SUMMARY
Purpose
In the context of the transcrestal maxillary sinus lift a wide variety of biomaterials have been used to fill the subantral space over the years. In this study, two types of biomaterials were used in order to fill the maxillary sinus: a nano-crystallized hydroxyapatite in an aqueous solution and a micronized heterologous bone in a collagen matrix.
Materials and methods
The surgical procedures were designed and carried out using computer-guided surgery. The filling volume obtained was measured with a comparative software program.
Results
A ≥ 6 millimeter augmentation of osseous volume was obtained. This result is comparable to those obtained in lifts where conventional techniques were applied. The technique used was very precise and the difference between the projected and clinical outcome of the implant position had an average of less than 0.3 millimeters.
Conclusions
This technique allows for the surgery to be performed in a way which is both minimally traumatic and invasive, and represents a viable alternative to those surgical techniques for crestal sinus lift currently in use.
Keywords: Schneiderian, computer guided surgery, lift, sinus, maxillary, graft, membrane
Introduction
When there is increased pneumatisation of the maxillary sinus various surgical lifting techniques of the maxillary sinus floor lift are required.
These techniques involve filling part of the maxillary sinus with biocompatible material which, after some months, due to reabsorption and remodelling processes are transformed into bone, resulting in adequate bone volume for implant-prosthesis.
When large cavity fillings are required, it is generally necessary to resort to vestibular approaches to the maxillary sinus.
The crestal approach is increasingly employed where small quantities of biomaterial are required to fill the sinus cavity. In these cases it is generally possible to insert the implants immediately. These ‘mini lifts’ are therefore advisable when the initial osseous thickness is between 5 and 6 millimetres (1).
Over the years, numerous maxillary sinus lift techniques using a crestal approach have been described in the literature (2).
A transcrestal hydraulic lift technique, recently proposed, has been used in this study. A specially made injector, named ML, linked to a micrometric piston, named ML easy, allows for the elevation of the membrane and the filling of the sinus cavity simultaneously (3, 4).
One of the most frequent reasons for failure during a maxillary sinus floor lift operation is connected to the possibility of a rupture of the Schneiderian membrane which, if lacerated, cannot perform the function of graft containment. For this reason, it is important to choose smooth biomaterials in order to avoid damaging the membrane (2–4).
In order to reduce the incidence of complications, it is necessary to cut the hard tissue with extreme accuracy, and as little trauma as possible, while saving the soft tissue.
The precision of pre-operation measurements obtained through endoral X-rays, dental-scans and cone-beam CT allows us to approach and cut the sinus cortical floor with delicacy.
With the advent of computer-guided surgery, it is possible to design and carry out minimally traumatic and invasive procedures that allow for the filling of the maxillary sinus via a crestal approach, which is precisely calibrated, thus reducing the risk of failure (5–10).
In this study, using a fluido-dynamic technique of transcrestal maxillary sinus lift performed with the aid of computer guided surgery, two different biomaterials in two different clinical cases are used: a nano-crystallized hydroxyapatite in an aqueous solution and a micronized heterologous bone in a collagen matrix.
The cortical of the maxillary sinus is reduced through the use of calibrated burs and a profiler so as to obtain a hole that enables both access to the maxillary sinus and, subsequently, the lifting of the Schneiderian membrane.
In order to facilitate the detachment of the membrane, a gel form of hyaluronic acid was also used, a viscous material, with reepitalization properties.
Ballini et al. (11) suggested that autologous bone combined with an esterified low-molecular HA preparation seems to have good capabilities in accelerating new bone formation in infrabony defects (11–17).
Each stage in the operation was monitored and all the devices used passed through a custom-made template, which acts as a surgical guide. The sinuses were filled using two different fluid biomaterial distributed through a dispenser, which had been created specifically for the technique.
Due to the reduction in trauma and the fact that the process is much less invasive, this technique could be a valid alternative to the techniques known and used to date.
Work time is reduced in the cortical thinning operation and percussive trauma is avoided, while it is important to fill the sinus slowly and with care, in the cases described, the filling process took three minutes to fill the sinus with both biomaterials by a specific micrometric device.
The volume of the sinus to be filled was calculated using a specific software programme that, in addition to an accurate volume measurement, allows for an accurate comparison between the projected and clinical outcome of the implant position.
The aim of this study is to compare the volumetric measurement and the behaviour of two different biomaterials used as a sinus filler using a fluido-dynamic technique for transcrestal maxillary sinus lift performed with the aid of computer-guided surgery that allows for a precise reduction in the cortical thickness without entering the sinus.
Materials and methods
In case study number 1, the operation field was prepared using hyaluronic acid, which was delivered in a nebulized form using a patented device (Yabro spray sol, IBSA, Italy). The cortical of the maxillary sinus was reduced through the use of calibrated burs and a profiler that allows for an opening to be created to give access to the maxillary sinus and, subsequently, the lifting of the Schneiderian membrane can be achieved (FMD Rome, Italy). In case number 1, the detachment and lifting was achieved using HA gel and micronized heterologous bone in an 80% collagen matrix whose granulometry was less than 300 microns (Putty by Osteobiol-Tecnoss, Italy). While in the second case, a nano-crystallized hydroxyapatite in an aqueous solution (Nanogel, TEKNIMED, France) was used.
Each stage in the operative process was monitored and all the devices used passed through a custom-made template, which acted as a surgical guide (BIONOVA, La Spezia, Italy). Fluid biomaterials, distributed through a dispenser, which had been created specifically for this technique, were used to fill the sinus.
Case study
Two cases with similar clinical situations were treated and have been described in detail below to illustrate the method used. Both patients were not-smokers, with no systemic disease, nor on regular prescribed medication. Both patients were periodontally treated. The first patient was a healthy, 55-year-old male whose first upper right molar had been extracted 6 months previous and showed approx. four-millimetre residual bone height with a horizontal thickness of nine millimetres. The second was a 60-year-old male whose second upper left premolar had been extracted 6 months previous and showed approx. 8-millimetre residual bone height with a horizontal thickness of seven millimetres. Neither patients showed signs of inflammation of the sinus membrane.
Two different dental surgeons performed the operations. The preoperative planning was carried out in the following phases:
Two high accuracy addition silicone impressions were taken. During the cone beam CT (GENDEX GXCB-500) scan, there was a universal stent (BIONOVA, La Spezia, Italy), with three spheres on which 8 thousands landmarks were detected by the software, which was fixed in the mouth of the patient with the use of an impression material (impregum, ESPE).
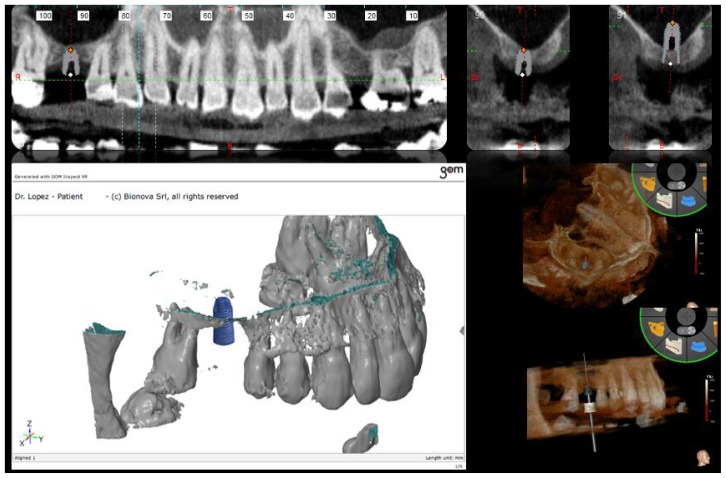
The Dicom files obtained were processed with specialised software (ModelGuide, BIONOVA, La Spezia, Italy). The residual ridge measurement was found to be four millimetres in patient 1, and 8 millimetres in patient 2, with a horizontal thickness of nine millimetres and seven millimetres, respectively. The positioning of a 10-millimetre implant (FMD, Italy; cylindrical i-fix) was planned to obtain a sinus floor elevation of six millimetres in patient 1, and six millimetres in patient 2 in order to completely cover the implant with biomaterial (Figures 1, 4).
Using a gypsum model as a guide, a custom-made template was produced by a 3D printer for each patient. These templates were used as a surgical guide and had a bushing on site 16 for patient 1, and 25 for patient 2 with a diameter of 5.5 millimetres, a height of 4 millimetres and a distance from the bone crest of 4 millimetres (which equates to the thickness of soft tissue). The surgical templates, named ‘easy’ by the company (BIONOVA), were designed to have only dental supports and do not require the use of endosseous fixers (Figure 1).
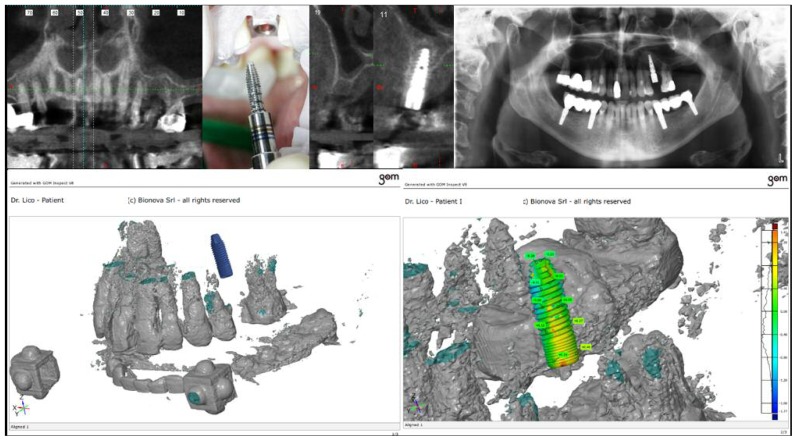
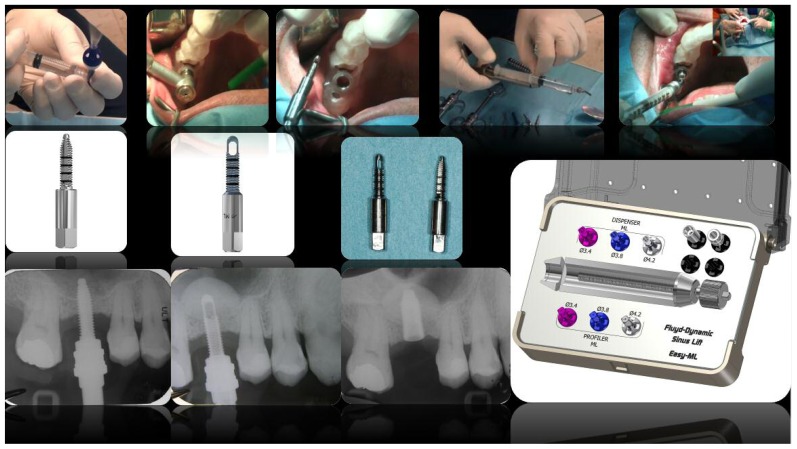
A computer-guided surgery kit was used and two instruments specifically created for this technique were used: a calibrated profiler and a ML dispenser (FMD, Italy) both of which were capable of passing through the bushing (Figure 2).
An operculectomy was carried out with the use of a rotating circular tissue punch mounted on a hand-piece, after which a pilot drill was used to cut the cortical bone and assess the consistency of the bone. In the case of patient 1, nebulized HA was used to prepare the operative field (Yabro spray sol, IBSA, Italy). Subsequently, two burs, with a height of 6 millimetres and respective diameters of 2.3 millimetres and 2.8 millimetres, were used to enlarge the hole and to reach the sinus floor (Figure 2).
Through these two steps the complete erosion of the cortical bone of the maxillary sinus was achieved, after which it was possible to insert an ML profiler with a smooth, convex apex, with a diameter of 3.4 millimetres and a calibrated height that would allow it to penetrate precisely six millimetres into the maxillary sinus in the case of patient 1, and five millimetres in the case of patient 2 (Figures 2, 3, 4). After removing the profiler, a calibrated smooth, convex apex ML dispenser was used to enter six millimetres inside the sinus of patient 1, and five millimetres in patient 2. This tool allowed for both the detachment and the lifting of the Schneiderian membrane and the simultaneous insertion of a fluid biomaterial. In patient 1, there was the pre-detachment of the membrane using HA gel and a mixture of micronized heterologous bone in an 80% collagen matrix. The granulometry was less than 300 microns (Putty by Osteobiol-Tecnoss, Italy). In patient 2, an a nano-crystallized hydroxyapatite in an aqueous solution (Nanogel, TEKNIMED, France) was used. These materials were chosen due to the fact that the small size of the granules better facilitates the injection process (Figure 2).
The accuracy of the measurements was monitored using an endoral X-ray (Figure 2).
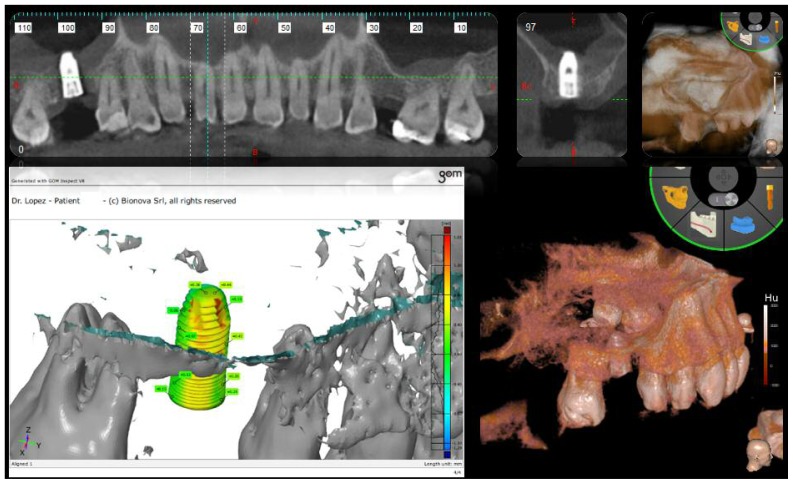
After the first filling, the ML injector was rotated by 90° to obtain a radial filling of the sinus, which formed a dome shape. The process of filling was performed slowly, with great care, in order to avoid rupturing the membrane, and took about three minutes. A specially designed micrometric piston (ML easy) was used (Figure 2). After the ML dispenser had been removed, two further burs with a diameter of 3.2 millimetres and 3.7 millimetres, respectively, and a height of 6 millimetres were used in order to enlarge the hole in the case number 1. An implant was inserted with a diameter of 4.2 millimetres and height of 10 millimetres with an aggressive thread in the upper crestal region, which gave higher primary stability, thus reducing the risk of the implant accidentally sliding into the sinus (FMD, Italy; cylindrical i-fix). The implant was placed through a mounter, which was the surgical kit, which was removed using a special extractor that allowed the final position of the fixture to remain unchanged. Finally, the healing screw was positioned. In the case of patient 1, nebulized HA was used on the tissue after suturing (Yabro spray sol, IBSA Italy) (Figures 2, 3). In the case number 2 an implant with a diameter of 3.8 millimetres and height of 12 millimetres with a spiral design was used (FMD, Italy; Adapta i-fix) (Figure 4).
The precision of the final implant position was monitored through an endoral X-ray (Figures 2, 4).
A comparison between the project and the clinical outcome was carried out using specific software (GOM, BIONOVA, La Spezia, Italy) The same software was used to measure the filling volume of the biomaterial in the sinus (Figures 1, 3, 4).
A measurement of the filled final volume was carried using the ellipsoid formula and taking into account the measurements of the three antero-posterior diameters. In the first case the volume was 0.716 cubical centimeters and in second case was 0.614 cubical centimeters.
Figure 1.
First projected plan of sinus floor operation with a 10 mm implant.
Figure 4.
Gom comparison and final 3D results in patient number 2.
Figure 2.
The custom-made template, Nebulized HA with Yabro-spray-sol and injection with of viscous HA and pasty biomaterial with ML Easy Kit. The ML calibrated profiler with smooth convex apex. Rotating circular tissue punch and the profiler. X-ray check during the filling of the sinus with ML profiler.
Figure 3.
Gom comparison and final 3D results in patient number 1.
During each of the phases outlined above it was possible to remove and replace the template with ease in order to better monitor and verify the site of the future implant.
Results
In the cases described above a ≥ 6 millimetre augmentation of osseous volume was obtained. This result is comparable to those obtained in lifts where conventional techniques were applied. The technique used was very precise and the difference between the projected and clinical outcome of the implant position had an average of less than 0.3 millimetres (Figures 3, 4).
In both cases, the distribution of biomaterial in the sinus was very regular and the quantity of biomaterial used was enough to create a dome, which formed a cover over the implant, as well as being capable of supporting the elevation of the membrane.
The biomaterial, after becoming stable and remodelled, transforms into bone acting as a filler and support to the implants located in the sinus.
Discussion
In recent years there has been an increased tendency to use instruments and techniques so as to reduce the incidence of the type of complication, such as membrane laceration, as mentioned above.
Biomaterials that have a pasty consistency, and are smooth and free from lumps are the most suitable to come into contact with the Schneiderian membrane, which is true for the two different biomaterials used in these two different clinical cases: a nano-crystallized hydroxyapatite in an aqueous solution and a micronized heterologous bone in a collagen matrix.
In particular, the aim of this study is to compare the final filling volume of the sinus with the fluido-dynamic technique of transcrestal maxillary sinus lift performed with the aid of computer-guided surgery, which allows for a precise reduction in the cortical thickness without entering the sinus, while concurrently saving the soft tissues.
Such characteristics not only meet the requirements of maxillary sinus surgery, but are also much less traumatic than more traditional techniques. Even the risk of membrane rupture is reduced due to the high-precision system used and thanks to the smooth consistency of the biomaterials without lumps.
The improvement in diagnostic tests and new biomaterials available, allow the clinician to use computer-guided surgery in the case of crestal sinus lift operations in order to increase accuracy and reduce trauma.
In addition, there is the elimination of percussion, with a direct decrease in the patient’s discomfort. Furthermore, the technique reduces the number of radiographic intraoperative checks necessary during the procedure, with consequent reduction in post-operative recovery time.
Topical HA can be useful as a coadjutant treatment in gingivitis, chronic periodontitis, as well as during the post-operative period, both for implant and sinus lift procedures, for faster healing and to reduce the patients’ discomfort during the postoperative period.
The measurement of the filled volume is compatible with the amount of material in the biomaterial syringe and the amount retained in the ML and in his connector.
The use of various biomaterials in computer-guided crestal sinus lift procedures may affect prosthodontic (18–21) and endodontic (22, 23) clinical outcomes. In addiction the use of general and local anesthesia may have side effects (24–27) and severe complications (28–30).
Conclusions
Even with the limited number of case studies carried out to date, it is possible to foresee that the results obtained with the technique described above are encouraging.
The two biomaterials used have the same pasty consistency, and seem to have the same clinical behaviour, however, the results must be monitored during the remodelling time.
Both the reduced percussive trauma and the low invasiveness mean such a technique should be considered a valid and concrete alternative to those known about and used thus far.
Further studies are necessary in order to investigate the higher or lower efficacy in comparison with statistically significant success and to check the filling volume of the sinus over time. Histologic studies will also be needed in order to confirm the quality of bone formed.
References
- 1.Chiapasco M, Romeo E. The implant-prosthesis rehabilitation in complex cases. Place. 2003 [Google Scholar]
- 2.Lopez MA, Andreasi Bassi M, Confalone L, Lico S, Carinci F. Crestal Sinus Lift Using a Fluido-Dynamic Computer Guided Precise and Atraumatic Technique. J Biol Regul Homeost Agents. 2015;29:67–73. [PubMed] [Google Scholar]
- 3.Andreasi Bassi M, Lopez MA. Hydraulic sinus lift: a new method proposal. Journal of Osteology and Biomaterials. 2010;1:93–101. [Google Scholar]
- 4.Lopez MA, Andreasi Bassi M, Confalone L, Carinci F. Maxillary sinus floor elevation via crestal approach: the evolution of the hydraulic pressure technique. J Craniofac Surg. 2014;25:e127–32. doi: 10.1097/SCS.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 5.Grecchi F, Danza M, Bianco R, Parafioriti A, Carinci F. Computer planned implant-orthognathic rehabilitation: a case of one step surgical procedure with implants insertion, Le Fort I advancement, grafting and immediate loading. J Osseointegration. 2009;3 [Google Scholar]
- 6.Danza M, Zollino I, Carinci F. Comparison between implants inserted with and without computer planning and custom model coordination. J Craniofac Surg. 2009;20:1086–92. doi: 10.1097/SCS.0b013e3181abb322. [DOI] [PubMed] [Google Scholar]
- 7.Danza M, Carinci F. Flapless surgery and immediately loaded implants: a retrospective comparison between implantation with and without computer-assisted planned surgical stent. Stomatologija. 2010;12:35–41. [PubMed] [Google Scholar]
- 8.Engelke W, Capobianco M. Flapless sinus floor augmentation using endoscopy combined with CT scan-designed surgical templates: method and report of 6 consecutive cases. Int J Oral Maxillofac Implants. 2005;20:891–7. [PubMed] [Google Scholar]
- 9.Pozzi A, De Vico G, Sannino G, Spinelli D, Schiavetti R, Ottria L, Barlattani A. Flapless Transcrestal Maxillary Sinus Floor Elevation: computer guided implant surgery combined with expanding-condensing osteotomes protocol. Oral Implantol (Rome) 2011;4:4–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Guarnieri R, Turchini F, Ceccherini A. Valutazione in vitro dell’accuratezza del sistema di chirurgia guidata implantare. Model Guide Implant 3D Implantologia. 2014;1:11–22. [Google Scholar]
- 11.Ballini A, Cantore S, Capodiferro S, Grassi FR. Esterified hyaluronic acid and autologous bone in the surgical correction of the infra-bone defects. Int J Med Sci. 2009;6:65–71. doi: 10.7150/ijms.6.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Araujo Nobre M, Cintra N, Malo P. Peri-implant maintenance of immediate function implants: a pilot study comparing hyaluronic acid and chlorhexidine. Int J Dent Hyg. 2007;5:87–94. doi: 10.1111/j.1601-5037.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 13.Vanden Bogaerde L. Treatment of infrabony periodontal defects with esterified hyaluronic acid: clinical report of 19 consecutive lesions. Int J Periodontics Restorative Dent. 2009;29:315–23. [PubMed] [Google Scholar]
- 14.Koray M, Ofluoglu D, Onal EA, Ozgul M, Ersev H, Yaltirik M, Tanyeri H. Efficacy of hyaluronic acid spray on swelling, pain, and trismus after surgical extraction of impacted mandibular third molars. Int J Oral Maxillofac Surg. 2014;43:1399–403. doi: 10.1016/j.ijom.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Romeo U, Libotte F, Palaia G, et al. Oral soft tissue wound healing after laser surgery with or without a pool of amino acids and sodium hyaluronate: a randomized clinical study. Photomed Laser Surg. 2014;32:10–6. doi: 10.1089/pho.2013.3509. [DOI] [PubMed] [Google Scholar]
- 16.Galli F, Zuffetti F, Capelli M, Fumagalli L, Parenti A, Testori T, Esposito M. Hyaluronic acid to improve healing of surgical incisions in the oral cavity: a pilot multicentre placebo-controlled randomised clinical trial. Eur J Oral Implantol. 2008;1:199–206. [PubMed] [Google Scholar]
- 17.Avantaggiato A, Palmieri A, Carinci F, Pasin M, Bertuzzi GL. Biostimulation and biorevitalization: effects on human skin fibroblasts. Annals of Oral & Maxillofacial Surgery. 2013;1(11) [Google Scholar]
- 18.Ottria L, Zavattini A, Ceruso FM, Gargari M. Maxillofacial prosthesis (P.M.F): in a case of oral-nasal communication post-surgery and post-radiotherapy. Oral Implantol (Rome) 2014;7:46–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Gargari M, Gloria F, Cappello A, Ottria L. Strength of zirconia fixed partial dentures: review of the literature. Oral Implantol (Rome) 2010;3:15–24. [PMC free article] [PubMed] [Google Scholar]
- 20.De Vico G, Ottria L, Bollero P, Bonino M, Cialone M, Barlattani A, Jr, Gargari M. Aesthetic and functionality in fixed prosthodontic: sperimental and clinical analysis of the CAD-CAM systematic 3Shape. Oral Implantol (Rome) 2008;1:104–15. [PMC free article] [PubMed] [Google Scholar]
- 21.Moretto D, Gargari M, Nordsjo E, Gloria F, Ottria L. Immediate loading: a new implant technique with immediate loading and aesthetics: Nobel Active. Oral Implantol (Rome) 2008;1:50–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Fanucci E, Nezzo M, Neroni L, Montesani L, Jr, Ottria L, Gargari M. Diagnosis and treatment of paranasal sinus fungus ball of odontogenic origin: case report. Oral Implantol (Rome) 2013;6:63–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Gargari M, Ottria L, Nezzo M, Neroni L, Fanucci E. Cone Beam CT use in the pre-prosthetic evaluation of endodontically treated of the rear maxilla. Oral Implantol (Rome) 2012;5:42–6. [PMC free article] [PubMed] [Google Scholar]
- 24.Feltracco P, Gaudio RM, Barbieri S, et al. The perils of dental vacation: possible anaesthetic and medicolegal consequences. Med Sci Law. 2013;53:19–23. doi: 10.1258/msl.2012.012047. [DOI] [PubMed] [Google Scholar]
- 25.Feltracco P, Barbieri S, Galligioni H, et al. A fatal case of anaphylactic shock during paragliding. J Forensic Sci. 2012;57:1656–8. doi: 10.1111/j.1556-4029.2012.02187.x. [DOI] [PubMed] [Google Scholar]
- 26.Feltracco P, Gaudio RM, Avato FM, Ori C. Authors’ Response (Letter) Journal of Forensic Sciences. 2012;57 doi: 10.1111/j.1556-4029.2012.02187.x. [DOI] [PubMed] [Google Scholar]
- 27.Gaudio RM, Barbieri S, Feltracco P, et al. Traumatic dental injuries during anaesthesia. Part II: medico-legal evaluation and liability. Dent Traumatol. 2011;27:40–5. doi: 10.1111/j.1600-9657.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- 28.Gaudio RM, Barbieri S, Feltracco P, et al. Impact of alcohol consumption on winter sports-related injuries. Med Sci Law. 2010;50:122–5. doi: 10.1258/msl.2010.010007. [DOI] [PubMed] [Google Scholar]
- 29.Giannitelli SM, Basoli F, Mozetic P, Piva P, Bartuli FN, Luciani F, Arcuri C, Trombetta M, Rainer A, Licoccia S. Graded porous polyurethane foam: a potential scaffold for oro-maxillary bone regeneration. Mater Sci Eng C Mater Biol Appl. 2015 Jun;51:329–35. doi: 10.1016/j.msec.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Bramanti E, Matacena G, Cecchetti F, Arcuri C, Cicciù M. Oral health-related quality of life in partially edentulous patients before and after implant therapy: a 2-year longitudinal study. Oral Implantol (Rome) 2013 Oct 15;6(2):37–42. [PMC free article] [PubMed] [Google Scholar]