SUMMARY
Purpose
The aim of this paper was to evaluate the histological and histomorphometric outcome of Preformed Titanium Foil (PTF) to perform Guided Bone Regeneration (GBR) in posterior mandibular atrophies.
Materials and methods
10 subjects (1 male; 9 females; mean age 58±11.37 years), with distal mandibular atrophies were selected to perform GBR by means of PTF, using a moldable allograft paste as graft material. The devices, made of a 0,2 mm thick pure titanium foil, were pre-shaped using stereolithographic models obtained from CT-scan of the patients’ recipient site. In the second stage, performed at 6.7±2.33 months, 18 cylindrical two-piece implants were placed and the devices removed, at the same time bone biopsies were harvested. At 4 months, the implants were exposed and submitted to progressive prosthetic load for a span of 4 months. The cases were finalized by means of metal-ceramic cementable restorations. The post finalization follow-up was at 12 months.
Results
Survival rate (i.e. SVR) was 100% since none fixtures were lost. At the one-year follow up the clinical appearance of the soft tissues was optimal and not pathological signs on probing were recorded. The success rate (i.e. SCR) was 88.2% and the average peri-implant bone reabsorption was 1.17±0.41 mm. The average rate of graft contraction was 19.4±10.55%. The mean percentage occupied by mineralized bone was 48.03±5.93%, while the bone marrow and graft material were 36.1±2.81% and 15.87±4.87 %, respectively.
Conclusion
The results suggest good potentialities of the method for GBR in distal mandibular atrophies, allowing to maximize the outcome and simplifying the surgical phase.
Keywords: guided bone regeneration, moldable allograft paste, titanium membrane, preformed titanium foil, stereolithographic model, histomorphometric analysis
Introduction
Severe vertical alveolar bone loss in the edentulous posterior mandibular areas, dramatically limiting the bone height available for proper implant placement, constitutes one of the major challenge for the rehabilitation of these areas (1).
Often, in these cases, the bone segment required the adoption of a bone augmentation procedure. There are many different techniques to reconstruct deficient alveolar ridges that are operator-experience- and technique- sensitive. These cases frequently need autogenous bone onlay graft in order to improve implant success and prevent pathologic fracture before prosthetic rehabilitation (2). Concerning the autograft, as a bone block, it has the advantage of being osteogenic and osteoinductive, but increases surgery time, creates a second operative site and can cause complications and morbidity. In case of severe atrophy, only bone from extraoral sites is available, making the surgery not feasible in ambulatory setting. Guided Bone Regeneration (GBR) techniques seem to provide predictable and favorable results in long-term follow-up studies, in particular when performed with non-resorbable Titanium-reinforced e-PTFE membranes (1, 3).
Most titles in literature report that the graft used, under such devices, vary among autogenous bone graft, blood clot, deproteinized bovine bone matrix or demineralized freeze-dried bone allograft (1).
Membranes are used to hinder the migration of undesirable cell types allowing the repopulation of the wound with the desirable cell type. The objectives of such kind of devices are: space maintenance; clot protection; barrier formation; stopping graft resorption (4, 5).
For these reasons vertical bone augmentation is usually performed with non-resorbable Titanium-reinforced e-PTFE membranes, so that the barrier effect can last until the second stage surgery, that usually takes place after at least 6 months from the first stage (6).
Currently dense PTFE (d-PTFE) has been proposed in order to prevent the contamination of the graft in case of undesired exposure of the device (7).
This material in the same manner as the e-PTFE, can be advantageously used in combination with titanium framework in order to give malleability and stability to the device (8, 9).
The favorable clinical performance of d-PTFE showed that one of the major unsuspected properties of the non-resorbable membrane is that of the complete impermeability, preventing the bacterial contamination of the graft (10, 11).
Unfortunately the stiffness of these non-resorbable membranes, which can be increased with the simultaneous implant placement, can be compromised by the chewing and also the adaptation and fixation of the device to the recipient site is a time consuming procedure (12).
A Preformed Titanium Foil (PTF) could be advantageously used in order to obtain a stiff, biocompatible, impermeable and customizable device for GBR (13, 14).
In present paper the histomorphometric outcome of a GBR case series, in distal mandibular atrophies, performed with PTF, combined with a moldable allograft paste, is proposed.
Materials and methods
Patient selection and assessment
Candidates for GBR, were screened according to the following inclusion criteria: controlled oral hygiene and absence of any lesions in the oral cavity.
The exclusion criteria were as follows: bruxism, consumption of alcohol higher than 2 glasses of wine per day, localized radiation therapy of the oral cavity, antitumor chemotherapy, liver, blood and kidney diseases, immunosuppressed patients, patients taking corticosteroids, pregnant women, inflammatory and autoimmune diseases of the oral cavity.
In the selected patients the Cone Beam Computed Tomography (CBCT) (NewTom 5G®, QR, Verona, Italy) was carried out. The CBCT showed, in the edentulous sites, a reduced thickness of the residual bone (Figure 4a).
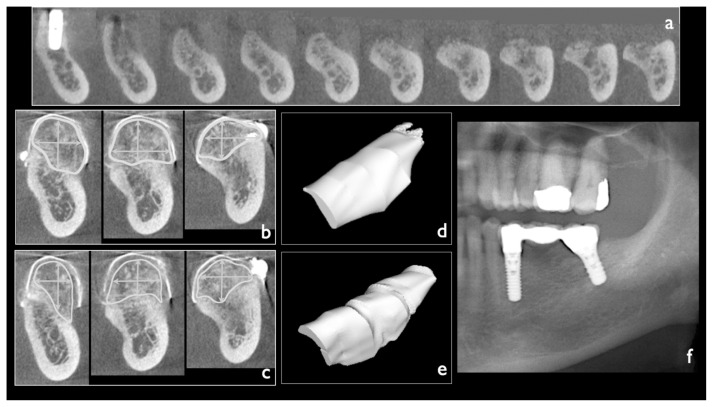
Figure 4.
a) CBCT-scan of the recipient site immediately before the first stage surgery (FSS); b, c) CBCT-scan of the grafted area was carried out immediately before the second stage surgery (SSS), the reached increment was measured vertically and horizontally, then ROIs were selected in order to evaluate the volume of the grafted area, at the FSS as well as the SSS; d) the volume of the graft at FSS; e) the volume of the graft at SSS, a slight contraction is evident; f) panoramic X-ray at one-year follow-up, after prosthetic finalization.
For each CBCT a three-dimensional stereolithographic model, of the recipient site, was performed in order both to evaluate the case and to perform the PTF device (Figure 1b) (13, 14).
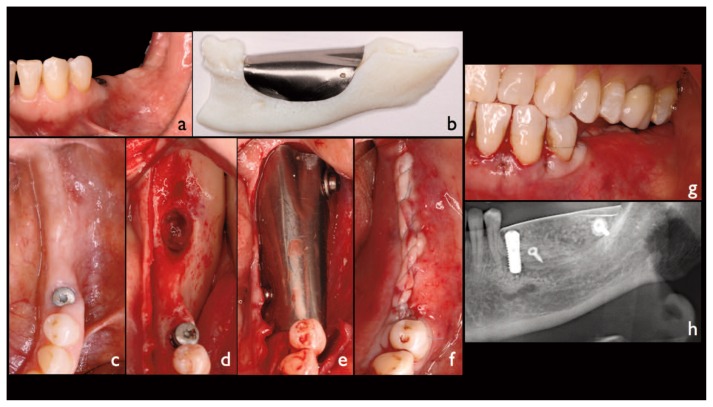
Figure 1.
Description of a clinical case; a, c) intraoral view of the case; b) the stereolithographic model obtained by the CT-scan, the titanium foil is moulded on the recipient site, a variable number of holes are usually performed for its stabilization via fixation screws; d) a full thickness flap was reflected exposing the recipient site; e) the inner surface of the device was filled with the graft material and then immediately placed on the recipient site and fixed via screws; f, g) the flap is passivated and then sutured over the device; h) post-operative panoramic X-ray.
Devices Preparation
The GBRs were planned choosing a PTF as membrane barrier. A pure titanium foil (grade 4; 0.2 mm thick) was moulded on each stereolithographic model. A variable number of holes (ranging between 1 and 3) were performed, mainly in the lower-anterior portion of each device, for its stabilization via fixation screws (Figure 1b). The devices were then cleaned (denatured alcohol 90% 30 min, sodium hypochlorite 5% 1h, chloridric acid 10% 1h) and sterilized by means of an autoclave (Domina Plus, Dental X, Vicenza, Italy) before of the surgery (13, 14).
Clinical Procedure
All patients underwent the same surgical protocol: an antimicrobial prophylaxis was administered with amoxicillin clavulanate (Clavulin, GlaxoSmithKline, Italy), 1g every 8h for 7 days, begun 3 hours before the operation. After an initial rinse with chlorhexidine digluconate 0.2% (Corsodyl Mouthwash, GlaxoSmithKline, Italy) for 1 minute to disinfect the mouth, loco-regional anesthesia was performed with articaine hydrochloride 4% with epinephrine 1:100,000 (Citocartin, Molteni Dental, Italy). The bony area was exposed through reflection of a mucoperiosteal envelope flap (Figure 1d).
The cortical bone, of the recipient site, was then repeatedly punched until bleeding with a ball bur mounted on a contra-angle handpiece. The PTF was first tried, in order to verify the correct fitting and matching, and then placed on the recipient site previously filled with a thermoplastic moldable allograft paste (TMAP) provided with osteogenic properties (Regenaform, RTI Surgical Inc., USA). The amount of TMAP used for each case was ranging between 1 cc and 4 cc, depending on the dimension of the bone defect. The graft material was previously placed in a syringe, and then heated at 48°C, in order to facilitate its placement into the inner surface of the PTF. After the placement, the device was immobilized via fixation screws (Figure 1e). Flap closure, without tension, was performed with a double-layered continuous suture (Figure 1f, g). Ibuprofen (Brufen 600 mg, Abbot, Italy), every 8–12 hours for 5 days was administered to control postoperative pain and edema. Rinses with chlorhexidine digluconate 0.2% (Corsodyl Mouthwash, GlaxoSmithKline, Italy) were prescribed for the disinfection of the surgical wound, 2/3 times/day for 7 days. After 14 days the sutures were removed and oral hygiene instructions were provided. The potential occurrence of major post-operative complications such as early and late exposure of the device, respectively within 1 month and after 1 or more months post-operative and failure of graft integration was evaluated.
After a suitable period of time needed for the consolidation of the graft (mean value 6.7±2.33 months), the second stage surgery (SSS) was performed.
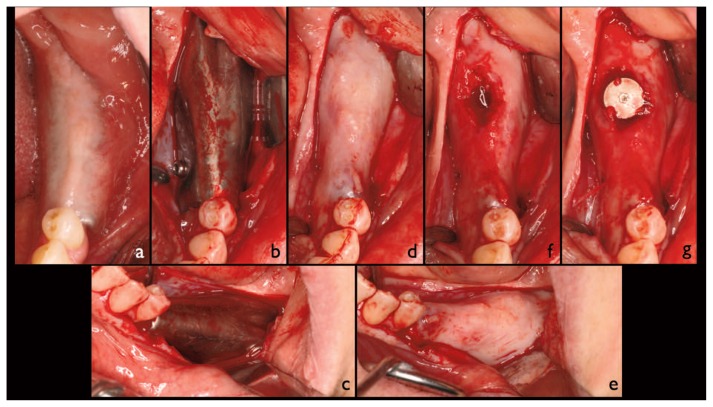
At re-entry the implant placement was performed with a full thickness flap elevation approach also in order to remove the device (Figure 2a–e). Two-piece cylindrical implants (FMD, Rome, Italy) were placed submerged in the regenerated sites (Figure 2g). In each case, for at least an implant site, the initial implant tunnel preparation was carried out by means of a trephine drill, in order to harvest a biopsy of the regenerated area (Figure 2f). Drug prescriptions before and after surgery were identical to those of the first stage surgery (FSS).
Figure 2.
a) Intraoral condition at the re-entry; b, c) a full thickness flap is reflected and the device exposed; d, e) after device removal the underlining regenerated area is exposed; f) the white connective, covering the graft, was not removed, the initial implant tunnel preparation was carried out with a trephine drill in order to harvest a bone biopsy; g) after the implant tunnel preparation the implants is placed then the flap is sutured.
In case of early exposure, the device was removed at 4 months post-operative and the implants were placed 2 months after waiting complete healing of both soft and hard tissues. In case of late exposure the usual time for the SSS was respected (i.e. 6 months post-operative). In case of both early and late exposure the patients were provided with oral hygiene domiciliary instructions, typical of the immediate post-operative period, associated with the use of an extra soft toothbrush for the entire period in which the devices would be on site, in order to minimize the deposition of microbial plaque on the exposed titanium surface.
After 4 months from the placement, all the implants were exposed and then a progressive management of the prosthetic load was carried out and protracted for a period of 4 months, by means of temporary resin crowns or bridges screwed on preformed Peek abutments. The cases were finalized with cemented metal-ceramic crowns or bridges, on preformed titanium screwed abutments. All patients were included in a strict hygiene recall and provided with oral hygiene domiciliary instructions. One year after prosthetic finalization, clinical and radiographic follow-up of was performed for all the cases, in order to verify the condition of the soft and hard peri-implants tissues (Figure 4f).
Data assessment
For each clinical case two panoramic X-ray exams were performed (GXDP-700, Gendex Dental Systems, Hatfield, USA), one immediately after SSS and one after one year from the prosthetic finalization, in order to evaluate the stability of the grafted area and the implant success rate (SCR) (Figure 1h, 4f). The SCR was evaluated according to the absence of persisting peri-implant bone resorption greater than 1.5 mm during the first year of loading. The distance between the implant-abutment junction and the bone crestal level was defined as the Bone Junction Distance (BJD) and calculated at the time of operation and at the end of the follow-up analysing the digital radiographs. The delta BJD (ΔBJD) is the difference between the BJD at the last check-up and the BJD recorded just after the operation. The measurements were carried out mesially and distally to each implant, calculating the distance between the implant neck and the most coronal point of contact between the bone and implant. The bone level recorded just after the surgical insertion of the implant was the reference point for the following measurements. The measurement was approximated to the nearest 0.1 mm. The digital radiographs were analyzed by means of a dedicated software (Vixwin Platinum, Gendex Dental Systems, Hatfield, USA). Knowing the dimensions of the implant, it was possible to establish the mathematical correlation between the distance from the mesial and distal edges of the implant platform to the point of bone-implant contact (expressed in tenths of a millimeter).
Together with the SCR the percentage of implants still in place at the end of the follow-up period (i.e. survival rate-SVR) was used as clinical outcome predictor (15, 16).
Graft increments evaluation
The graft increments were evaluated, by the calibrated digital examination of the paraxial tomograms of CBCT performed immediately before the SSS (OsiriX v. 3.5.1 32bit, Pixmeo, Switzerland). For each grafted area the higher vertical distance between the lower level of the recipient site and the top of the graft as well as the greater horizontal distance reached by bone augmentation were measured (Figure 4b, c). The measurements were approximated to the nearest 0.1 mm.
Furthermore thanks to the CBCT the volume of each grafted areas, reached at the time of SSS, as well as the volume of each grafts, at the time of FSS were automatically calculated by the software, selecting for all the paraxial tomograms two areas of interest (ROIs). The difference between the volume of the graft at FSS and the volume of the graft at SSS gave the volume of graft lost due to its contraction (Figure 4d, e).
In all the paraxial tomograms, for each grafted area, the two ROIs were selected as follows: one identifying the space initially occupied the graft at the time of the FSS (Figure 4b); the other identifying the final volume reached, by the graft at the time of SSS (Figure 4c). The perimeter of the first ROI was delimited, on the top, by the inner limit of the titanium barrier device while the bottom was delimited by the bone profile of the recipient site (Figure 4b). The perimeter of the second ROI was delimited, on the top, by the coronal limit of the new regenerated bone while the bottom was delimited, by the bone profile of the recipient site (Figure 4c).
Considering that the space, immediately situated below the titanium device, was completely filled by the graft, at the time of FSS, the sum of the first ROIs identifies the initial volume of the graft, while the sum of the second ROIs identifies the final volume of the graft, when its integration occurred.
Samples processing and analysis
The biopsies, harvested from the sites of implant placement, were fixed in 10% formalin buffered with phosphate buffer (pH 7), demineralized with a descaling solution containing EDTA (Kaltek, Padua, Italy), dehydrated in a scale of increasing alcohol content, embedded in paraffin and, finally, sectioned along the major axis of the biological samples using a microtome (Leitz 1512, Germany). The sections obtained were stained with hematoxylin-eosin and observed under an optical microscope and polarized transmitted light (Leitz Dialux, Germany) (Figure 3). The aforementioned microscope was equipped with a digital camera (Nikon Coolpix 990, Japan) making it therefore possible to photograph the samples, in uncompressed TIFF format, at different magnifications (10X-25X-40X-100X-250X). The images at lower magnification (10X-25X) were initially processed with digital image processing software (Adobe Photoshop CS3 Extended vers. 10.0.1, Adobe Systems Inc, U.S.) in order to allow discrimination between the mineralized bone, the bone marrow and the graft material. Subsequently, the images were subjected to histomorphometric analysis, using dedicated software (Image-Pro Plus 4.1, Media Cybernetics Inc., U.S.), first converted into a 8-bit grayscale and afterwards to pseudo-colors in order to assign a percentage to the areas occupied respectively by the three materials in question. The areas occupied by each color were thus calculated and weighted on the total area of sampling (Figure 3a) (17).
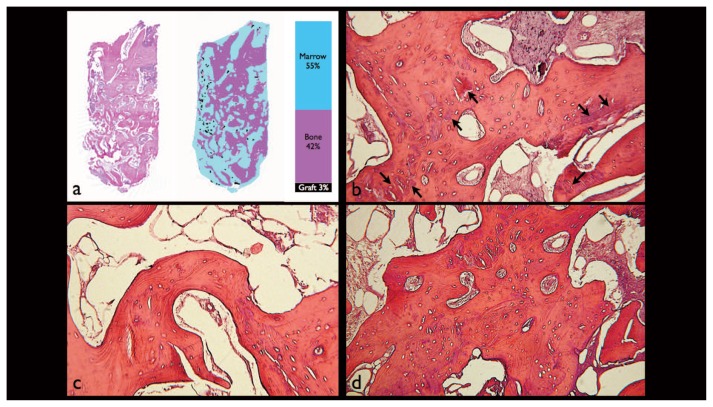
Figure 3.
a) Histomorphometric analysis of a sample. After identification and discriminative coloring of the 3 components (bone, marrow, graft), the image is primarily converted into an 8-bit grayscale and then into pseudo-colors in order to calculate the percentage extension of each area; b) the graft material granules are dispersed (arrows) and completely surrounded by bone trabeculae formed by secondary osteons (stained with hematoxylin-eosin, original magnification 100X); c, d) trabecular bone in various stages of remodeling (stained with hematoxylin-eosin, original magnification 100X).
In case of Guided Bone Regeneration local anesthesia can be performed to sampling patients but it may have relevant side effect (17–20) and severe complications (21).
This topic can be also potentially investigated with immunofluorescence techniques which are well known since the nineties (22, 23).
Results
Clinical results
Ten subjects (1 male; 9 females; mean age 58±11.37 years) were enrolled in the study. The CBCT of the 10 edentulous sites showed a reduced thickness of the residual bone. According to the implant dentistry bone volume classification, developed by Misch and Judy in 1985, the cases were classified as follows: 6 Class II Div. C; 2 Class I Div. C; 1 Class III Div. C; 1 Class I Div. C, D.
Eighteen two-piece implants (FMD, Rome, Italy) were inserted during the second stage surgery. The fixtures replaced first premolars (i.e. 3.4, 4.4), first molars (i.e. 3.6, 4.6) and second molars (i.e. 3.7, 4.7) in respectively in 16.67, 50 and 33.33% cases.
Implants length was x≤8 mm, x=10 mm and x ≥11.5 mm in respectively 33.33, 55.55 and 11.12% cases. Implants diameter was x≤3.8 mm, x=4.2 mm and x≥4.8 mm in respectively 11.12, 44.44 and 44.44% cases. Three implants were restored with single crowns and 15 with bridges.
At the one year follow up, after prosthetic finalization, all implants were in place (survival rate-SVR=100%). The clinical appearance of the soft tissues was optimal and no pathological signs were recorded upon probing. Two fixtures presented a peri-implant bone loss, around the implant neck, greater than 1.5 mm. Thus the success rate (i.e. SCR) was 88.2% and the average ΔBJD was 1.17±0.41 mm.
The occurrence of major post-operative complications was limited to early and late exposure in respectively 20 and 10% of cases. The early exposures (2 cases) were observed, both after 14 days, at the moment of the sutures remotion, while late exposure (1 case) was observed 5 months post-operative.
In particular the early exposures increased the width of attached gingiva on the grafted area.
Both early and late exposures did not seem to compromise the healing and integration of the surrounding graft and they were not associated with particular patient’s symptoms.
At re-entry the regenerated area was made of well-vascularized bone, its consistency and appearance was similar to the adjacent bone tissue.
The average volume of the graft, at the FSS, was 2.0±0.88 cm3, while the average volume of the graft, at the SSS, was 1.5±0.62 cm3, thus the calculated average graft contraction was 0.43±0.42 cm3, corresponding to an average rate of 19.4±10.55%. The average vertical and horizontal bone augmentation, in the grafted sites, were respectively 6.1±2.46 mm and 8.6± 3.96 mm.
Histological and histomorphometric results
The histological examination of the samples shows trabecular bone tissue in various stages of remodelling, there are areas of newly formed bone in which the cellular components are visibly active and dispersed areas in which the biomaterial particles appears perfectly osseointegrated in the trabecular bone (Figure 3b–d). In the connective tissue there are no visible cellular elements indicating inflammation or immune reaction.
Histomorphometric analysis of all samples, examined in their entirety, shows that the mean percentage occupied by mineralized bone was 48.03±5.93%, while the bone marrow and graft material were 36.1±2.81% and 15.87±4.87%, respectively.
Discussion
GBR is the most widely used augmentation technique for alveolar ridge augmentation in case of jaws atrophy, in particular it is has detailed documentation and long-term follow-up studies in comparison to other bone augmentation techniques (3). Recently the use of d-PTFE as membrane barrier has been proposed as alternative to e-PTFE because, in case of exposure of the device to the oral environment, using the latter the graft integration would be compromised (7). In fact the permeability of e-PTFE allows the undesired passage of oral bacteria, compromising the underlying graft (8–11). The use of impermeable barriers is progressively spreading in the clinical practice (8–11). Titanium-reinforced PTFE membranes (TRPTFE) are often used in case of GBR for large defects to increase the stiffness of the device. For the same reason, TRPTFE membranes are increasingly used in combination with implant placement, in order to both reduce the number of surgeries and increase the rigidity of the device, because the implant works as a tenting screw, stabilizing the device and reducing the mechanical solicitation to the underlining graft during chewing (12).GBR like the other alveolar ridge augmentation procedures is technique- and operator-experience-sensitive, for this reason new procedures and materials are often proposed in order to reach more predictable results and to simplify the clinician’s work (3).
Titanium mesh (TM) were initially developed in the ’60s as a restraining device of vital organs in trauma patients as well as with reconstructive purposes in oncology patients as a device of restraint and immobilization of particulate autogenous bone grafts, taken from extraoral sites. In the ’80s their use has been extended to bone augmentation to enable dental implant placement (24).
Substantial bone augmentation can be achieved using TM in conjunction with bone grafting. Despite the fact that the major complication associated to this device is its exposure in the oral environment, this event does not necessarily compromise the final treatment outcome (25, 26). Theoretically the use of TM without holes can give both the advantages of TM and TRPTFE in the same device.
As described in previous papers the use of a PTF membrane barrier in GBR is proposed for its features of stiffness and adaptability to the recipient site. Also the impermeability and biocompatibility must be considered as strong points of this device (13, 14). In case of limited availability of intraoral grafting bone, especially in edentulous patients with severe maxillary atrophy, bone augmentation procedure performed in ambulatory setting require the use of allograft materials, which are provided of osteoconductive and osteoinductive properties (13, 14, 27). These materials must be combined with a membrane barrier in order to prevent the competitive growth of soft tissues in the area of bone augmentation. The use of PTF seems to maximize the outcome simplifying the surgical phase. In these cases the Authors suggest the use of a moldable allograft paste combined with PTF (13, 14).
In our clinical experience the absence of holes (characteristic of meshes) prevents connective tissue growth and infiltration, promoting bone regeneration and facilitating the removal of the device in the second-stage surgery (13, 14). A number of different techniques have been described to allow the pre-shaping of both the barrier-membranes and the allograft-blocks, by means of stereolithographic models (SMs) obtained from TC-Scan of the patient (28). These procedures allow to maximize the outcome of the GBR and to simplify the surgical phase. Must be said that this method requires a careful and precise construction of the device on the SM, also the TC-scan images must be free of artifacts to not affect the fidelity of the model and consequently of the device that may therefore not fit perfectly on the recipient site. In fact in case of mismatching between the device and the recipient site, it would be complicated to get the desired adaptation, compromising the final outcome (13, 14).
According our clinical experience the procedure is indicated in case of bone augmentation in edentulous distal mandibular atrophies when a rigid device as barrier is required in order to protect and stabilize the underlying graft. Requirements for the technique are represented by supporting surfaces or bone pillars to stabilize the device. In addition the use of PTF facilitates the suture of the soft tissues and their healing over it (13, 14).
Even both early and late exposures of the PTF to the oral environment, if properly cleansed by the patient, do not compromise the clinical outcome, giving predictable results. In particular, the early exposure increased the thickness of the attached gingiva on the grafted area, consequent to second intention healing of the wound (13, 14).
In the present paper, although at one year followup no implants were lost (SVR 100%), 2 fixtures had a peri-implant bone loss greater than 1.5 mm, around implant neck (SCR 88,2%). This occurrence is mainly attributable to the bone graft remodelling. In the distal region of the mandible the remodelling process is principally influenced by the mobility of the bone segment (mandibular bending) due to muscular forces that act on it (2, 3, 13, 14, 16).
The histological and histomorphometric analysis of the samples demonstrate the effectiveness of GBR by means of PTF, as barrier membrane, in combination with the TMAP, as graft material.
The results suggest good potentialities of the method for the augmentation of bone volume in the distal mandibular atrophies, encouraging further studies aimed to its validation.
References
- 1.Rocchietta I, Fontana F, Simion M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: a systematic review. J Clin Periodontol. 2008;35(8 Suppl):203–15. doi: 10.1111/j.1600-051X.2008.01271.x. [DOI] [PubMed] [Google Scholar]
- 2.Misch CE. The Contemporary Implant Dentistry. Mosby Elsevier; 2008. Place. [Google Scholar]
- 3.Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007;22(Suppl):49–70. [PubMed] [Google Scholar]
- 4.Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81(5):672–6. doi: 10.1097/00006534-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Buser D, Bragger U, Lang NP, Nyman S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implants Res. 1990;1(1):22–32. doi: 10.1034/j.1600-0501.1990.010104.x. [DOI] [PubMed] [Google Scholar]
- 6.Simion M, Scarano A, Gionso L, Piattelli A. Guided bone regeneration using resorbable and nonresorbable membranes: a comparative histologic study in humans. Int J Oral Maxillofac Implants. 1996;11(6):735–42. [PubMed] [Google Scholar]
- 7.Cucchi A, Ghensi P. Vertical Guided Bone Regeneration using Titanium-reinforced d-PTFE Membrane and Prehydrated Corticocancellous Bone Graft. Open Dent J. 2014;8:194–200. doi: 10.2174/1874210601408010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TM, Kowolik MJ, Janowski GM. Recent advances in the development of GTR/GBR membranes for periodontal regeneration-a materials perspective. Dent Mater. 2012;28(7):703–21. doi: 10.1016/j.dental.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 2013;57(1):3–14. doi: 10.1016/j.jpor.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Carbonell JM, Martin IS, Santos A, Pujol A, Sanz-Moliner JD, Nart J. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: a literature review. Int J Oral Maxillofac Surg. 2014;43(1):75–84. doi: 10.1016/j.ijom.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hezaimi K, Rudek I, Al-Hamdan KS, Javed F, Nooh N, Wang HL. Efficacy of using a dual layer of membrane (dPTFE placed over collagen) for ridge preservation in fresh extraction sites: a micro-computed tomographic study in dogs. Clin Oral Implants Res. 2013;24(10):1152–7. doi: 10.1111/j.1600-0501.2012.02526.x. [DOI] [PubMed] [Google Scholar]
- 12.Buser D, Dula K, Belser UC, Hirt HP, Berthold H. Localized ridge augmentation using guided bone regeneration. II. Surgical procedure in the mandible. Int J Periodontics Restorative Dent. 1995;15(1):10–29. [PubMed] [Google Scholar]
- 13.Andreasi Bassi M, Andrisani C, Lopez MA, Gaudio RM, Lombardo L, Lauritano D. Guided bone regeneration in distal mandibular atrophy by means of a preformed titanium foil: a case series. J Biol Regul Homeost Agents. 2016;30(2 Suppl 1):61–8. [PubMed] [Google Scholar]
- 14.Andreasi Bassi M, Andrisani C, Lopez MA, Gaudio RM, Lombardo L, Carinci F. Guided bone regeneration by means of a preformed titanium foil: A case of severe atrophy of edentulous posterior mandible. J Biol Regul Homeost Agents. 2016;30(S2):35–41. [PubMed] [Google Scholar]
- 15.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11–25. [PubMed] [Google Scholar]
- 16.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Effect of distance between one piece implants on crestal bone resorption. European Journal of Inflammation. 2011;9(3 S):1–6. [Google Scholar]
- 17.Feltracco P, Barbieri S, Galligioni H, Pasin L, Gaudio RM, Tommasi A, Zucchetto A, Trevisiol P, Ori C, Avato FM. A fatal case of anaphylactic shock during paragliding. J Forensic Sci. 2012;57(6):1656–8. doi: 10.1111/j.1556-4029.2012.02187.x. [DOI] [PubMed] [Google Scholar]
- 18.Feltracco P, Gaudio RM, Avato FM, Ori C. Authors’ Response (Letter) Journal of Forensic Sciences. 2012;57(5) doi: 10.1111/j.1556-4029.2012.02187.x. [DOI] [PubMed] [Google Scholar]
- 19.Feltracco P, Gaudio RM, Barbieri S, Tiano L, Iacobone M, Viel G, Tonetti T, Galligioni H, Bortolato A, Ori C, Avato FM. The perils of dental vacation: possible anaesthetic and medicolegal consequences. Med Sci Law. 2013;53(1):19–23. doi: 10.1258/msl.2012.012047. [DOI] [PubMed] [Google Scholar]
- 20.Gaudio RM, Barbieri S, Feltracco P, Tiano L, Galligioni H, Uberti M, Ori C, Avato FM. Traumatic dental injuries during anaesthesia. Part II: medico-legal evaluation and liability. Dent Traumatol. 2011;27(1):40–5. doi: 10.1111/j.1600-9657.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- 21.Gaudio RM, Barbieri S, Feltracco P, Spaziani F, Alberti M, Delantone M, Trevisiol P, Righini F, Talarico A, Sanchioni R, Spagna A, Pietrantonio V, Zilio G, Dalla Valle R, Vettore G, Montisci M, Bortoluzzi A, Sacco A, Ramacciato G, Pasetti A, Mognato E, Ferronato C, Costola A, Ori C, Avato FM. Impact of alcohol consumption on winter sports-related injuries. Med Sci Law. 2010;50(3):122–5. doi: 10.1258/msl.2010.010007. [DOI] [PubMed] [Google Scholar]
- 22.Petruzzi M, Campus G, Paparusso F, Lucchese A, Lauritano D, De Benedittis M, Serpico R. Analysis of plasma fibronectin levels in patients affected by oral lichen planus. European Journal of Inflammation. 2012;10(1):45–50. [Google Scholar]
- 23.Petruzzi M, Lucchese A, Nardi GM, Lauritano D, Favia G, Serpico R, Grassi FR. Evaluation of autofluorescence and toluidine blue in the differentiation of oral dysplastic and neoplastic lesions from non dysplastic and neoplastic lesions: a cross-sectional study. J Biomed Opt. 2014;19(7):76003. doi: 10.1117/1.JBO.19.7.076003. [DOI] [PubMed] [Google Scholar]
- 24.Misch CE, Judy KW. Classification of partially edentulous arches for implant dentistry. Int J Oral Implantol. 1987;4(2):7–13. [PubMed] [Google Scholar]
- 25.Her S, Kang T, Fien MJ. Titanium mesh as an alternative to a membrane for ridge augmentation. J Oral Maxillofac Surg. 2012;70(4):803–10. doi: 10.1016/j.joms.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Louis PJ, Gutta R, Said-Al-Naief N, Bartolucci AA. Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J Oral Maxillofac Surg. 2008;66(2):235–45. doi: 10.1016/j.joms.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 27.Toscano N, Holtzclaw D, Mazor Z, Rosen P, Horowitz R, Toffler M. Horizontal ridge augmentation utilizing a composite graft of demineralized freeze-dried allograft, mineralized cortical cancellous chips, and a biologically degradable thermoplastic carrier combined with a resorbable membrane: a retrospective evaluation of 73 consecutively treated cases from private practices. J Oral Implantol. 2010;36(6):467–74. doi: 10.1563/AAID-JOI-D-09-00100. [DOI] [PubMed] [Google Scholar]
- 28.Jacotti M, Wang HL, Fu JH, Zamboni G, Bernardello F. Ridge augmentation with mineralized block allografts: clinical and histological evaluation of 8 cases treated with the 3-dimensional block technique. Implant Dent. 2012;21(6):444–8. doi: 10.1097/ID.0b013e31826f7a67. [DOI] [PubMed] [Google Scholar]