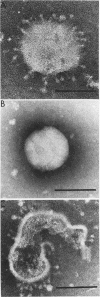
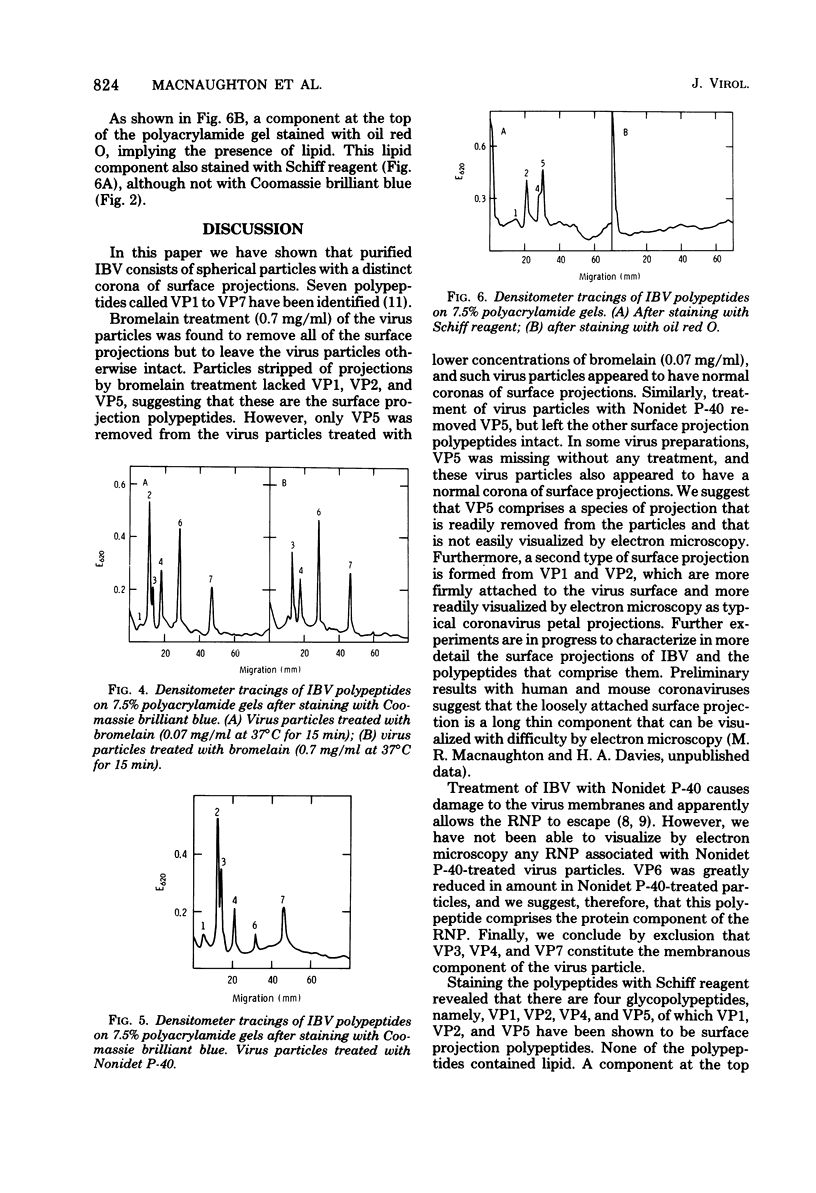
Abstract
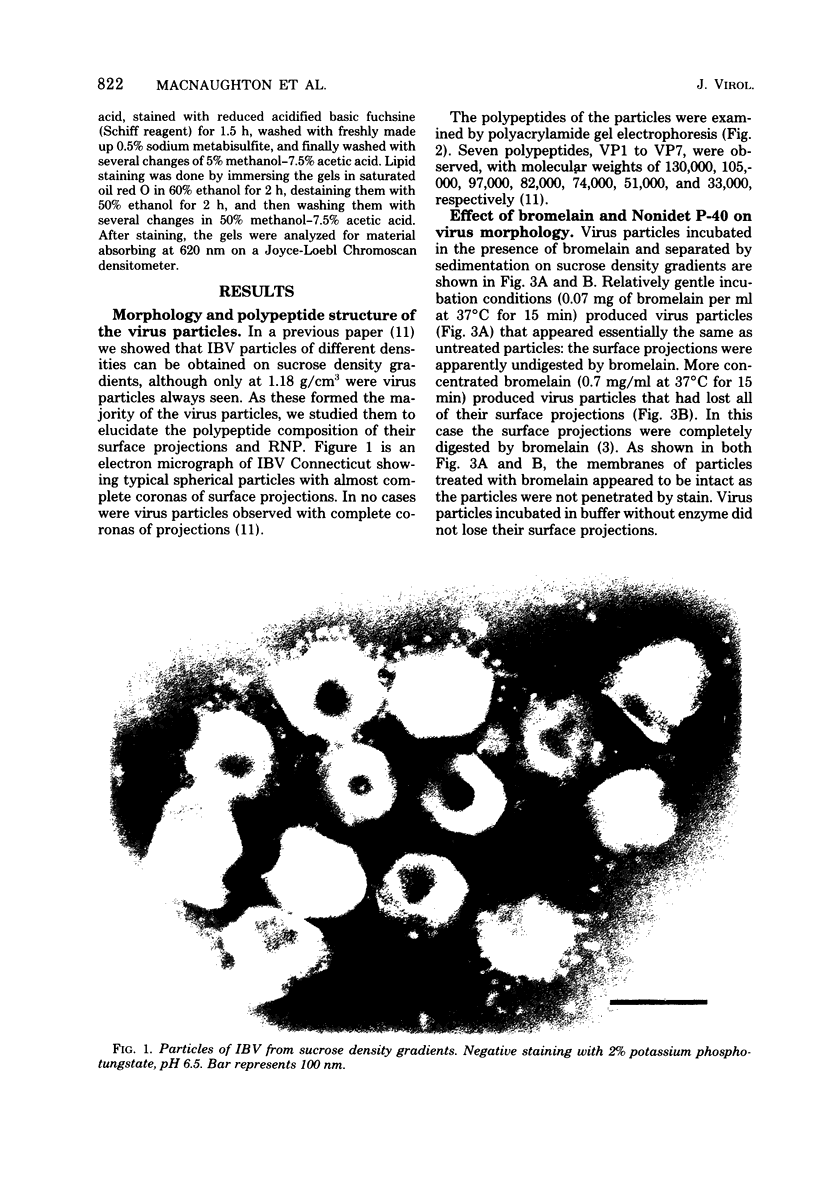
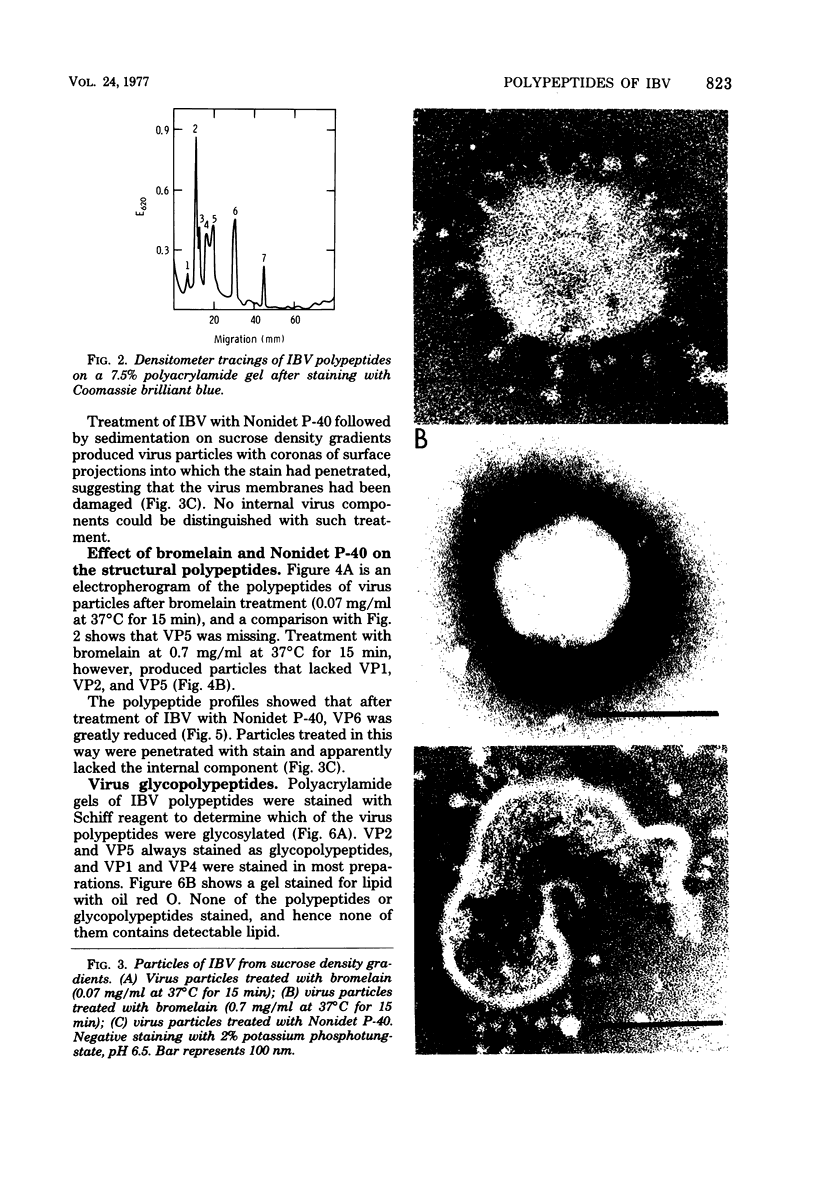
Purified avian infectious bronchitis virus was digested with bromelain (0.7 mg/ml), and the surface projections were removed. Polyacrylamide gel electrophoresis of the polypeptides from these bromelain-treated particles showed that VP1, VP2, and VP5 were missing from the seven polypeptides. VP1 to VP7, that were present in untreated virus preparations. Milder bromelain treatment (0.07 mg/ml) left visible surface projections and polypeptides comprising VP1 and VP2 intact, but removed VP5. Thus, there are apparently two types of surface projections on the virus particle. The ribonucleoprotein complex was released from virus particles disrupted with 1% Nonidet P-40. The proportion of VP6 in such preparations was greatly reduced, implying that VP6 is the structural polypeptide of the ribonucleoprotein. Polypeptides VP1, VP2, VP4, and VP5 are glycosylated, but none of the polypeptides contains lipid.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham R. W. The polypeptide composition of avian infectious bronchitis virus. Arch Virol. 1975;49(2-3):207–216. doi: 10.1007/BF01317539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. S., Alexander D. J., Harkness J. W. Heterogeneity of infectious bronchitis virus grown in eggs. Arch Virol. 1976;50(1-2):55–72. doi: 10.1007/BF01318001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W. Location of the glycoprotein in the membrane of Sindbis virus. Nat New Biol. 1971 Jan 27;229(4):114–116. doi: 10.1038/newbio229114a0. [DOI] [PubMed] [Google Scholar]
- Garwes D. J., Pocock D. H., Pike B. V. Isolation of subviral components from transmissible gastroenteritis virus. J Gen Virol. 1976 Aug;32(2):283–294. doi: 10.1099/0022-1317-32-2-283. [DOI] [PubMed] [Google Scholar]
- Garwes D. J., Pocock D. H. The polypeptide structure of transmissible gastroenteritis virus. J Gen Virol. 1975 Oct;29(1):25–34. doi: 10.1099/0022-1317-29-1-25. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Palmer E. L., Whitfield S. G., Kaye H. S., Dowdle W. R. Protein composition of coronavirus OC 43. Virology. 1972 May;48(2):516–527. doi: 10.1016/0042-6822(72)90062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C. Purification and biophysical properties of human coronavirus 229E. Virology. 1976 Nov;75(1):155–165. doi: 10.1016/0042-6822(76)90014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye H. S., Hierholzer J. C., Dowdle W. R. Purification and further characterization of an "IBV-like" virus (coronavirus). Proc Soc Exp Biol Med. 1970 Nov;135(2):457–463. doi: 10.3181/00379727-135-35074. [DOI] [PubMed] [Google Scholar]
- Kennedy D. A., Johnson-Lussenburg C. M. Isolation and morphology of the internal component of human coronavirus, strain 229E. Intervirology. 1975;6(4-5):197–206. doi: 10.1159/000149474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M. B., Burnstein T., Haelterman E. O. Cell culture-adapted SH strain of transmissible gastroenteritis virus of pigs: in vivo and in vitro studies. Am J Vet Res. 1970 Apr;31(4):771–781. [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. Characterization of coronavirus II. Glycoproteins of the viral envelope: tryptic peptide analysis. Virology. 1977 Apr;77(2):650–660. doi: 10.1016/0042-6822(77)90489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S. I. Structural proteins: effects of preparative conditions on the migration of protein in polyacrylamide gels. Virology. 1977 Apr;77(2):637–649. doi: 10.1016/0042-6822(77)90488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]