Abstract
Background
Callous-unemotional (CU) traits represent a significant risk factor for severe and persistent conduct problems in children and adolescents. Extensive neuroimaging research links CU traits to structural and functional abnormalities in the amygdala and ventromedial prefrontal cortex. In addition, adults with psychopathy (a disorder for which CU traits are a developmental precursor) exhibit reduced integrity in uncinate fasciculus, a white-matter (WM) tract that connects prefrontal and temporal regions. However, research in adolescents has not yet yielded similarly consistent findings.
Method
We simultaneously modeled CU traits and externalizing behaviors as continuous traits, while controlling for age and IQ, in order to identify the unique relationship of each variable with WM microstructural integrity, assessed using diffusion tensor imaging. We used tract-based spatial statistics to evaluate fractional anisotropy, an index of WM integrity, in uncinate fasciculus and stria terminalis in 47 youths aged 10–17 years, of whom 26 exhibited conduct problems and varying levels of CU traits.
Results
Whereas both CU traits and externalizing behaviors were negatively correlated with WM integrity in bilateral uncinate fasciculus and stria terminalis/fornix, simultaneously modeling both variables revealed that these effects were driven by CU traits; the severity of externalizing behavior was not related to WM integrity after controlling for CU traits.
Conclusions
These results indicate that WM abnormalities similar to those observed in adult populations with psychopathy may emerge in late childhood or early adolescence, and may be critical to understanding the social and affective deficits observed in this population.
Keywords: Callous-unemotional traits, conduct problems, diffusion tensor imaging, uncinate fasciculus, white matter
Introduction
Conduct problems in children and adolescents are characterized by disruptive and externalizing behaviors and are among the primary reason that youths are referred to mental health practitioners (Kazdin, 1995; Turgay, 2004). However, the effectiveness of treatments for youth conduct problems remains poor relative to treatments for other common pediatric psychological disorders (Kazdin, 2000). Among the reasons for ongoing difficulties in treating conduct problems is heterogeneity among affected children (Eyberg et al. 2008; Masi et al. 2013; Hawes et al. 2014). One significant source of heterogeneity among children with conduct problems is the presence or absence of callous-unemotional (CU) traits, a constellation of affective and personality traits in children that represent a precursor to adult psychopathy (Barry et al. 2000; Crowe & Blair, 2008; Pardini & Frick, 2013). CU traits include deficits in prosocial emotions such as empathy, guilt, and remorse; an uncaring nature; and shallow patterns of emotional responding, particularly fear-based responding (Frick & White, 2008; Marsh et al. 2011; Sylvers et al. 2011). The presence of elevated CU traits is associated with more severe and persistent disruptive behavior problems in childhood and adolescence, as well as higher risk for persistent aggressive, antisocial, and criminal behavior in adulthood (Pardini, 2006; Kahn et al. 2013). CU traits are highly heritable (Viding et al. 2005, 2008) and are associated with distinct patterns of behavioral and neural dysfunction (Crowe & Blair, 2008). Neuroscience research has begun to delineate the specific abnormalities in neural anatomy and functioning that may be associated with CU symptomology. In particular, evidence is accumulating that reduced amygdala responsiveness to fear-relevant stimuli is a central feature of CU traits (Marsh et al. 2008; Jones et al. 2009; Viding et al. 2012), and may be important to understanding patterns of aggression in these youths (Lozier et al. 2014). As yet unclear, however, is the role of white-matter (WM) abnormalities in CU traits in children and adolescents. In the present study, we aimed to clarify the relationship between CU traits and WM integrity in adolescent youths with conduct problems.
Dysfunction in the amygdala and ventromedial prefrontal cortex (vmPFC) have been reliably associated with CU traits in children and adolescents (Marsh et al. 2008; Sebastian et al. 2012) and with psychopathy in both youths and adults (Finger et al. 2008; Glenn et al. 2009; Harenski et al. 2010; Decety et al. 2013). The amygdala is a temporal lobe structure involved in coding the affective significance of external stimuli, and information from this structure is fed forward to vmPFC to guide decision-making (Cardinal et al. 2002). It is theorized that reduced responsiveness in the amygdala to affective stimuli, combined with inadequate signaling of reinforcement expectancies between amygdala and the vmPFC, underlies the major social and behavioral deficits observed in CU youths (Blair, 2007). This renders possible aberrations in the WM tracts that connect prefrontal and temporal regions of particular interest with respect to the etiology of CU traits.
Multiple studies have now found reduced integrity in fronto-temporal WM tracts in adults with psychopathy. The uncinate fasciculus is a WM tract of particular significance because it is the primary pathway connecting vmPFC with structures in the anterior temporal lobe, including the amygdala (Petrides & Pandya, 2007), and because it has been shown to play a critical role in emotional empathy (Oishi et al. 2015). Accordingly, three recent studies of male psychopathic offenders found psychopathy to be associated with reduced WM integrity in right uncinate fasciculus (Craig et al. 2009; Motzkin et al. 2011; Hoppenbrouwers et al. 2013). Motzkin and colleagues also linked deficits in WM integrity in this tract to reduced functional connectivity between amygdala and vmPFC.
Efforts to delineate WM abnormalities in youths have not yielded similarly consistent patterns. One study of boys with psychopathic traits revealed decreased WM concentrations in several regions, including right superior frontal lobe and right superior temporal gyrus, but also identified increased WM concentrations bilaterally in middle frontal gyrus (De Brito et al. 2011). In a mixed gender sample of adolescents with conduct disorder undifferentiated with respect to CU or psychopathic traits, widespread patterns of reduced integrity across multiple WM tracts were identified (Haney-Caron et al. 2014). By contrast, two studies of adolescent boys with conduct disorder have found increased WM integrity in the uncinate fasciculus relative to controls (Passamonti et al. 2012; Sarkar et al. 2013). In one, WM integrity in uncinate fasciculus was found to be unrelated to psychopathic or CU traits within the subset of boys with conduct disorder (Sarkar et al. 2013). A fifth study found no group differences in WM integrity in any region when comparing a mixed gender sample of adolescents with disruptive behavior disorders and CU traits with controls (Finger et al. 2012), although this study did identify reduced ventromedial prefrontal-amygdala functional connectivity in participants with CU traits. Because these studies vary in many respects, the underlying reasons for the inconsistencies in their findings are not clear. Possible causes may include heterogeneous sampling strategies and diffusion tensor imaging (DTI) analysis methodologies. In addition, the design of these studies may not have been optimized for dissociating the neurobiological characteristics of CU traits from those of externalizing behaviors. This is potentially important because although externalizing behaviors like aggression, destructiveness, and rule breaking are a common manifestation of CU traits, these behaviors can also result from other etiological factors (Frick, 2012).
We aimed to clarify the relationship between CU traits and WM integrity using a different approach than previous studies, one which simultaneously models both conduct problems and CU traits as continuous measures, in keeping with the continuously distributed nature of these variables in the population (Hicks & Patrick, 2006; Sebastian et al. 2012). CU traits are positively correlated with externalizing behaviors in youths, but these variables frequently show opposite patterns of covariance with various physiological, behavioral, and neurobiological outcome measures (Crowe & Blair, 2008; Frick, 2012). Among youths with conduct problems who engage in externalizing behaviors, those with low levels of CU traits exhibit elevated emotional reactivity and responsiveness in the amygdala and related structures to affective stimuli, whereas those with elevated CU traits show reduced emotional reactivity and decreased amygdala responsiveness to affective stimuli (Anastassiou-Hadjicharalambous & Warden, 2008; Viding et al. 2012; Lozier et al. 2014; Seara-Cardoso et al. in press). This suggests that CU traits and externalizing behaviors are dissociable, and that dissociating these variables may reveal distinct patterns of neurobiological dysfunction with potentially important treatment implications.
In the present study, we used DTI (Le Bihan et al. 2001) to assess WM integrity in regions of interest (ROIs) previously identified as aberrant in adult populations with psychopathy (right and left uncinate fasciculus) as well as in the stria terminalis/fornix, a WM tract that represents a major output pathway of the amygdala, and that projects from this structure to brain stem and hypothalamic regions involved in regulating fear responding (Davis et al. 1997; Walker et al. 2003). In light of past findings in adult psychopathy and in light of the important role fronto-temporal WM tracts play in emotional empathy (Oishi et al. 2015), we hypothesized that WM integrity in these regions would be specifically predicted by CU traits. Furthermore, although both fronto-temporal connectivity deficits and amygdala dysfunction in response to fear-relevant stimuli have been investigated separately in youths with conduct problems and CU traits, it is as yet unclear how these two phenomena are related. We therefore also assessed the relationship between WM abnormalities and functional activation in the amygdala.
Method and materials
Participants
Forty-seven participants aged 10–17 (mean = 14.37, S.D. = 2.61), including 26 male and female youths with conduct problems and 21 healthy male and female control youths were recruited from the Washington, DC, metropolitan region using fliers, brochures, and advertisements in local publications. Study procedures were explained to each participant and a parent or legal guardian who provided, respectively, informed assent and consent before the commencement of testing. Participants and parents/guardians then completed measures to assess conduct problems, CU traits, and demographic information (Table 1). Cognitive intelligence was assessed in all participants using the Kaufman Brief Intelligence Test-2 (K-BIT; Kaufman & Kaufman, 2004) to ensure that all participants’ IQ was ≥80. Qualified participants reported no history of head trauma or neurological disorders. Youths were medication-free at the time of scanning, with the exception of three youths with conduct problems taking, respectively, aripiprazole, methylphenidate and quetiapine fumarate, and one youth taking divalproex sodium, guanfacine, methylphenidate, and trazodone; for these youths, medications could not be withheld prior to scanning. Pregnancy testing prior to scanning ensured that female participants were not pregnant.
Table 1.
Demographic and clinical characteristics of control participants and participants with conduct problems
| Participant characteristics | Healthy controls (n = 21) | Conduct problems (n = 26) | p value |
|---|---|---|---|
| Demographic variable, mean (S.D.) | |||
| Male:female ratio | 12:9 | 13:13 | 0.77 |
| Age, years | 13.55 (2.32) | 15.05 (2.67) | <0.05a |
| IQb | 110.38 (14.90) | 98.19 (11.34) | <0.005a |
| Race | |||
| White | 11 | 9 | >0.05a |
| Black or African American | 9 | 16 | |
| Asian | 1 | 1 | |
| Behavioral measures, mean (S.D.) | |||
| Externalizing behavior (CBCL) | 42.86 (8.33) | 73.04 (5.02) | <0.001a |
| Internalizing behavior (CBCL) | 46.95 (10.63) | 64.46 (12.35) | <0.001a |
| Attention problems, raw score (CBCL) | 2.43 (2.69) | 10.00 (4.50) | <0.001a |
| Callous-unemotional traits (ICU) | 25.38 (6.70) | 44.54 (9.42) | <0.001a |
| Strengths and Difficulties Questionnaire (SDQ) – conduct problems subscale |
0.81 (1.03) | 6.35 (1.44) | <0.001a |
| Reactive aggressionc | 4.86 (3.88) | 12.90 (4.60) | <0.001a |
| Proactive aggressionc | 0.86 (1.20) | 6.81 (5.50) | <0.001a |
| Substance used | 0 | 5 | >0.05a |
CBCL, Child Behavior Checklist; ICU, Inventory of Callous-Unemotional Traits.
Group differences were assessed with two-tailed t tests, χ2 tests, or Fisher’s exact tests for categorical variables.
Cognitive intelligence measured from the full-scale IQ on the Kaufman Brief Intelligence Test-2.
Measures of aggression are subscales from the Reactive-Proactive Aggression Questionnaire.
Substance use was determined by parent reports of ‘frequent use’.
Ethical standards
The protocol was approved by the Georgetown University Institutional Review Board. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Clinical measures
Conduct problems were assessed using the Strengths and Difficulties Questionnaire (SDQ; Goodman & Scott, 1999) and Child Behavior Checklist (CBCL; Achenbach, 1991), which were completed by a parent/guardian. The SDQ measures overall stress, emotional symptoms, hyperactivity, peer problems, prosocial behavior and conduct problems. The CBCL measures internalizing (i.e. anxious, depressive) and externalizing (i.e. rule-breaking and aggressive) behavior in children and adolescents. Participants were classified using established cut-offs on these measures (SDQ Conduct Problems score >3; CBCL age- and gender-normed Externalizing score >98th percentile). CU traits were assessed using the Inventory of Callous-Unemotional Traits (ICU), a measure adapted for use in children and adolescents from the CU subscale of the APSD (Kimonis et al. 2008; Frick & Hare, 2001). Consistent with established procedures, the ICU was scored by taking the highest item rating from either the child or parent/guardian version and summing the total of these scores (Jones et al. 2009; Sebastian et al. 2012; Lozier et al. 2014). This scoring method is recommended to account for reporter bias and discrepancies that can emerge following a summative or averaging approach (Roose et al. 2010). Reliability of ICU scores was acceptable among both control participants (α = 0.67) and those with conduct problems (α = 0.70).
Functional magnetic resonance imaging (fMRI) task
Thirty-three youths in our sample (22 conduct problems, 11 controls) completed an fMRI face-emotion processing task, the results of which have been previously reported (Lozier et al. 2014). During the scan, participants viewed images of ten men and women from the Pictures of Facial Affect series (Ekman & Friesen, 1976) displaying positive-neutral, fearful, and angry expressions. Faces appeared for 2 s in randomized order, followed by a 1-s fixation cross. The emotion processing component of the task was implicit such that participants responded by button press to the gender of each face rather than the expression (Marsh et al. 2008; Jones et al. 2009; Lozier et al. 2014). The task included four 5.5-min consecutive runs, each containing 80 face trials and 20 jittered interstimulus interval trials.
Imaging protocol
MRI scanning was performed using a 3-T Siemens Tim Trio. DTI data were acquired using two runs of an echo planar imaging (EPI) sequence (TE = 86 ms, TR = 6300 ms; 2.5 mm3 spatial resolution), each lasting 4:01 min. For each run, five non-diffusion-weighted (b = 0 s/mm2) and 30 directionally orthogonal, diffusion-weighted (b = 1000 s/mm2) images were collected. The four runs of fMRI data (task described above) were collected using a T2*-weighted EPI sequence with the following parameters: TR = 3000 ms, TE = 30 ms, 3.0 mm voxels, 56 slices, 64 × 64 matrix, FOV = 192 mm. Finally, a high-resolution T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) structural scan was collected for each subject (TR = 1900 ms, TE = 5.52 ms, 1.03 mm voxels, 176 slices, 246 × 256 matrix, FOV = 250 mm). Due to ongoing changes in scanning protocols, an 8-channel phased array head coil was used with the 33 participants (22 conduct problems, 11 controls) who completed the face-emotion task, whereas for the remaining 14 participants (4 conduct problems, 10 controls), a 12-channel head coil was used. All analyses control for head coil type and/or separately analyzed only those youths scanned using the 8-channel coil.
Analysis of DTI data
Four participants with excessive movement parameters (>3 mm) during image acquisition were excluded from further analysis to avoid biasing subsequent statistical analysis with motion artifact. FMRIB’s Diffusion Toolbox, a part of FSL (http://www.fmrib.ox.ac.uk/fsl), was used to correct the remaining 47 participants’ images for eddy current distortion and head motion, and create individual fractional anisotropy (FA) images from the DTI data. Tract-Based Spatial Statistics (TBSS), also a part of FSL, was used to create a standard space skeletonized FA image for each participant. FA images were aligned into a common space with FNIRT (a nonlinear registration tool), and normalized to a Montreal Neurological Institute (MNI) template. The mean FA image across all participants was then thinned to create a skeleton image that represented the tracts common to the group, and thresholded at an FA of 0.2 to restrict the skeleton to WM. Each participant’s FA image was projected onto this common skeleton. ROIs in right and left uncinate fasciculus and stria terminalis/fornix were created from the ICBM-DTI-81 WM atlas (Mori et al. 2005; Oishi et al. 2008). The mean FA within these ROIs was computed for the statistical analyses below.
Analysis of fMRI data
As reported previously (Lozier et al. 2014) fMRI data were processed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK). Prior to preprocessing, the first four volumes of each functional run were excluded. Echoplanar images were slice-time corrected, realigned, co-registered, normalized into MNI space, and smoothed with an 8 mm full width at half maximum Gaussian kernel. Realignment parameters were inspected to ensure head motion did not exceed one voxel/TR. Four participants were excluded from activation analyses due to excessive motion. For each participant a general linear model was run that included regressors for each stimulus type (fear, anger, etc.), incorrect/non-response trials, and six motion parameters. Contrast images were generated for fearful faces over implicit baseline. Mean unthresholded values within bilateral anatomically defined amygdala ROIs were extracted from these contrast images to derive amygdala activation in response to fearful faces for each participant.
Group analysis
A series of analyses were performed to identify relationships between psychological and behavioral variables and WM integrity. Multiple regression analyses were computed to assess the relationship between externalizing behavior and CU traits and WM structural integrity in the ROIs. Both externalizing behaviors and CU traits were entered simultaneously into multiple regression models so that the unique contributions of each variable could be assessed while controlling for the other. Regression analyses were conducted across the entire sample as well as in only the sample of youths with conduct problems. Finally, to assess potential relationships between WM amygdala connections and functional activation in the amygdala in response to fearful faces, we conducted additional multiple regression analyses to determine whether amygdala activation can be predicted based on the integrity of uncinate fasciculus and/or stria terminalis/fornix. Follow-up bootstrap mediation analyses were conducted using the PROCESS macro implemented in SPSS 22 (Hayes, 2013).
Finally, following our previous work (Lozier et al. 2014) we also computed analyses of variance (ANOVA) in SPSS 22 (IBM Corp., USA) to compare the efficacy of this approach with that of a regression model approach. For these analyses, youths with conduct problems were divided into two post-hoc groups using a median split on ICU scores (median = 44), resulting in a low CU traits group (n = 14) and a high CU traits group (n = 12).
Results
Group comparisons indicated that children with conduct problems differed from controls in both age (t45 = 2.04, p < 0.05), and IQ (t45 = 3.19, p < 0.05). Post-hoc correlational analyses determined that neither age nor IQ showed any significant associations with WM integrity in our neuroanatomical ROIs. We nevertheless included age and IQ, as well as a dichotomous head coil variable, in all regression analyses to control for identified group differences.
We next conducted separate multiple regression analyses assessing the associations between externalizing behavior and CU traits, respectively, and WM integrity. Across the entire sample, WM integrity was negatively associated with externalizing behaviors in both right (t42 = −2.44, p < 0.05, β = −0.40), and left (t42 = −2.17, p < 0.05, β = −0.37) uncinate fasciculus (Fig. 1), and right stria terminalis/fornix (t42 = −3.04, p < 0.005, β = −0.47) (Fig. 2). WM integrity was also negatively associated with CU traits in both right (t42 = −3.19, p < 0.005, β = −0.48) and left (t42 = −2.99, p < 0.01, β = −0.48) uncinate fasciculus (Fig. 1), and right (t42 = −3.91, p < 0.001, β = −0.56), and left (t42 = −2.20, p < 0.05, β = −0.37), stria terminalis/fornix (Fig. 2). We next conducted multiple regression analyses that simultaneously included both externalizing behaviors and CU traits. Results indicated that, when these variables were entered together, CU traits were more closely associated with reductions in WM integrity in right (t41 = −1.91, p = 0.06, β = −0.44), and left (t41 = −1.92, p = 0.06, β = −0.47) uncinate fasciculus, and right stria terminalis/fornix (t41 = −2.24, p < 0.05, β = −0.48) (Fig. 3a).1 By contrast, no associations were found between WM integrity and externalizing behavior after controlling for CU traits (right uncinate fasciculus, p = 0.81; left uncinate fasciculus, p = 0.96; right stria terminalis/fornix, p = 0.66).2
Fig. 1.
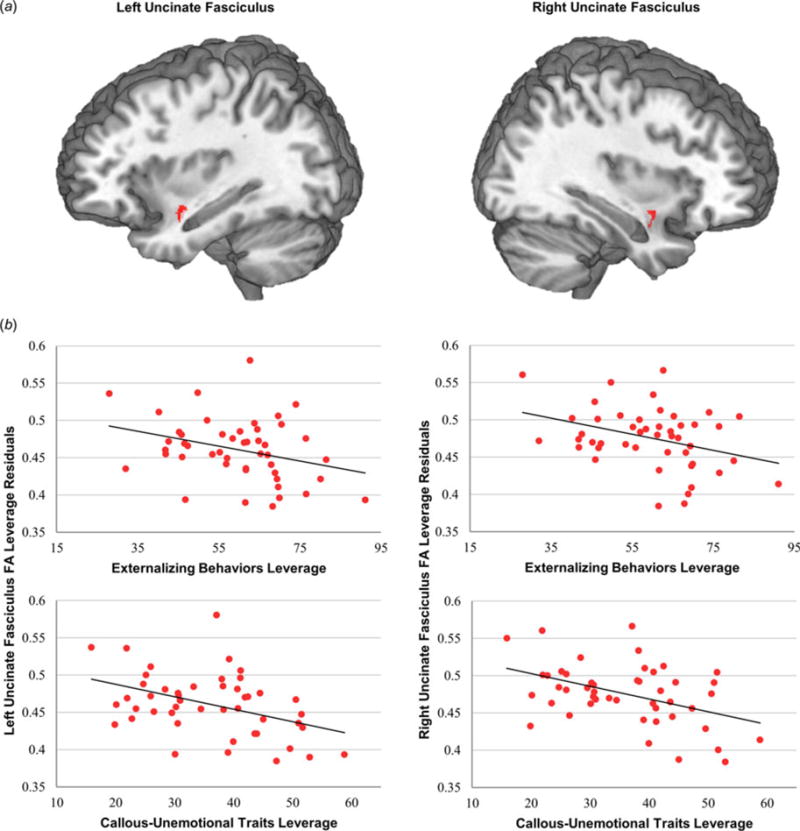
Uncinate fasciculus white-matter integrity is related separately to externalizing behaviors and callous-unemotional (CU) traits. (a) Mean white-matter integrity was extracted from uncinate fasciculus anatomical regions of interest. (b) Separate regression analyses demonstrated that externalizing behaviors and CU traits are related to white-matter integrity in both left and right uncinate fasciculus. Leverage plots show the unique association between each predictor (i.e. CU traits), and white-matter integrity, controlling for covariates of no interest.
Fig. 2.
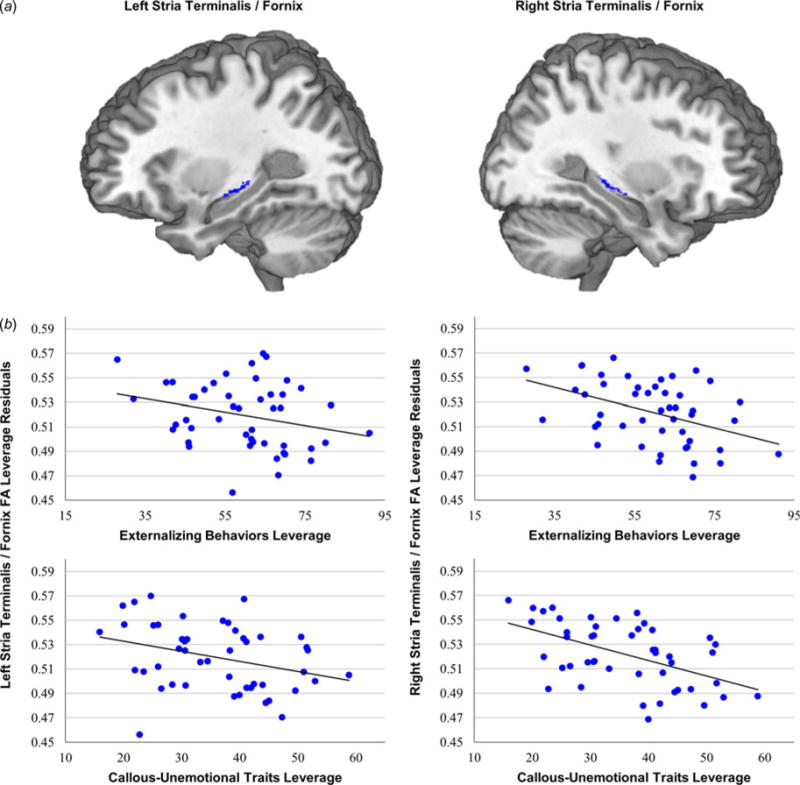
Stria terminalis/fornix white-matter integrity is related both to externalizing behaviors and callous-unemotional (CU) traits. (a) Mean white-matter integrity was extracted from stria terminalis/fornix anatomical regions of interest. (b) Separate regression analyses demonstrated that CU traits, but not externalizing behaviors, were related to white-matter integrity in the left stria terminalis/fornix. Both externalizing behaviors and CU traits were related to white-matter integrity in the right stria terminalis/fornix.
Fig. 3.
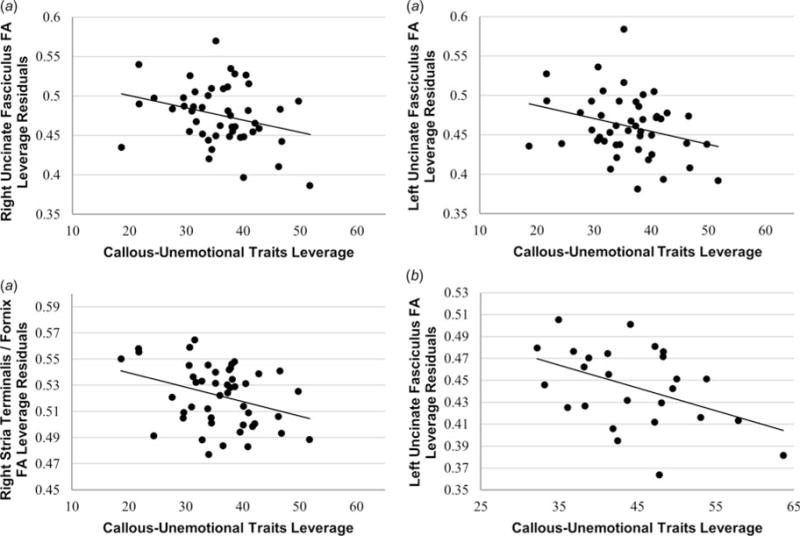
Callous-unemotional (CU) traits are uniquely associated with white-matter integrity after controlling for severity of externalizing behaviors. Leverage plots demonstrate that the unique variance associated with CU traits, controlling for externalizing behaviors, is negatively related to white-matter integrity, (a) both across the entire group, and (b) within the conduct problems group. By contrast, the unique variance associated with externalizing behaviors did not relate to white-matter integrity. Note that values reflected in these leverage plots reflect residual values rather than raw scores, in keeping with our analytic model.
In light of new diagnostic recommendations that CU traits represent an important distinction within the population of conduct disordered children and adolescents (Frick & Moffitt, 2010), we repeated our primary regression analyses examining only youths with conduct problems. These analyses obtained similar results in left uncinate fasciculus, with WM integrity in this region again negatively associated with CU traits (t20) −2.20, p < 0.05, β = −0.54) (Fig. 3b). Although previous research has suggested gender may significantly moderate the biological correlates of CU traits among youths with conduct problems (Meier et al. 2011), these results persisted after controlling for gender in this analysis (t19 = −2.22, p < 0.05, β = −0.53), and gender was not itself a significant predictor of WM integrity in the analysis (p = 0.15).
We also conducted multiple regression analyses in the portion of our sample who had completed the fMRI face-emotion processing task (all scanned using the same head coil). Results showed that, among youths with conduct problems, reduced activation in the left amygdala was associated with reduced WM integrity in both left uncinate fasciculus (t18 = 2.72, p < 0.05, β = 0.512), and left stria terminalis/fornix (t18 = 2.74, p < 0.05, β = 0.560). Reduced activation in right amygdala was predicted by reduced WM integrity in right uncinate fasciculus (t18 = 2.11, p < 0.05, β = 0.413) (Fig. 4). The results of bootstrap mediation analyses conducted to test the hypothesis that the relationship between WM integrity and CU traits is mediated by amygdala activation (with IQ and age included as covariates) did not, however, reveal an indirect effect of left uncinate fasciculus FA [bias-corrected 95% confidence interval (CI) −68.9 to 111] or left stria/terminalis/fornix FA (bias-corrected 95% CI −137 to 53.9) on CU traits through left amygdala activation. Results also did not reveal an indirect effect of right uncinate fasciculus FA (bias-corrected 95% CI −94.7 to 52.3) on CU traits through right amygdala activation. There were no significant mediation effects when externalizing behavior was added as an additional covariate to these analyses.
Fig. 4.
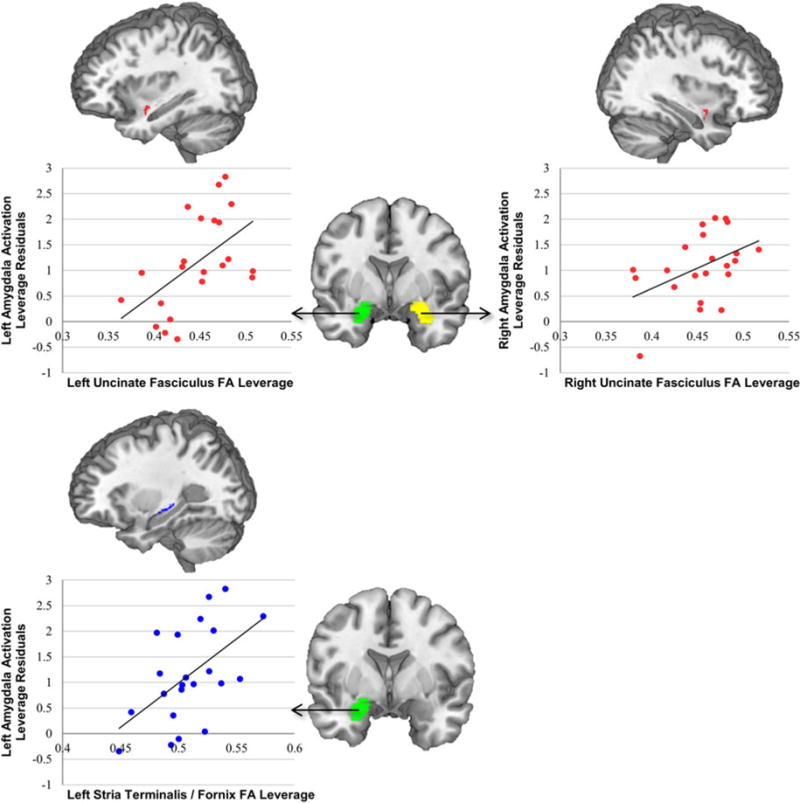
White-matter integrity and amygdala activation to fearful faces are related in youths with conduct problems. Mean amygdala activation to fearful faces was extracted from the AAL anatomical regions of interest pictured in the center of the figure. Among youths with conduct problems, left amygdala activation was related to white-matter integrity in the left uncinate fasciculus and left stria terminalis/fornix. Right amygdala activation was related to white-matter integrity in the right uncinate fasciculus.
Finally, we computed 3 × 1 ANOVAs to assess group differences in FA across all participants (control, conduct problems/high CU, conduct problems/low CU), once again controlling for age, IQ, and coil. Results showed group differences in FA in all neuroanatomical ROIs, including the right (F1,41 = 4.66, p < 0.05), and left (F1,41 = 3.62, p < 0.05) uncinate fasciculus, and right (F1,41 = 6.55, p < 0.005), and left (F1,41 = 2.86, p = 0.075), stria terminalis (the latter at the trend level). Follow-up pairwise contrast tests revealed reduced FA in low CU participants compared to healthy control participants in right uncinate fasciculus (p < 0.05) and right stria terminalis (p < 0.05). High CU participants also exhibited reduced FA compared to healthy control participants in the right (p < 0.01) and left (p < 0.05) uncinate fasciculus and right (p < 0.005) and left (p < 0.05) stria terminalis. However, comparisons between low and high CU participants did not show significantly different FA in any of the examined WM tracts (all Ps > 0.05).
Discussion
The results of this study reinforce the importance of considering CU traits as a continuous variable that is dissociable from the severity of externalizing behavior in youths. Results of regression analyses indicated that both the severity of externalizing and the severity of CU traits were individually associated with lower WM integrity in bilateral uncinate fasciculus and stria terminalis. Simultaneously modeling both variables, however, suggests it is the unique variance associated with CU traits that drives these results, as WM integrity was no longer predicted by the severity of externalizing behavior after controlling for CU traits, whereas the inverse was not true. We found that group-based analyses were less sensitive to these differences, showing only FA differences between healthy control youths and youths with conduct problems, but no significant FA differences among youths with conduct problems as a function of CU traits. Finally, we identified the first evidence that, among youths with conduct problems, WM integrity in the uncinate fasciculus is related to patterns of functional activation in the amygdala in response to fearful facial expressions, although mediation analyses did not indicate a causal pathway whereby structural differences influence CU traits through their impact on activation patterns.
Our approach yielded the first results that identify similar WM deficits in youths with CU traits as have been consistently found in adult psychopaths. This is significant because these findings indicate continuity between pediatric CU populations and adult psychopathic populations in the development of temporal-prefrontal WMs connections, consistent with the characterization of CU traits as the pediatric precursor to adult psychopathy (Barry et al. 2000). Whereas three studies have now found reduced WM integrity in the uncinate fasciculus of adult male psychopaths (Craig et al. 2009; Motzkin et al. 2011; Hoppenbrouwers et al. 2013), previous studies in adolescents have yielded less consistent results, with studies variably finding increased, decreased, or no difference in WM integrity in youths with conduct problems (Finger et al. 2012; Passamonti et al. 2012; Sarkar et al. 2013; Haney-Caron et al. 2014).
How can the divergence of our findings from prior studies in youths be explained? Clearly one distinction between the adult and youth findings is that, whereas all adult studies have assessed participants for psychopathy, most studies in youths have variably assessed youths for only undifferentiated conduct disorder (Passamonti et al. 2012; Haney-Caron et al. 2014), or have limited their samples to only youths with conduct disorder and high CU traits (Finger et al. 2012). Differences in participant classification across studies may have affected the consistency of results across the studies. In addition, the designs of the individual studies may not have been optimized for dissociating the neurobiological characteristics of externalizing behaviors from those of CU traits. Youths with undifferentiated conduct disorder may or may not vary significantly in CU traits, depending on how they were sampled, because youths with elevated CU traits comprise a minority of all children with conduct problems (Frick & Moffitt, 2010). Youths selected to have Conduct Disorder and high CU traits are also unlikely to vary substantially in CU traits.
It is difficult to definitively evaluate this hypothesis by assessing the means and ranges of CU traits across studies, however, due to the use of different measurement instruments. Previous DTI studies of youths have assessed CU and/or psychopathic traits using the Youth Psychopathic Traits Inventory (YPI; Passamonti et al. 2012), Antisocial Process Screening Device (APSD; Sarkar et al. 2013), and the Psychopathy Checklist: Youth Version (PCL:YV; Finger et al. 2012). Compared to the YPI, the ICU assessment used in this study has the advantage of combining information from two reporters – both parent and child – which is recommended particularly for this population to account for reporter bias and discrepancies (Roose et al. 2010). The ICU also provides a more detailed inventory of CU traits that is designed to overcome the restricted range and limited response items of the CU subscale of the APSD (Roose et al. 2010). Therefore, it is plausible that both our sampling strategy and our strategy for assessing CU traits may have increased the sensitivity of our analyses for detecting the hypothesized relationships. On the other hand, it should be noted that in prior studies that compared youths with conduct disorder to controls, the comparison of these extreme groups could nevertheless have been capable of detecting effects related to externalizing and CU traits when these effects are correlated. Given this, the issue of variability in CU traits may not be the primary contributing factor to the divergence of prior results.
Another potential reason for the discrepancy between our results and those of previous studies is our choice of DTI analysis methodology. Two previous studies using a tractography approach found results opposite to ours, such that youths with conduct disorder (who also showed increased CU traits relative to controls) exhibited increased FA within the uncinate fasciculus (Passamonti et al. 2012; Sarkar et al. 2013). Our methodology specified pre-defined ROIs within the center of major WM tracts, whereas the two aforementioned studies defined the uncinate fasciculus as all WM fibers passing through one or more seed regions. These studies therefore averaged FA across larger and more diffuse portions of fiber tracts than our ROI tract-based spatial statistic approach. It may be that examining the center of tracts that were consistent across our participants produced more reliable intra-individual FA measurements, resulting in fundamentally different WM integrity estimates than those obtained through methods such as tractography. Supporting this view, one previous study that used a tract-based spatial statistics approach similar to ours found lower FA bilaterally within the uncinate fasciculus in youths with conduct disorder (Haney-Caron et al. 2014). One limitation to this explanation for the observed discrepancies is that both tract-based spatial statistics and tractography have yielded convergent findings of reduced fronto-temporal WM integrity in studies of adult psychopaths (Craig et al. 2009; Motzkin et al. 2011; Hoppenbrouwers et al. 2013). It may be, however, that imaging methodology and clinical characterizations are especially important in pediatric samples because of developmental heterogeneity. DTI studies of related disorders such as attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders have also obtained heterogeneous findings, and developmental WM changes and potentially different developmental trajectories in clinical populations have been suggested as points of concern (Van Ewijk et al. 2012; Aoki et al. 2013). In addition, one previous DTI study in youths with conduct problems and psychopathic traits used both tractography and tract-based spatial statistics, and these methods both failed to identify WM aberrations (Finger et al. 2012). However, the sensitivity of this study may have been reduced by its relatively small sample size and by the sample including only youths with both conduct problems and psychopathic traits, thus potentially confounding these variables. This also precluded an effective analysis of the unique effects of either variable, or an effective analysis of psychopathic traits as a continuous variable. Together, these issues suggest that both the choice of DTI analysis strategy and sampling strategy may be important features in deriving and interpreting DTI results in this population.
Several limitations of this study should also be considered. First, due to ongoing changes in scanning protocols, youths in this study were scanned using different MRI head coils. We took several steps to mitigate this concern, including controlling for head coil type in all regression analyses and conducting supplementary regression analyses restricted to only the 8-channel coil. Results of all supplementary analyses confirmed results of analyses conducted across the full sample and heighten our confidence that this variable minimally influenced our findings. Second, medication could not be withheld from three participants prior to scanning. However, previous studies have not found a significant impact of psychotropic medications on fractional anisotropy (Kyriakopoulos et al. 2011; Hafeman et al. 2012). Third, our groups differed in cognitive intelligence and age. To account for this, we included these variables as covariates in all analyses. We also did not exclude or control for various co-morbities such as internalizing or anxiety or ADHD. It has been suggested that ADHD symptoms are important to consider when examining youths with conduct problems (Sarkar et al. 2013). However, we were concerned about limiting the number of regressors in our analyses to only those that were essential due to statistical problems that arise as the number of regressors increases (Breiman & Freedman, 1983). Because a recent meta-analysis of DTI studies in ADHD found the most consistent WM aberrations in this population in the anterior corona radiata, right forceps minor, bilateral internal capsule, and left cerebellum, rather than the uncinate fasciculus or stria terminalis/fornix (Van Ewijk et al. 2012), we concluded that this variable was not likely closely related to our hypotheses. It should also be noted that our study employed research assessments of conduct problems rather than clinical assessments of conduct disorder. Although previous studies have found convergent neuroimaging results when similar paradigms have been employed across youths classified using clinical (Marsh et al. 2008) and research (Jones et al. 2009) assessments, it is nonetheless important to confirm that the present findings apply to clinically assessed participants. Finally, in addition to the separate effects of CU traits and externalizing behaviors these variables may also interact, and this additive effect may place youths at significant risk for poor outcomes (Andershed et al. 2002; Byrd et al. 2012). We did not include an interaction term in our regression models due again to concerns regarding the number of independent variables, but future studies with larger sample sizes may want to consider this interaction. Despite these limitations, this study extends our understanding regarding the distinct associations between externalizing behaviors and CU traits and WM integrity in major amygdala connections.
Conclusions
Persistent conduct problems in youth are associated with adverse outcomes such as aggressive behavior and criminality (Babinski et al. 1999; Moffitt et al. 2008). This, together with the current dearth of effective treatment options for youths with severe conduct problems, reinforces the importance of better understanding their origins with an eye to improved treatments informed by neurobiological data. Converging evidence indicates that conduct problems that co-occur with elevated CU traits are associated with a unique neurobiological phenotype characterized by amygdala hypoactivation (Sebastian et al. 2012; Viding et al. 2012; Lozier et al. 2014) and low fear arousal (Pardini & Frick, 2013). The present results extend these findings, demonstrating that CU traits may also reflect reduced microstructural integrity in major WM amygdala connections, including the uncinate fasciculus and stria terminalis/fornix. Because they are consistent with previous findings in adult populations, our results suggest observed patterns in adults may reflect developmental WM abnormalities that may emerge as early as late childhood or early adolescence. Our mediation analyses did not support the possibility that WM impairments relate to CU traits indirectly through their influences on amygdala activity. However, participants with conduct problems who displayed the lowest WM integrity also exhibited the most pronounced amygdala hypoactivation to fearful faces, indicating that structural and functional brain impairments may be related. Although both structural and functional impairments have been previously implicated in youths with conduct problems and CU traits, our results bridge these two findings and indicate that functional and structural fronto-temporal impairments may represent a broader neurobiological phenotype. This finding is consistent with theories that reduced responsiveness in the amygdala to affective stimuli, combined with inadequate signaling between amygdala and the prefrontal cortex, underlie the major social and behavioral deficits observed in CU youths (Blair, 2007).
Acknowledgments
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (grant R03 HD064906–01); and the National Center for Advancing Translational Sciences/National Institutes of Health (grant 1KL2RR031974-01).
Footnotes
Declaration of Interest
None.
Previous work indicates that FA abnormalities in youths with conduct problems may be diffuse (Haney-Caron et al. 2014). We therefore tested whether our results reflected global differences, or were indicative of specific WM abnormalities in our tracts of interests, above and beyond any potential global differences. We averaged FA for each participant inside our group-level TBSS mask, and added this as a control in our analyses. Results remained similar even after controlling for global FA. Both left (t41 = −1.77, p = 0.08, β = −0.27) and right (t41 = −2.41, p < 0.05, β = −0.38) uncinate fasciculus FA, and right stria terminalis FA (t41 = −2.78, p < 0.01, β = −0.38) remained related to callous-unemotional traits above and beyond global WM FA. By contrast, FA in other tracts with no theoretical relationship to externalizing behaviors or CU traits, such as the body of the corpus callosum (p = 0.82) or posterior corona radiata (p = 0.72), were unrelated to CU traits after controlling for global WM FA. This indicates that, although general WM integrity problems may exist, our primary WM tracts of interest have a specific relationship to callous unemotional traits.
In order to be conservative, we also repeated our analyses including only those participants scanned in the 8-channel head coil, and again found that when externalizing behavior and CU traits were entered simultaneously into the model, only CU traits were associated with reductions in WM integrity in right (t28 = −3.28 p < 0.005, β = −0.80), and left (t28 = −2.91, p < 0.01, β = −0.78), uncinate fasciculus, and right stria terminalis/fornix (t28 = −2.10, p < 0.05, β = −0.53). Again, no associations were found between WM integrity and externalizing behavior in any region after controlling for CU traits (all ps > 0.20).
References
- Achenbach TM. Integrative Guide for the 1991 CBCL/4–18, Ysr, and Trf Profiles. University of Vermont/Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- Anastassiou-Hadjicharalambous X, Warden D. Physiologically-indexed and self-perceived affective empathy in Conduct-Disordered children high and low on Callous-Unemotional traits. Child Psychiatry and Human Development. 2008;39:503–517. doi: 10.1007/s10578-008-0104-y. [DOI] [PubMed] [Google Scholar]
- Andershed H, Gustafson SB, Kerr M, Stattin H. The usefulness of self-reported psychopathy-like traits in the study of antisocial behaviour among non-referred adolescents. European Journal of Personality. 2002;16:383–402. [Google Scholar]
- Aoki Y, Abe O, Nippashi Y, Yamasue H. Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Molecular Autism. 2013;4:25. doi: 10.1186/2040-2392-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinski LM, Hartsough CS, Lambert NM. Childhood conduct problems, hyperactivity-impulsivity, and inattention as predictors of adult criminal activity. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1999;40:347–355. [PubMed] [Google Scholar]
- Barry CT, Frick PJ, DeShazo TM, McCoy MG, Ellis M, Loney BR. The importance of callous-unemotional traits for extending the concept of psychopathy to children. Journal of Abnormal Psychology. 2000;109:335–340. doi: 10.1037/0021-843X.109.2.335. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Breiman L, Freedman D. How many variables should be entered in a regression equation? Journal of the American Statistical Association. 1983;78:131–136. [Google Scholar]
- Byrd AL, Loeber R, Pardini DA. Understanding desisting and persisting forms of delinquency: the unique contributions of disruptive behavior disorders and interpersonal callousness. Journal of Child Psychology and Psychiatry. 2012;53:371–380. doi: 10.1111/j.1469-7610.2011.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and Biobehavioral Reviews. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Craig MC, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, Picchioni M, McGuire PK, Fahy T, Murphy DGM. Altered connections on the road to psychopathy. Molecular Psychiatry. 2009;14:946–953. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Crowe SL, Blair RJR. The development of antisocial behavior: what can we learn from functional neuroimaging studies? Development and Psychopathology. 2008;20:1145–1159. doi: 10.1017/S0954579408000540. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito SA, McCrory EJ, Mechelli A, Wilke M, Jones AP, Hodgins S, Viding E. Small, but not perfectly formed: decreased white matter concentration in boys with psychopathic tendencies. Molecular Psychiatry. 2011;16:476–477. doi: 10.1038/mp.2010.74. [DOI] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski C, Kiehl KA. An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Frontiers in Human Neuroscience. 2013;7:489. doi: 10.3389/fnhum.2013.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of Facial Affect. Consulting Psychologists; Palo Alto, CA: 1976. [Google Scholar]
- Eyberg SM, Nelson MM, Boggs SR. Evidence-based psychosocial treatments for children and adolescents with disruptive behavior. Journal of Clinical Child and Adolescent Psychology. 2008;37:215–237. doi: 10.1080/15374410701820117. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh A, Blair KS, Majestic C, Evangelou I, Gupta K, Schneider MR, Sims C, Pope K, Fowler K, Sinclair S, Tovar-Moll F, Pine D, Blair RJ. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Research. 2012;202:239–244. doi: 10.1016/j.pscychresns.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS, Chen G, Towbin KE, Leibenluft E, Pine DS, Blair JR. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives of General Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ. Developmental pathways to conduct disorder: implications for future directions in research, assessment, and treatment. Journal of Clinical Child and Adolescent Psychology. 2012;41:378–389. doi: 10.1080/15374416.2012.664815. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Hare RD. The Antisocial Process Screening Device. Multi-Health Systems; Toronto, Ontario, Canada: 2001. [Google Scholar]
- Frick PJ, Moffitt TE. A Proposal to the DSM-V Childhood Disorders and the ADHD and Disruptive Behavior Disorders Work Groups to Include a Specifier to the Diagnosis of Conduct Disorder based on the Presence of Callous-Unemotional Traits. American Psychiatric Association; Washington, DC: 2010. [Google Scholar]
- Frick PJ, White SF. Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49:359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Molecular Psychiatry. 2009;14:5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Goodman R, Scott S. Comparing the strengths and difficulties questionnaire and the child behavior checklist: is small beautiful? Journal of Abnormal Child Psychology. 1999;27:17–24. doi: 10.1023/a:1022658222914. [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disorders. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Haney-Caron E, Caprihan A, Stevens MC. DTI-measured white matter abnormalities in adolescents with Conduct Disorder. Journal of Psychiatric Research. 2014;48:111–120. doi: 10.1016/j.jpsychires.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. Journal of Abnormal Psychology. 2010;119:863–874. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes DJ, Price MJ, Dadds MR. Callous-unemotional traits and the treatment of conduct problems in childhood and adolescence: a comprehensive review. Clinical Child and Family Psychology Review. 2014;17:248–267. doi: 10.1007/s10567-014-0167-1. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. 1st. The Guilford Press; New York: 2013. [Google Scholar]
- Hicks BM, Patrick CJ. Psychopathy and negative emotionality: analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. Journal of Abnormal Psychology. 2006;115:276–287. doi: 10.1037/0021-843X.115.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppenbrouwers SS, Nazeri A, de Jesus DR, Stirpe T, Felsky D, Schutter DJLG, Daskalakis ZJ, Voineskos AN. White matter deficits in psychopathic offenders and correlation with factor structure. PLoS ONE. 2013;8:e72375. doi: 10.1371/journal.pone.0072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kahn RE, Byrd AL, Pardini DA. Callous-unemotional traits robustly predict future criminal offending in young men. Law and Human Behavior. 2013;37:87–97. doi: 10.1037/b0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. Kaufman Brief Intelligence Test – Second Edition (KBIT-2) American Guidance Service; Circle Pines, MN: 2004. [Google Scholar]
- Kazdin AE. Conduct Disorders in Childhood and Adolescence. 2nd. Sage Publications; London: 1995. [Google Scholar]
- Kazdin AE. Treatments for aggressive and antisocial children. Child and Adolescent Psychiatric Clinics of North America. 2000;9:841–858. [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Skeem JL, Marsee MA, Cruise K, Munoz LC, Aucoin KJ, Morris AS. Assessing callous-unemotional traits in adolescent offenders: validation of the Inventory of Callous-Unemotional Traits. International Journal of Law and Psychiatry. 2008;31:241–252. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Samartzis L, Dima D, Hayes D, Corrigall R, Barker G, Correll CU, Frangou S. P03-111 – Does antipsychotic medication affect white matter in schizophrenia and bipolar disorder? a review of diffusion tensor imaging literature. European Psychiatry. 2011;26(Suppl. 1):1280. [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, Marsh AA. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71:627–636. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJR. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Schechter JC, Jurkowitz ITN, Reid ME, Blair RJR. Adolescents with psychopathic traits report reductions in physiological responses to fear. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52:834–841. doi: 10.1111/j.1469-7610.2010.02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi G, Muratori P, Manfredi A, Lenzi F, Polidori L, Ruglioni L, Muratori F, Milone A. Response to treatments in youth with disruptive behavior disorders. Comprehensive Psychiatry. 2013;54:1009–1015. doi: 10.1016/j.comppsych.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Meier MH, Slutske WS, Heath AC, Martin NG. Sex differences in the genetic and environmental influences on childhood conduct disorder and adult antisocial behavior. Journal of Abnormal Psychology. 2011;120:377–388. doi: 10.1037/a0022303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Jaffee SR, Kim-Cohen J, Koenen KC, Odgers CL, Slutske WS, Viding E. Research review: DSM-V conduct disorder: research needs for an evidence base. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49:3. doi: 10.1111/j.1469-7610.2007.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI Atlas of Human White Matter. Elsevier; Amsterdam: 2005. [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. The Journal of Neuroscience. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria AV, Hsu J, Tippett D, Mori S, Hillis AE. Critical role of the right uncinate fasciculus in emotional empathy. Annals of Neurology. 2015;77:68–74. doi: 10.1002/ana.24300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, Akhter K, Hua K, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Zhang J, Huang H, Miller MI, van Zijl PCM, Mazziotta J, Mori S. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. NeuroImage. 2008;43:447–457. doi: 10.1016/j.neuroimage.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini DA. The callousness pathway to severe violent delinquency. Aggressive Behavior. 2006;32:590–598. [Google Scholar]
- Pardini D, Frick PJ. Multiple developmental pathways to conduct disorder: current conceptualizations and clinical implications. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2013;22:20–25. [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Fornito A, Goodyer IM, Nimmo-Smith I, Hagan CC, Calder AJ. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS ONE. 2012;7:e48789. doi: 10.1371/journal.pone.0048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. Journal of Neuroscience. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose A, Bijttebier P, Decoene S, Claes L, Frick PJ. Assessing the affective features of psychopathy in adolescence: a further validation of the inventory of callous and unemotional traits. Assessment. 2010;17:44–57. doi: 10.1177/1073191109344153. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Craig MC, Catani M, Dell’Acqua F, Fahy T, Deeley Q, Murphy DGM. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychological Medicine. 2013;43:401–411. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Seara-Cardoso A, Viding E, Lickley RA, Sebastian CL. Neural responses to others’ pain vary with psychopathic traits in healthy adult males. Cognitive, Affective & Behavioral Neuroscience. doi: 10.3758/s13415-015-0346-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJP, Cecil CAM, Lockwood PL, De Brito SA, Fontaine NMG, Viding E. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Archives of General Psychiatry. 2012;69:814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Sylvers PD, Brennan PA, Lilienfeld SO. Psychopathic traits and preattentive threat processing in children: a novel test of the fearlessness hypothesis. Psychological Science. 2011;22:1280–1287. doi: 10.1177/0956797611420730. [DOI] [PubMed] [Google Scholar]
- Turgay A. Aggression and disruptive behavior disorders in children and adolescents. Expert Review of Neurotherapeutics. 2004;4:623–632. doi: 10.1586/14737175.4.4.623. [DOI] [PubMed] [Google Scholar]
- Van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews. 2012;36:1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Viding E, Blair RJR, Moffitt TE, Plomin R. Evidence for substantial genetic risk for psychopathy in 7-year-olds. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46:592–597. doi: 10.1111/j.1469-7610.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- Viding E, Jones AP, Frick PJ, Moffitt TE, Plomin R. Heritability of antisocial behaviour at 9: do callous-unemotional traits matter? Developmental Science. 2008;11:17–22. doi: 10.1111/j.1467-7687.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CAM, De Brito SA, McCrory EJ. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. The American Journal of Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]


