Abstract
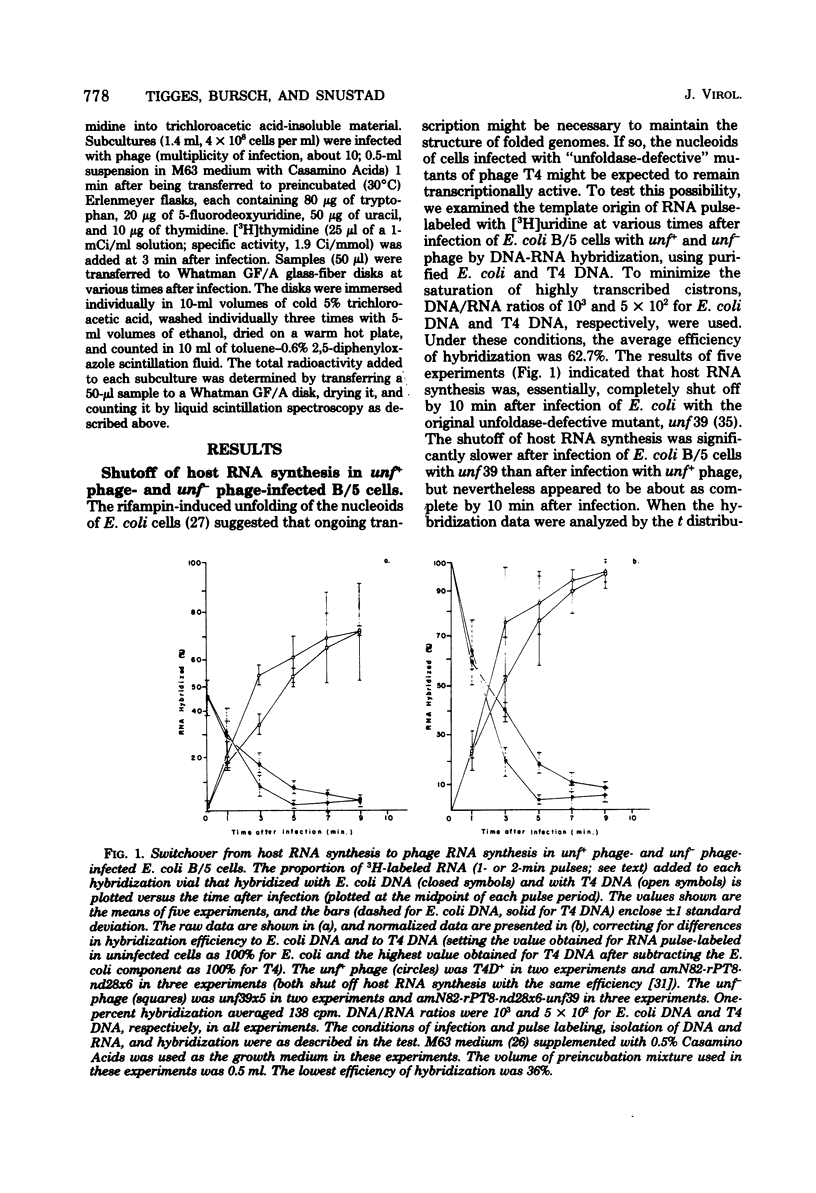
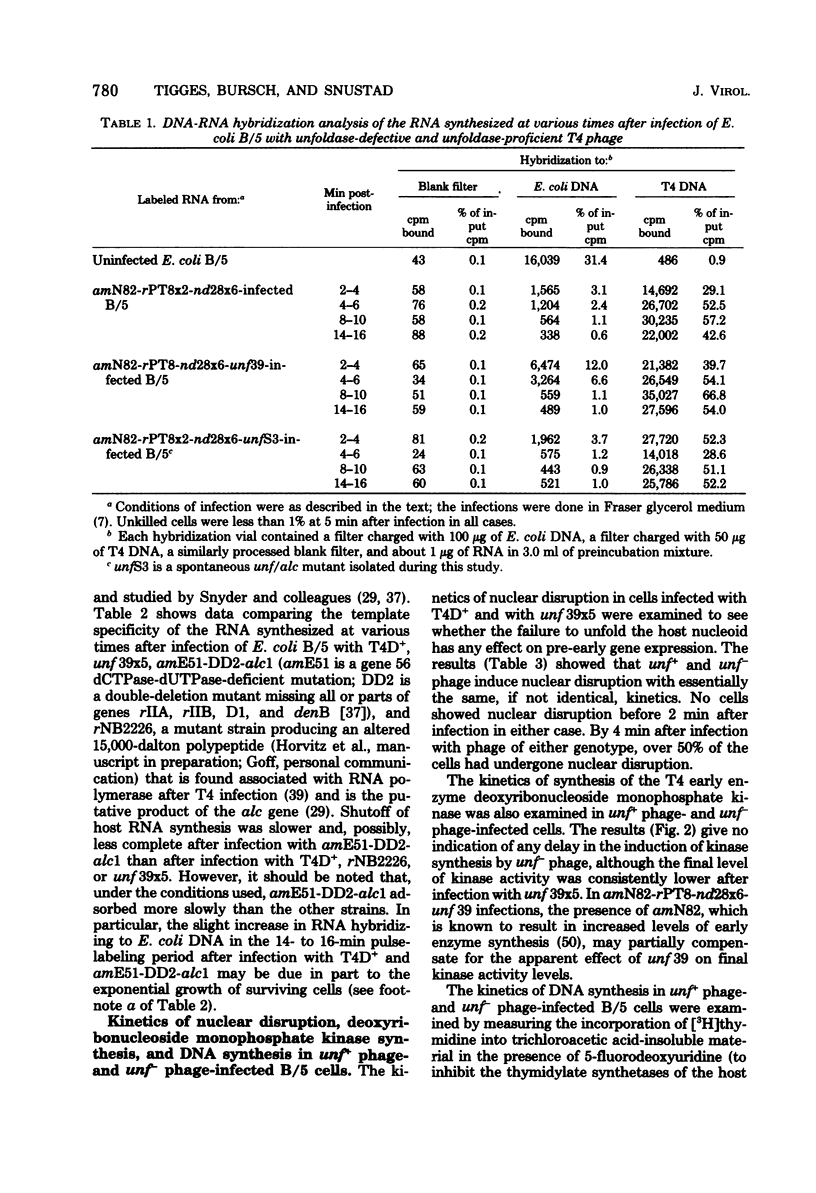
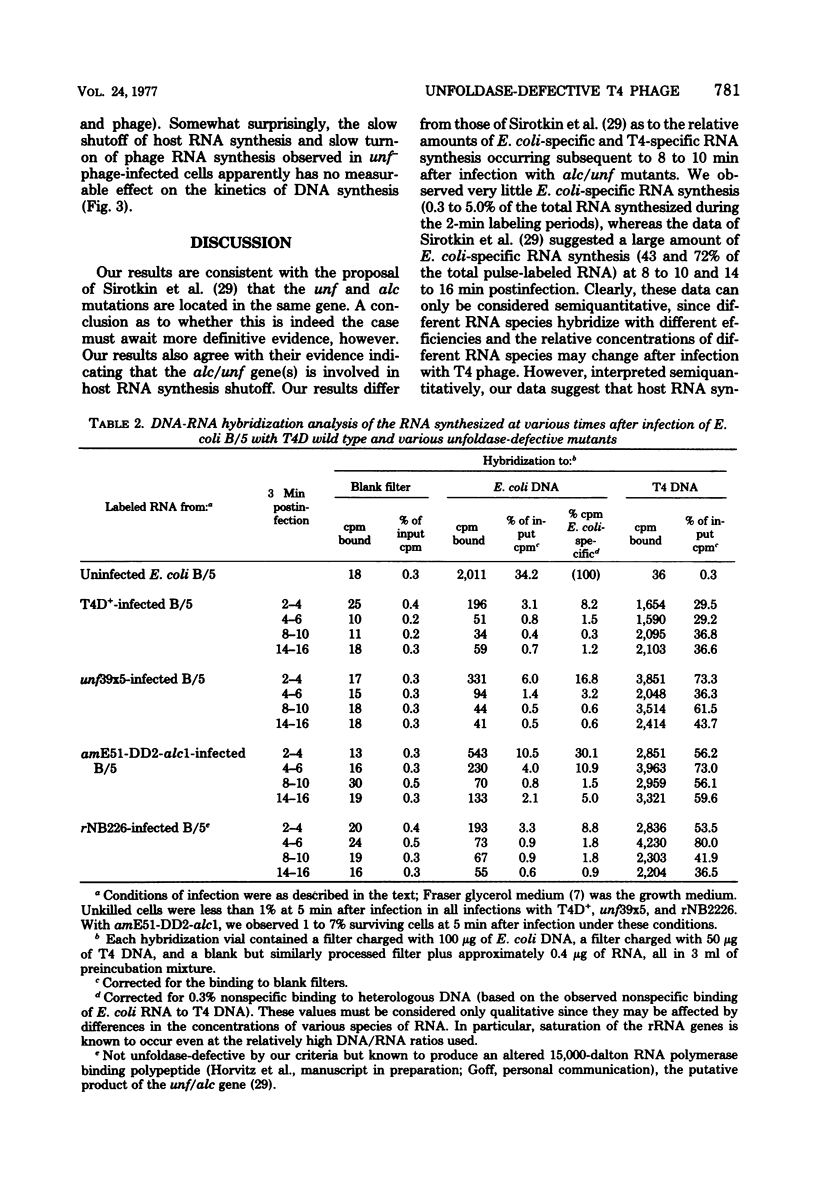
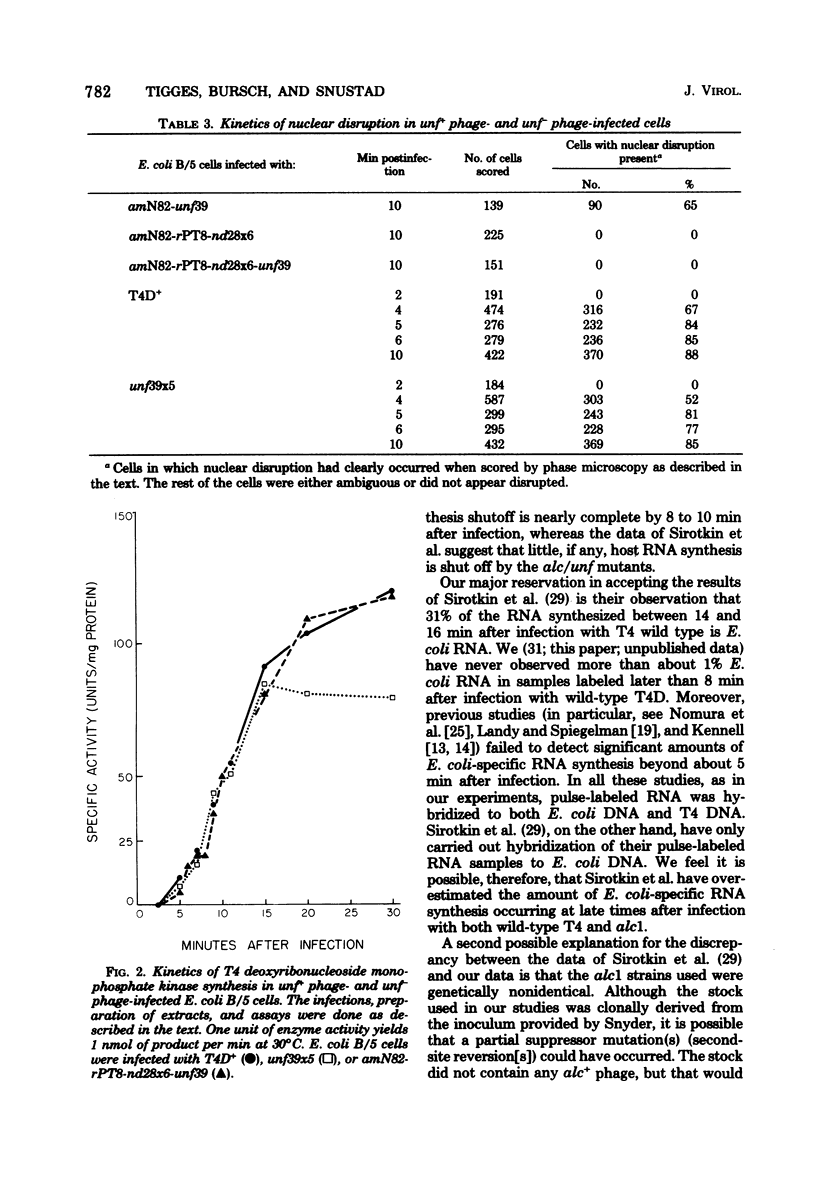
Most, if not all, host RNA synthesis was shut off after infection of Escherichia coli strain B/5 with a bacteriophage T4 multiple mutant defective in the abilities to induce (i) unfolding of the host nucleoid (unf-), (ii) nuclear disruption (ndd-), and (iii) host DNA degradation (denA-, denB-). The shutoff of host RNA synthesis and turn-on of phage RNA synthesis were slower after infection of E. coli with unf- phage than after infection with unf+ phage. This delay in the switchover from host RNA synthesis to phage RNA synthesis in unf- infections did not result in a measurable delay in the onset of nuclear disruption, deoxyribonucleoside monophosphate kinase synthesis, or DNA synthesis. unf39 did not complement alc (allows late transcription on cytosine-containing DNA) mutants, supporting the proposal of Sirotkin et al. [Nature (London) 265:28-32, 1977] that alc and unf are possibly the same gene.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONIFAS V., KELLENBERGER E. Etude de l'action des membranes du bactériophage T2 sur Escherichia coli. Biochim Biophys Acta. 1955 Mar;16(3):330–338. doi: 10.1016/0006-3002(55)90234-1. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baralle F. E., Travers A. Phage T4 infection restricts rRNA synthesis by E. coli RNA polymerase. Mol Gen Genet. 1976 Sep 23;147(3):291–297. doi: 10.1007/BF00582880. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Goff C. G. Chemical structure of a modification of the Escherichia coli ribonucleic acid polymerase alpha polypeptides induced by bacteriophage T4 infection. J Biol Chem. 1974 Oct 10;249(19):6181–6190. [PubMed] [Google Scholar]
- HERSHEY A. D., DIXON J., CHASE M. Nucleic acid economy in bacteria infected with bacteriophage T2. I. Purine and pyrimidine composition. J Gen Physiol. 1953 Jul;36(6):777–789. doi: 10.1085/jgp.36.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Munro J. L., Mendelsohn S., Wiberg J. S. Mutants in a nonessential gene of bacteriophage T4 which are defective in the degradation of Escherichia coli deoxyribonucleic acid. J Virol. 1971 Jan;7(1):95–105. doi: 10.1128/jvi.7.1.95-105.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J., RYTER A. Electron microscopical studies of phage multiplication. IV. The establishment of the DNA pool of vegetative phage and the maturation of phage particles. Virology. 1959 Aug;8:478–498. doi: 10.1016/0042-6822(59)90050-9. [DOI] [PubMed] [Google Scholar]
- KOCH A. L., PUTNAM F. W., EVANS E. A., Jr Biochemical studies of virus reproduction. VIII. Purine metabolism. J Biol Chem. 1952 May;197(1):113–120. [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. I. Continued synthesis of host ribonucleic acid. J Virol. 1968 Nov;2(11):1262–1271. doi: 10.1128/jvi.2.11.1262-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. II. Induction of host messenger ribonucleic acid and its exclusion from polysomes. J Virol. 1970 Aug;6(2):208–217. doi: 10.1128/jvi.6.2.208-217.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Titration of the gene sites on DNA by DNA-RNA hybridization. I. Problem of measurement. J Mol Biol. 1968 May 28;34(1):71–84. doi: 10.1016/0022-2836(68)90235-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., HUMAN M. L. Chromatin staining of bacteria during bacteriophage infection. J Bacteriol. 1950 Apr;59(4):551–560. doi: 10.1128/jb.59.4.551-560.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy A., Spiegelman S. Exhaustive hybridization and its application to an analysis of the ribonucleic acid synthesized in T4-infected cells. Biochemistry. 1968 Feb;7(2):585–591. doi: 10.1021/bi00842a011. [DOI] [PubMed] [Google Scholar]
- Lee M., Miller R. C., Jr T7 exonuclease (gene 6) is necessary for molecular recombination of bacteriophage T7. J Virol. 1974 Nov;14(5):1040–1048. doi: 10.1128/jvi.14.5.1040-1048.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G. E., GILLEN D. H., HEAGY F. C. Cytological changes in Escherichia coli produced by infection with phage T2. J Bacteriol. 1950 May;59(5):603–615. doi: 10.1128/jb.59.5.603-615.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhammer R., Yang H. L., Reiness G., Zubay G. Effects of bacteriophage T4-induced modification of Escherichia coli RNA polymerase on gene expression in vitro. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4928–4932. doi: 10.1073/pnas.72.12.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Witten C., Mantei N., Echols H. Inhibition of host nucleic acid synthesis by bacteriophage T4: effect of chloramphenicol at various multiplicities of infection. J Mol Biol. 1966 May;17(1):273–278. doi: 10.1016/s0022-2836(66)80107-9. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Wei J., Snyder L. T4 Bacteriophage-coded RNA polymerase subunit blocks host transcription and unfolds the host chromosome. Nature. 1977 Jan 6;265(5589):28–32. doi: 10.1038/265028a0. [DOI] [PubMed] [Google Scholar]
- Snustad D. P., Bursch C. J., Parson K. A., Hefeneider S. H. Mutants of bacteriophage T4 deficient in the ability to induce nuclear disruption: shutoff of host DNA and protein synthesis gene dosage experiments, identification of a restrictive host, and possible biological significance. J Virol. 1976 Apr;18(1):268–288. doi: 10.1128/jvi.18.1.268-288.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P., Bursch C. J. Shutoff of host RNA synthesis in bacteriophage T4-infected Escherichia coli in the absence of host DNA degradation and nuclear disruption. J Virol. 1977 Mar;21(3):1240–1242. doi: 10.1128/jvi.21.3.1240-1242.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P., Conroy L. M. Mutants of bacteriophage T4 deficient in the ability to induce nuclear disruption. I. Isolation and genetic characterization. J Mol Biol. 1974 Nov 15;89(4):663–673. doi: 10.1016/0022-2836(74)90043-6. [DOI] [PubMed] [Google Scholar]
- Snustad D. P. Dominance interactions in Escherichia coli cells mixedly infected with bacteriophage T4D wild-type and amber mutants and their possible implications as to type of gene-product function: catalytic vs. stoichiometric. Virology. 1968 Aug;35(4):550–563. doi: 10.1016/0042-6822(68)90285-7. [DOI] [PubMed] [Google Scholar]
- Snustad D. P., Parson K. A., Warner H. R., Tutas D. J., Wehner J. M., Koerner J. F. Mutants of bacteriophage T4 deficient in the ability to induce nuclear disruption. II. Physiological state of the host nucleoid in infected cells. J Mol Biol. 1974 Nov 15;89(4):675–687. doi: 10.1016/0022-2836(74)90044-8. [DOI] [PubMed] [Google Scholar]
- Snustad D. P., Tigges M. A., Parson K. A., Bursch C. J., Caron F. M., Koerner J. F., Tutas D. J. Identification and preliminary characterization of a mutant defective in the bacteriophage T4-induced unfolding of the Escherichia coli nucleoid. J Virol. 1976 Feb;17(2):622–641. doi: 10.1128/jvi.17.2.622-641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P., Warner H. R., Parson K. A., Anderson D. L. Nuclear disruption after infection of Escherichia coli with a bacteriophage T4 mutant unable to induce endonuclease II. J Virol. 1972 Jul;10(1):124–133. doi: 10.1128/jvi.10.1.124-133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L., Gold L., Kutter E. A gene of bacteriophage T4 whose product prevents true late transcription on cytosine-containing T4 DNA. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3098–3102. doi: 10.1073/pnas.73.9.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A., Rhoton J. C. Characterization of an inhibitor causing potassium chloride sensitivity of an RNA polymerase from T4 phage-infected Escherichia coli. Biochemistry. 1975 Nov 18;14(23):5074–5079. doi: 10.1021/bi00694a007. [DOI] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. Modulation of RNA polymerase specificity by ppGpp. Mol Gen Genet. 1976 Aug 19;147(2):225–232. doi: 10.1007/BF00267575. [DOI] [PubMed] [Google Scholar]
- Tutas D. J., Wehner J. M., Koerner J. F. Unfolding of the host genome after infection of Escherichia coli with bacteriophage T4. J Virol. 1974 Feb;13(2):548–550. doi: 10.1128/jvi.13.2.548-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R., Hochhauser S., Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEED L. L., COHEN S. S. The utilization of host pyrimidines in the synthesis of bacterial viruses. J Biol Chem. 1951 Oct;192(2):693–700. [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Evidence for a dual role for the bacteriophage T4-induced deoxycytidine triphosphate nucleotidohydrolase. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1233–1240. doi: 10.1073/pnas.56.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Snustad D. P., Koerner J. F., Childs J. D. Identification and genetic characterization of mutants of bacteriophage T4 defective in the ability to induce exonuclease A. J Virol. 1972 Mar;9(3):399–407. doi: 10.1128/jvi.9.3.399-407.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Snustad P., Jorgensen S. E., Koerner J. F. Isolation of bacteriophage T4 mutants defective in the ability to degrade host deoxyribonucleic acid. J Virol. 1970 Jun;5(6):700–708. doi: 10.1128/jvi.5.6.700-708.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S. Amber mutants of bacteriophage T4 defective in deoxycytidine diphosphatase and deoxycytidine triphosphatase. On the role of 5-hydroxymethylcytosine in bacteriophage deoxyribonucleic acid. J Biol Chem. 1967 Dec 25;242(24):5824–5829. [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]


