings.
In conclusion, in our experiment we found that maternal exposure to ionizing radiation had persistent negative effects on the ontogeny of progeny. The effect seems to be strongest during early development (i.e. germination), and persists as a complex developmental trajectory that depends on the timing of early ontogenetic stages. We hypothesize that low quality of resources stored in seeds, damaged DNA, epigenetic factors or their combination may delay development, and in some cases completely halt germination in seeds from plants exposed to elevated ionizing radiation. Multigenerational common garden and transplant experiments will be necessary to determine if the first generation variation observed under common garden conditions has a genetic basis (i.e. reflects the effects of natural selection) and has any adaptive significance. Moreover, as wild carrots accumulate substantial amounts of radionuclides in tissues, this shows great promise as a useful model system for investigating the genetic, ecological and evolutionary consequences of long-term radioactive contamination in the Chernobyl region.
Material and Methods
Study design
Wild carrots (Daucus carota subsp. carota, Apiaceae) were used for this study. This species is a common weed throughout temperate regions of the world. For the experiment, seeds were collected from a population growing at abandoned field (51°21′N, 30°0′E, September 2012) located 7.5 km SW of the destroyed reactor 4 of the Chernobyl nuclear power plant11,40, an area characterized by very local heterogeneous levels of radioactive contamination. This local scale of variation of exposure to radioactive contamination is not visible on the general overview maps (e.g. http://radatlas.isgeo.com.ua/). The only way to depict such local variation is conducting localized hand dosimetry measurements. Radiation measurements of the soil were made exactly under focal plants to estimate exposure of particular plants to radioactive particles using hand-held dosimeters placed on the ground (Inspector, International Medcom, Sebastopol, CA, USA) and calibrated to measure Gray (Gy). The ground level measurement of α, β, and γ radiation with a hand-held dosimeter provide highly reliable field measurements of level of radiation11, and in plants it can be related to both external and internal potential dose exposures (Figure S1). The radioactive exposure per unit time (μGy/h), rather than cumulative dose exposure, was used as predictor in all analyses to avoid artificial inflation of exposure differences between parental plants caused by spurious estimation of unknown vegetative period of a particular plant. Prior to the common garden (same environment) experiment in the greenhouse, collected seeds were weighed in groups of 5, and this total mass was divided by five to obtain individual mean seed mass. We randomly selected 660 seeds originating from 33 maternal plants (20 seeds per plant) collected across a radiation gradient, and these seeds were subsequently planted. Seeds were placed on the soil surface in individual pots after an initial 3-day cold treatment at 5 °C, simulating natural conditions prior to germination. Pots were randomly distributed and randomized daily by mixing the position of at least 30 pots to homogenize germination/growing conditions. The greenhouse was equipped with an automatic water vaporiser, and thus humidity (but not temperature) was controlled throughout the experiment, starting from 5 October 2015. The greenhouse was inspected daily (excluding Sundays) until 10 December 2015 and dates of emergence of subsequent developmental stages were recorded: emergence of radicle (indicator and timing of successful germination), cotyledons and subsequent adult leaves, respectively.
Dosimetry
Samples from 21 plants were used to estimate accumulation of radionuclides in soil, root and plants above ground parts, using gamma spectrometry. Activity of 137Cs, in 63 soil samples (3 per location of sampled carrots), 21 above ground parts of carrots and 21 carrot roots, was measured with the Berkeley Nucleonics SAM 940 radionuclide identifier system equipped with a 10 × 10 × 10 cm NaI detector. This detector was chosen because of its very high efficiency allowing for very rapid measurements of gamma emitters. The detector was enclosed in 5–10 cm thick lead shielding (about 400 kg) to reduce the noise from background radioactivity. Given the high sensitivity and large amounts of shielding, we are able to accurately measure radioactivity levels to less than 1 or 2 bq per sample (i.e. calculated errors are typically around 0.5 bq). The system was calibrated with reference standard sources. Samples of soil, and entire roots and plants were measured separately for 120 s (soil) and 180 s (roots and plants, longer measures up to 900 s gave similar results). With corrections for laboratory background, the activity of 137Cs was evaluated. Samples were weighed prior to measurements on a Radan electronic balance and individual mass was used to standardize radioactivity across samples. Estimations of exposure to ground contamination for Cs137 were recalculated from soil dose-rates41.
Statistical analyses
Generalized linear mixed model analyses were used to test for effects of contamination level and seed mass on germination success and time, and time of subsequent developmental stages. The identity of the maternal plant was included as a random effect in all models to control for non-independence of related seeds. Separate models were tested for germination rate, time of germination, and time of emergence of cotyledons and subsequent adult leaves. Due to the large number of tested iterations, data sets with a large number of observations (>100, until 7th leaf) were included in the analyses. The first model included a binary dependent variable describing germination of seeds, or lack there-of. Subsequent models included time of development of a particular stage since seeding as the dependent variable. Models for a given developmental stage were built hierarchically to include information about previous stages. Basic initial models included seed mass, radiation level at the collection location, and interaction between these variables, and for models other than germination success and time of the previous developmental stages and the interaction between those and seed mass and radiation level, respectively. For statistical tests a non-linear mixed model procedure was used in the R (version 3.2.3) package “lme4” with the glmer function and Laplace approximation (although Adaptive Gauss-Hermite Quadrature, nAGQ = 50, gave similar results)42. A bimodal distribution with logit link function was applied to analyse germination success, and a Gaussian with log link function (lognormal) was used for all developmental time analyses. All independent variables were log transformed prior to analysis to reduce the kurtosis of the distribution of radiation and seed mass. Statistical significance of the effects, and backward model reductions (selection of fixed effects), were performed by log likelihood ratio test with the “LMERConvenienceFunctions” R package. Differences in mean radiation and seed mass of individuals surviving between developmental stages were also tested.
Additional Information
How to cite this article: Boratyński, Z. et al. Ionizing radiation from Chernobyl affects development of wild carrot plants. Sci. Rep. 6, 39282; doi: 10.1038/srep39282 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We gratefully acknowledge the help and support of Gennadi Milinevsky, Laura Martínez-Rodríguez, Margarida Isabel Oliveira Barros and Ricardo Guerreiro during field work and greenhouse experiment, and Nikolaos Evangeliou for help in calculations of exposure to ground contamination (http://radio.nilu.no). The study was financially supported by the Academy of Finland to TM (Grant No. 268670). AJMP and ZB were funded by the Portuguese Foundation for Science and Technology (FCT: RH/BPD/84822/2012 and SFRH/BPD/111015/2015). CG was supported by the Portuguese Foundation for Science and Technology (FCT) through the Investigador Programme and two research grants (FCT-ANR/BIA-BIC/0010/2013 and PTDC/BIA-BIC/5223/2014) co-funded by the European Program COMPETE (FCOMP-01-0124-FEDER-019772), and by the Project “Genomics and Evolutionary Biology” cofinanced by North Portugal Regional Operational Programme 2007/2013 (ON.2 – O Novo Norte), under the National Strategic Reference Framework (NSRF), through the European Regional Development Fund (ERDF). MP was supported by the Polish National Science Centre (grant no. 2015/18/E/NZ8/00716). Additional support to TAM and APM came from the Samuel Freeman Charitable Trust, the CNRS (France), the American Council of Learned Societies, and the University of South Carolina College of Arts and Sciences.
Footnotes
Author Contributions All authors planned the study. Z.B., T.M., T.A.M. and A.M. participated in field work. Z.B., J.M.A., A.J.M.P., C.G.P. and M.P. conducted greenhouse experiment. Z.B. and A.P.M. prepared dataset and conducted statistical analyses. E.T. collected dosimetry measures and calculated dose exposure levels. All authors prepared the final version of the manuscript.
References
- Roff D. A. R. Life history evolution (Sinauer, Sunderland, 2002). [Google Scholar]
- Stearns S. C. Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87, 476–486 (2000). [DOI] [PubMed] [Google Scholar]
- Smith S. A. & Donoghue M. J. Rates of molecular evolution are linked to life history in flowering plants. Science. 322, 86–89 (2008). [DOI] [PubMed] [Google Scholar]
- Prati D. & Schmid B. Genetic differentiation of life-history traits within populations of the clonal plant Ranunculus reptans. Oikos 90, 442–456 (2000). [Google Scholar]
- Hereford J. A quantitative survey of local adaptation and fitness trade-offs. Am. Nat. 173, 579–88 (2009). [DOI] [PubMed] [Google Scholar]
- Roff D. A. & Fairbairn D. J. The evolution of trade-offs: where are we? J. Evol. Biol. 20, 433–447 (2007). [DOI] [PubMed] [Google Scholar]
- Stearns S. C. Trade-offs in life-history evolution. Funct. Ecol. 3, 259 (1989). [Google Scholar]
- Monaghan P., Metcalfe N. B. & Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92 (2009). [DOI] [PubMed] [Google Scholar]
- Lind E. M. et al. Life-history constraints in grassland plant species: a growth-defence trade-off is the norm. Ecol. Lett. 16, 513–521 (2013). [DOI] [PubMed] [Google Scholar]
- Nakanishi T. M. & Tanoi K. Agricultural implications of the Fukushima nuclear accident. (Springer: Japan, Tokyo, 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau T. A. & Møller A. P. Genetic and ecological studies of animals in Chernobyl and Fukushima. J. Hered. 105, 704–709 (2014). [DOI] [PubMed] [Google Scholar]
- Boubriak I. I. et al. Adaptation and impairment of DNA repair function in pollen of Betula verrucosa and seeds of Oenothera biennis from differently radionuclide-contaminated sites of Chernobyl. Ann. Bot. 101, 267–76 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einor D., Bonisoli-Alquati A., Costantini D., Mousseau T. A. & Møller A. P. Ionizing radiation, antioxidant response and oxidative damage: A meta-analysis. Sci. Total Environ. 548–549, 463–471 (2016). [DOI] [PubMed] [Google Scholar]
- Møller A. P. Developmental instability of plants and radiation from Chernobyl. Oikos 81, 444 (1998). [Google Scholar]
- Møller A. P. & Mousseau T. A. Strong effects of ionizing radiation from Chernobyl on mutation rates. Sci. Rep. 5, 8363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller P. C. The effects of radiation on pollen grain development, differentiation, and germination. Proc. R. Soc. Edinburgh Sect. B. Biol. 61, 398–429 (1943). [Google Scholar]
- Sidler C., Li D., Kovalchuk O. & Kovalchuk I. Development-dependent expression of DNA repair genes and epigenetic regulators in Arabidopsis plants exposed to ionizing radiation. Radiat. Res. 183, 219–32 (2015). [DOI] [PubMed] [Google Scholar]
- Hayashi G. et al. Unraveling low-level gamma radiation-responsive changes in expression of early and late genes in leaves of rice seedlings at Iitate village, Fukushima. J. Hered. 105, 723–738 (2014). [DOI] [PubMed] [Google Scholar]
- Hayashi G. et al. 2D-DIGE-based proteome expression changes in leaves of rice seedlings exposed to low-level gamma radiation at Iitate village, Fukushima. Plant Signal. Behav. 10, e1103406 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow A. H., Cuany R. L., Miksche J. P. & Schairer L. A. Some factors affecting the responses of plants to acute and chronic radiation exposures. Radiat. Bot. 1, 10–34 (1961). [Google Scholar]
- Mousseau T. A. et al. Tree rings reveal extent of exposure to ionizing radiation in Scots pine Pinus sylvestris. Trees 27, 1443–1453 (2013). [Google Scholar]
- Hinton T. G. et al. Radiation-induced effects on plants and animals: findings of the United Nations Chernobyl Forum. Health Phys. 93, 427–40 (2007). [DOI] [PubMed] [Google Scholar]
- Galván I. et al. Chronic exposure to low-dose radiation at Chernobyl favours adaptation to oxidative stress in birds. Funct. Ecol. 28, 1387–1403 (2014). [Google Scholar]
- Leck M. A. & Simpson R. L. V. T. P. In Seedling Ecology and Evolution (Leck M. A. & Simpson R. L., P. V. T.) 3–13 (Cambridge University Press, 2008). [Google Scholar]
- Gray D., Ward J. A. & Steckel J. R. A. Endosperm and embryo development in Daucus carota L. J. Exp. Bot. 35, 459–465 (1984). [Google Scholar]
- Nascimento W. M., Huber D. J. & Cantliffe D. J. Carrot seed germination and respiration at high temperature in response to seed maturity and priming. Seed Sci. Technol. 41, 164–169 (2013). [Google Scholar]
- Lacey E. P. The genetic and environmental control of reproductive timing in a short-lived monocarpic species Daucus carota (Umbelliferae). J. Ecol. 74, 73–86 (1986). [Google Scholar]
- Møller A. P. Developmental selection against developmentally unstable offspring and sexual selection. J. Theor. Biol. 185, 415–422 (1997). [Google Scholar]
- Stearns S. C. In Evolution of Sex and its Consequences (Stearns S. C. ed.) 55, 337–349 (Birkhäuser, Basle, Switzerland, 1987). [Google Scholar]
- Woodwell G. M. & Miller L. N. Chronic gamma radiation affects the distribution of radial increment in Pinus rigida stems. Science. 139, 222–223 (1963). [DOI] [PubMed] [Google Scholar]
- Møller A. P. & Mousseau T. A. The effects of natural variation in background radioactivity on humans, animals and other organisms. Biol. Rev. Camb. Philos. Soc. 88, 226–54 (2013). [DOI] [PubMed] [Google Scholar]
- Monaghan P. Early growth conditions, phenotypic development and environmental change. Philos. Trans. R. Soc. Lond. B 363, 1635–1645 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. E., Blount J. D., Forbes S. & Royle N. J. Does oxidative stress mediate the trade-off between growth and self-maintenance in structured families? Funct. Ecol. 24, 365–373 (2010). [Google Scholar]
- Coley P. D. Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia 74, 531–536 (1988). [DOI] [PubMed] [Google Scholar]
- Kovalchuk I., Abramov V., Pogribny I. & Kovalchuk O. Molecular aspects of plant adaptation to life in the Chernobyl zone. Plant Physiol. 135, 357–63 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk O., Dubrova Y. E., Arkhipov A., Hohn B. & Kovalchuk I. Wheat mutation rate after Chernobyl. Nature 407, 583–4 (2000). [DOI] [PubMed] [Google Scholar]
- Sultan S. E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 5, 537–542 (2000). [DOI] [PubMed] [Google Scholar]
- Mousseau T. A. Intra- and interpopulation genetic variation: Explaining the past and predicting the future. In Adaptive Genetic Variation in the Wild (Mousseau T. A., Sinervo B. & Endler J. A. eds) pp. 219–250 (Oxford University Press, 2000). [Google Scholar]
- Mousseau T. A. & Fox C. W.. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 (1998). [DOI] [PubMed] [Google Scholar]
- Boratyński Z., Lehmann P., Mappes T., Mousseau T. A. & Møller A. P. Increased radiation from Chernobyl decreases the expression of red colouration in natural populations of bank voles (Myodes glareolus). Sci. Rep. 4, 7141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangeliou N. et al. Reconstructing the Chernobyl Nuclear Power Plant (CNPP) accident 30 years after. A unique database of air concentration and deposition measurements over Europe. Environ. Pollut. (2016). [DOI] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B. & Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 1–51 (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.