ABSTRACT
Semaphorins were originally identified as axon guidance cues that regulate the functional activity of axons in the nervous system. In addition, accumulating evidence indicates that semaphorins have multiple functions in physiological and pathogenic processes, including vascular development, tumor progression, and immune responses. Sema4A is a semaphorin expressed in immune cells, and is thus termed an “immune semaphorin.” Sema4A has 4 types of receptors: Plexin D family, Plexin B family, Tim-2, and Nrp-1. Recent studies suggest that Sema4A plays critical roles in many processes including cell–cell interactions, immune-cell activation, differentiation, and migration. In other studies, Sema4A is also associated with carcinogenesis and retinal systems. In this review, we summarize current knowledge regarding the biology of Sema4A in relation to angiogenesis, immune responses, colorectal cancer, and the retina.
KEYWORDS: angiogenesis, FCCTX, immune systems, retinal degeneration, Sema4A, semaphorin
Semaphorins and their receptors
Semaphorins is a family of proteins characterized by a conserved amino-terminal Sema domain. In physiological condition, semaphorins are present as either secretary or membrane bound proteins. They were originally described as membrane that provide both attractive and repulsive axon guidance cues during neural development.1-3 Over 30 semaphorins have been identified to date and classified into 8 subgroups. Invertebrate semaphorins are grouped into classes I and II, and vertebrate semaphorins are classified into classes III–VII.1 Plexins and neuropilins (Nrps) have been identified as the major semaphorin receptors. Plexins are transmembrane proteins with a Sema ligand–binding domain in their extracellular region. These receptors are classified into subclasses; Plexin A-D, Nrp-1 and -2. In most cases, members of the Plexin A family require neuropilins as ligand-binding partners, whereas members of other Plexin families are directly activated by semaphorins. Sema4A binds to members of the Plexin B and D family, Nrp-1, and also Tim-2 (T-cell, immunoglobulin and mucin domain protein 2) (Fig. 1). Although semaphorin–plexin interactions have been well characterized in the nervous system, more recent studies have explored these interactions in other systems, including cardiogenesis,4,5 angiogenesis,6,7 tumor progression and suppression,8 bone homeostasis,9,10 and immune responses. In particular, Sema4A, a class IV transmembrane semaphorin, plays various crucial roles in angiogenesis, immune cells, carcinogenesis, and retinal systems. In this review, we focus on the general features and functional roles of Sema4A.
Figure 1.
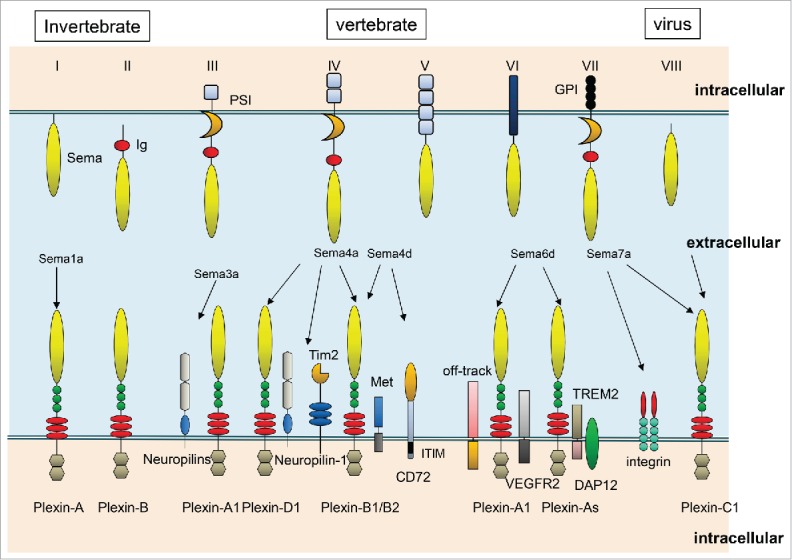
Representative semaphorins and their receptors. Class I and II semaphorins are found in invertebrates, whereas classes III–VII are vertebrate semaphorins. Sema3A interact with Nrp-1 and Class A plexin receptor complexes. Sema4A recognizes Plexin-B and D1, Nrp-1, and TIM-2 as receptors in the immune system. Sema4D binds to Plexin-B1 in neurons, and Plexin-B1 binds to Met in epithelial cells, thus inducing Sema4D-mediated cell outgrowth. In the immune system, Sema4D couples with CD72 and Plexin-B2. Sema6D binds to Plexin-A1. During cardiac development, Plexin-A1 interacts with Off-Track or VEGFR2, and these complexes have distinct functions. In the immune system, Plexin-A1 forms a receptor complex with Trem-2–DAP12, which plays important roles in activation of DCs and osteoclasts. Sema7A expressed in activated T cells binds to integrins to activate macrophages, and also binds to Plexin-C1 to induce monocyte. Viral semaphorin binds to Plexin-C1.
Sema4A and angiogenesis
Sema4A is normally expressed by endothelial cells, and Plexin-D1 is the functional receptor of Sema4A on these cells. Vascular endothelial growth factor (VEGF)-mediated endothelial cell migration and proliferation in vitro and embryonic vasculization in vivo are suppressed by Sema4A.6 Consistent with this, VEGF-mediated angiogenesis is enhanced in Sema4A-deficient mice. Sema4A-Fc inhibits VEGF-mediated activation of Rac, which regulates cytoskeletal remodeling and cell adhesion.11 Tyrosine phosphorylation of VEGFR2 is known to be followed by phosphorylation of Akt, which plays important roles in the survival and proliferation of endothelial cells.12 As with Rac, Sema4A-Fc inhibits phosphorylation of Akt. The inhibition of VEGF-induced Rac and Akt activation appears to affect vascular migration and cell survival. These results suggest that Sema4A–Plexin-D1 axis negatively regulates VEGF-induced signaling and subsequent angiogenesis.6
Sema4A and immune responses
Sema4A and dendritic cell functions
Sema4A is expressed on the surface of dendritic cells (DCs), and it is reported to be important in T-cell priming (Fig. 2). DCs derived from Sema4A-deficient mice stimulate allogeneic T cells less effectively than wild-type DCs.13 Moreover, when wild-type or Sema4A-deficient mice were immunized in the hind footpad with keyhole limpet hemocyanin (KLH) in complete Freund's adjuvant (CFA) to examine the effects of Sema4A deficiency on in vivo T-cell activation, CD4+ T-cell responses to KLH were significantly weaker in Sema4A-deficient mice than in wild-type mice. These results suggest that DC-derived Sema4A is directly and critically involved in the activation of T cells. In T-cell priming, TIM-2 appears to be the functional receptor for Sema4A.13 Actually, TIM-2-deficient mice have an enhanced Th2 T cell response to lung antigen challenge, suggesting that the TIM-2 pathway negatively regulates Th2 T cell responses.14,15 Consistent with this, several studies have reported that Sema4A is associated with multiple sclerosis (MS). MS is an inflammatory demyelinating disease of the central nervous system (CNS) that causes neurological disability in young adults. When genetically predisposed individuals are exposed to an environmental trigger, myelin-specific T cells are activated, and MS develops.16 Antigen presentation by DCs and activation and differentiation of CD4+ T cells in CNS most likely play an important role in the pathogenesis of MS. Experimental autoimmune encephalomyelitis (EAE), a widely used mouse model of MS,17 is induced in susceptible animals by immunization with myelin proteins, including myelin oligodendrocyte glycoprotein (MOG), proteolipid protein (PLP), and myelin basic protein (MBP), in combination with an adjuvant. This model reproduces many of the clinical and histopathological features of MS. Interestingly, the progression of MOG-induced EAE in wild-type mice can be suppressed by injection of Sema4A monoclonal antibody at the time of MOG immunization. Infiltration of mononuclear inflammatory cells in the spinal cord is reduced in Sema4A antibody–treated mice; moreover, CD4+ T cells in draining lymph nodes exhibit significantly reduced responses to the MOG peptide.18 However, Sema4A have no influence on the effector phase of the disease course, as the mdel mice developed MS after MOG-specific CD4+ T cells are transferred even with the Sema4A antibody. Thus, Sema4A plays an important role in the development of EAE in the priming phase rather than the effector phase.19 In patients with MS, serum Sema4A levels are markedly higher than in healthy control subjects or patients with other neurological diseases.19 While monocytes and DCs derived from healthy control subjects express moderate amounts of Sema4A, its expression levels are significantly higher in MS patients. The release of Sema4A can be prevented by inhibitors of proteases, including matrix metalloproteinases (MMPs) and ADAM metalloproteinases, suggesting that these enzymes play important roles in releasing Sema4A from the cell surface.19 Moreover, mRNA expression of MMPs and ADAM 10 is higher in peripheral blood mononuclear cells (PBMCs) from MS patients with high serum concentrations of Sema4A than in those from healthy controls or MS patients with lower serum Sema4A levels. Collectively, these findings indicate that Sema4A, which is highly expressed in DCs and monocytes in patients with MS, is enzymatically shed in a subgroup of the patients. Moreover, there are several important hallmarks of MS patients with high serumSema4A levels. For example, such patients have a significantly higher proportion of IL-17–producing CD4+ T cells than healthy subjects or patients with low serum Sema4A levels,19 and they also have higher IL-2 levels. Therefore, Sema4A levels in patients with MS seem to be involved in Th17-mediated MS pathogenesis. Moreover, the disease course is significantly more severe in MS patients with high Sema4A levels. In addition, MS patients with high Sema4A levels are resistant to first-line IFN-β therapy. These facts suggest that serum Sema4A could become a useful biomarker for response to IFN-β therapy. However, it remains unclear whether soluble and membrane-bound Sema4A have different functional effects on receptor-bearing cells. Further understanding of this will be necessary.
Figure 2.

Sema4A plays various roles in immune responses. (A) When DCs encounter T cells, Sema4A on DCs directly binds to TIM-2 on T cells, leading to optimal activation of antigen-specific T cells. (B) Sema4A promotes Th1 differentiation through TIM-2. (C) Sema4A on conventional T cells binds to Nrp-1 on Treg cells, leading to inhibition of Akt activation via recruitment of PTEN. Akt inactivation results in nuclear exclusion of FoxO molecules, thereby promoting the stability and function of Treg cells. (D) Sema4A on CD8+ T cells promotes optimal CD8+ T differentiation via Plexin-B2 by modulating mTOR-mediated signals.
Sema4A and CD4+ T-cell function
Sema4A is abundantly expressed in polarized T helper type 1 (Th1) cells and plays important roles in T-cell differentiation (Fig. 2).13 In Sema4A-deficient mice, Th1 responses to heat-killed Propionibacterium acne, a Th1-inducing bacterium, are impaired in vivo, whereas T helper type 2 (Th2) responses against Nippostrongylus brasiliensis, a Th2-inducing intestinal nematode, are augmented.13 Sema4A is also associated with pathogenesis of allergic airway hyperreactivity, such as allergic asthma, rhinitis, and atopic dermatitis. These diseases are thought to have onset involving both environmental and genetic factors,20,21 and they have several common features: eosinophil or mast cell infiltration, elevated levels of Th2-type cytokines, and elevated serum IgE levels.22 Th2 cells play important roles in allergic asthma,23,24 and IL-4 derived from Th2 cells is required for IgE class-switching and induction of strong Th2 responses. In a mouse model of OVA-specific experimental asthma, airway hyperreactivity is upregulated compared to that in wild-type mice. Furthermore, bronchoalveolar lavage (BAL) fluid of Sema4A-deficient mice contains elevated levels of Th2 cytokines and IgE, as well as higher levels of pulmonary eosinophil infiltration. These data suggest that Sema4A is involved in the regulation of Th2-driven pathophysiology in the lung.25,26 These results suggest that Sema4A plays a suppressive role in Th2-driven disease while driving Th1-driven diseases, such as EAE. Thus, Sema4A could be useful as a therapy of allergic diseases.
Sema4A/NRP-1 and regulatory T-cell stability
Regulatory T (Treg) cells play crucial roles in the immune system.27 They not only prevent autoimmunity, but also inhibit effective anti-tumor immunity. The most important regulator of Treg is the transcription factor Foxp3.28 In conventional T cells, Sema4A is associated with maintenance of Treg cells (Fig. 2).29 Sema4A directly interacts with the receptor neuropilin-1 (Nrp-1) on Treg cells, and this interaction enhances Treg-cell function and survival at inflammatory sites. The binding of Sema4A to Nrp-1 inhibits Akt phosphorylation by recruiting phosphatase and tensin homolog (PTEN) to Nrp-1, This leads to nuclear localization of the transcription factor Foxo3a, which plays an important role in the development and programming of Treg cells by promoting their exclusion from the nucleus.30,31 In fact, Nrp1f/fFoxp3Cre mice that allow Treg–specific Nrp-1 deletion, exhibit reduced or delayed tumor growth and increased survival in the presence of some cancers. In addition, blockade of this pathway using antibodies against Sema4 and Nrp1 significantly decreased tumor growth in wild-type mice.29 These findings suggest that Sema4A might help to regulate Treg cell–mediated tumor-induced tolerance without inducing autoimmunity.
Sema4A and CD8+ T-cell function
Sema4A is also associated with CD8+ T-cell differentiation through mTOR, a conserved serine/threonine kinase that plays a central role in regulation of cell growth and metabolism. mTOR consists of 2 multiprotein complexes, mTOR complex (mTORC) 1 and mTORC2. mTORC1 directly phosphorylates S6 kinase (S6K), resulting in an increase in ribosome biogenesis and translational initiation and elongation, ultimately leading to upregulation of protein synthesis.32-34 In the immune system, mTORC1 signaling is involved in CD8+ T-cell responses. Sema4A-deficient CD8+ T cells exhibit impairments in cytokine production and induction of effector molecules including Granzyme B, Perforin, and FAS-L. Upon infection with ovalbumin-expressing Listeria monocytogenes (LM-OVA), which initiates an acute infection and activates CD8+ T cells, Sema4a−/− mice have a lower frequency of CD8+ T cells producing IFN-γ or TNF-α. Although TCR signaling in Sema4a−/− CD8+ T cells is comparable to that in wild-type mice, Sema4A-deficient CD8+ T cells exhibit reduced mTORC1 activity and elevated mTORC2 activity. In addition, mTORC1 activity and cytokine production in Sema4A-deficient CD8+ T cells can be restored by addition of recombinant Sema4A protein. These results suggest that Sema4A is required for optimal activation of mTORC1 in CD8+ T cells. Plexin Bs, Plexin D1, TIM2, and NRP1 bind to SEMA4A. However, only Plexin B2 is expressed by CD8+ T cells. Consistently, Plexin B2–knockdown CD8+ cells produced significantly less IFN-γ. Thus, the functional receptor for Sema4A in CD8+ T cells is Plexin B2 (Fig. 2).35
Sema4A and macrophage function
Sema4A is expressed in activated macrophages. Moreover, the expression of Plexin D1, a receptor of Sema4A, is enhanced in activated macrophages. Soluble Sema4A protein stimulates macrophage migration in a dose-dependent manner. However, Sema4A does not significantly change the expression level of any inflammatory chemokine or cytokine. In addition, blocking anti-Plexin D1 mAb inhibits the migratory effect of Sema4A in macrophages.36 This chemotactic effect seems to be independent of inflammatory chemokines. Moreover, Sema4A-mediated macrophages enhance angiogenesis in vivo and in vitro through vascular endothelial growth factor-A (VEGF-A) / VEGFR-2 signaling. However, this is an opposite effect of Sema4A describe earlier, which suggests inhibitory function of Sema4A in experimental angogenesis.6 Further study is still needed to clarify the role of Sema4A in angiogenesis.
Sema4A and familial colorectal cancer type X
Familial colorectal cancer type X (FCCTX) is a disorder with clinical features of hereditary non-polyposis colorectal cancer. The genetic background of this disease has not yet been defined.37,38 Recently, SEMA4A mutation (V78M) was identified in one Austrian patients by integrative genomics analysis. In comparison with the wild-type protein, SEMA4AV78M markedly increased activation of MAPK/Erk and PI3K/Akt signaling, which play important roles in colorectal carcinogenesis. In addition, 2 additional SEMA4A mutations and a single-nucleotide polymorphism were identified in FCCTX patients. In particular, the SNP is highly associated with the FCCTX phenotype of elevated risk of colorectal cancer.39
Sema4A and retinal degeneration
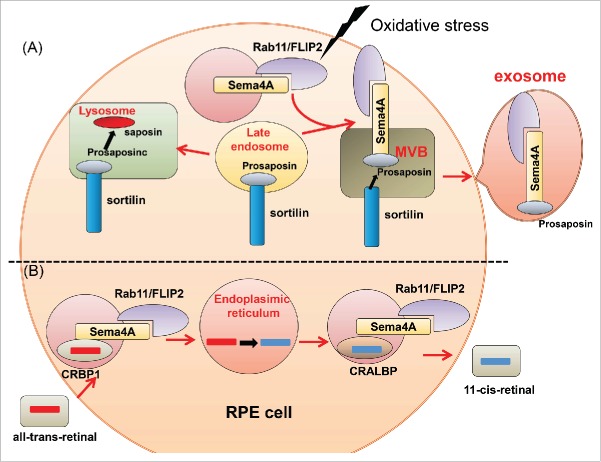
Retinitis pigmentosa (RP), which affects one in 3000 people, causes blindness and has no effective treatment. This disease is caused by abnormalities of photoreceptors (PRs) or retinal pigment epithelium (RPE). More than 100 associated mutations in RP patients have been discovered.40,41 Light exposure is one of the most important factors that make PRs vulnerable to degeneration. Visible and ultraviolet light ionize biomolecules, and oxygen enhances this effect. Consequently, retinal damage can occur when light acts on photosensitizing molecules including retinoids, thereby generating reactive oxygen and nitrogen species (RONS). Light damage to PRs is caused by the release of all-trans retinal from light-activated rhodopsin.42,43 In Sema4A-deficient mice, the outer segments of PRs are disrupted at postnatal day (P)14 and completely lost by P28. In response to illumination, the number of apoptotic cells significantly increases in the outer nuclear layer of Sema4A-deficient retinas before recovering to basal levels. Prosaposin is associated with procathepsin D in the Golgi membrane44 and can be targeted to lysosomes45,46 or secreted into the extracellular space. Such secreted lysosomal precursor proteins are antiapoptotic in various neuronal populations.46,47 Sema4A expressed in RPE is bound to prosaposin. Under oxidative stress caused by H2O2 treatment, prosaposin is transported to the cell periphery in wild-type RPE cells, but not in Sema4A-deficient RPE cells. Sema4A binds to a complex of Rab11 and the adaptor protein FIP2. Though these interactions, Sema4A-mediated prosaposin is transported to the cell periphery under oxidative stress48 (Fig. 3). Thus, Sema4A plays a crucial role in protecting the cell against H2O2. In addition, photo-excitation of all-trans retinal produces singlet dioxygen, which can lead to photo-oxidative damage. If the change from all-trans retinal to 11-cis retinal is inhibited, toxic bis-retinoids and adducts steadily accumulate over the course of aging. The levels of 11-cis retinal are markedly increased in wild-type retinas at P14 and P28. By contrast, these levels remain low in Sema4A-deficient retinas. Sema4A plays important roles in regulating the transport of retinoid-binding proteins in RPE cells. In the retinoid cycle, CRALBP transports 11-cis retinal, whereas CRBP1 transports all-trans retinal.49 These proteins, along with Sema4A, are associated with the endosomal sorting machinery. In Sema4A-deficient retinas, CRLBP1 is abnormally localized in the cell periphery and cannot interact with 11-cis retinal. As a result, all-trans retinal cannot covert to 11-cis retinal in the endoplasmic reticulum. Moreover, CRBP1 is abnormally retained at the endoplasmic reticulum and cannot interact with all-trans retinal imported from the extracellular space. Thus, Sema4A is involved in sorting of retinoid-binding proteins in the retinoid cycle48 (Fig. 3). In fact, 3 mutations in the Sema4A gene have been identified in patients with retinal degenerative diseases: D345H, F350C, and R713Q.50 Expression of Sema4A (F350C) causes severe retinal degeneration in a series of knock-in mouse lines carrying mutated alleles of Sema4A.51 In addition, Sema4A (F350C) tends to aggregate in the RPE, and this abnormal localization may lead to impaired endosomal sorting of molecules including prosaposin. Moreover, virus-mediated gene transfer of Sema4A into RPE in neonatal Sema4A-deficient mice successfully prevents retinal degeneration for at least 4 months after injection. Thus, it is possible that Sema4A might be efficacious as a replacement gene therapy in patients with retinal degenerative diseases.
Figure 3.
(A) Upon exposure to light, binding of prosaposin to Sema4A and the Rab11/FIP2 plays important roles in sorting of prosaposin to the exosomal pathway. (B) Sema4A regulates intracellular sorting of retinoid-binding proteins in the retinoid cycle.
Conclusion
In this review, we focused on the expression and functions of Sema4A and its receptors in the context of interactions in angiogenesis, immune responses, carcinogenesis, and retinal systems. Sema4A is widely expressed in immune cells, such as DCs, T cells, and macrophages in particular, and it is associated with various types of inflammatory disorders such as MS and allergic diseases. Moreover, recent work has shown that Sema4A is involved in rheumatoid arthritis and inflammatory bowel disease, including Crohn's disease and ulcerative colitis.52,53 Furthermore, Sema4A is involved in activation and differentiation of both CD4+ and CD8+ T cells. Thus, it appears that Sema4A is relevant to the induction phase of inflammation immune reactions. Indeed, Sema4A-deficent T cells exhibit impaired activation in response to TCR stimulation.13,32 On the other hand, Sema4A also seems to be involved in the progression of chronic inflammation. Consistent with this, Sema4A accumulates in the serum of patients with MS. Further evaluation is required to determine the phase of the immune response in which Sema4A is involved.
With respect to the relationship between Sema4A and retinal system, Sema4A mediates the exosomal release of prosaposin and endosomal sorting of retinoid-binding proteins. The fact that Sema4A functions as an intracellular guide for specific molecules is highly significant, because semaphorins and their receptor plexins are known to function as extracellular guidance molecules.
Although Sema4A plays various roles and has multiple functions, the signaling pathways that regulate the expression of semaphorins, including Sema4A, remain unknown. Elucidation of these pathways might facilitate development of therapeutic approaches for various diseases associated with semaphorins.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 1993; 31:1389-99; http://dx.doi.org/ 10.1016/0092-8674(93)90625-Z [DOI] [PubMed] [Google Scholar]
- [2].Tamagnone L, Comoglio PM. Signaling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol 2000; 10:377-83; PMID:10932095; http://dx.doi.org/ 10.1016/S0962-8924(00)01816-X [DOI] [PubMed] [Google Scholar]
- [3].Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol 2003; 13:79-89; PMID:12593985; http://dx.doi.org/ 10.1016/S0959-4388(03)00003-5 [DOI] [PubMed] [Google Scholar]
- [4].Toyofuku T, Kikutani H. Semaphorin signaling during cardiac development. Adv Exp Med Biol 2007; 600:109-17; PMID:17607950; http://dx.doi.org/ 10.1007/978-0-387-70956-7_9 [DOI] [PubMed] [Google Scholar]
- [5].Toyofuku T, Yoshida J, Sugimoto T, Yamamoto M, Makino N, Takamatsu H, Takegahara N, Suto F, Hori M, Fujisawa H, et al.. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev Biol 2008; 321:251-62; PMID:18625214; http://dx.doi.org/ 10.1016/j.ydbio.2008.06.028 [DOI] [PubMed] [Google Scholar]
- [6].Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, Hori M. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J 2007; 26:1373-84; PMID:17318185; http://dx.doi.org/ 10.1038/sj.emboj.7601589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al.. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 2003; 424:391-7; PMID:12879061; http://dx.doi.org/ 10.1038/nature01784 [DOI] [PubMed] [Google Scholar]
- [8].Luchino J, Hocine M, Amoureux MC, Gibert B, Bernet A, Royet A, Treilleux I, Lécine P, Borg JP, Mehlen P, et al.. Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell 2013; 24:673-85; PMID:24139859; http://dx.doi.org/ 10.1016/j.ccr.2013.09.010 [DOI] [PubMed] [Google Scholar]
- [9].Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med 2011; 17:1473-80; PMID:22019888; http://dx.doi.org/ 10.1038/nm.2489 [DOI] [PubMed] [Google Scholar]
- [10].Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature 2012; 485:69-74; PMID:22522930; http://dx.doi.org/ 10.1038/nature11000 [DOI] [PubMed] [Google Scholar]
- [11].Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 2004; 116:167-79; PMID:14744429; http://dx.doi.org/ 10.1016/S0092-8674(04)00003-0 [DOI] [PubMed] [Google Scholar]
- [12].Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003; 31:1171-7; PMID:14641020; http://dx.doi.org/ 10.1042/bst0311171 [DOI] [PubMed] [Google Scholar]
- [13].Kumanogoh A, Shikina T, Suzuki K, Uematsu S, Yukawa K, Kashiwamura S, Tsutsui H, Yamamoto M, Takamatsu H, Ko-Mitamura EP, et al.. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity 2005; 22:305-16; PMID:15780988; http://dx.doi.org/ 10.1016/j.immuni.2005.01.014 [DOI] [PubMed] [Google Scholar]
- [14].Rennert PD, Ichimura T, Sizing ID, Bailly V, Li Z, Rennard R, McCoon P, Pablo L, Miklasz S, Tarilonte L, Bonventre JV. T cell, Ig domain, mucin domain-2 gene-deficient mice reveal a novel mechanism for the regulation of Th2 immune responses and airway inflammation. J Immunol. 2006; 177:4311-21; PMID:16982865; http://dx.doi.org/ 10.4049/jimmunol.177.7.4311 [DOI] [PubMed] [Google Scholar]
- [15].Mogie G, Shanks K, Nkyimbeng-Takwi EH, Smith E, Davila E, Lipsky MM, DeTolla LJ, Keegan AD, Chapoval SP. Neuroimmune semaphorin 4A as a drug and drug target for asthma. Int Immunopharmacol. 2013; 17:568-75; PMID:23994348; http://dx.doi.org/ 10.1016/j.intimp.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 2007; 8:913-9; PMID:17712344; http://dx.doi.org/ 10.1038/ni1507 [DOI] [PubMed] [Google Scholar]
- [17].Mix E, Meyer-Rienecker H, Zettl UK. Animal models of multiple sclerosis for the development and validation of novel therapies - potential and limitations. J Neurol 2008; 255:7-14; PMID:19300954; http://dx.doi.org/ 10.1007/s00415-008-6003-0 [DOI] [PubMed] [Google Scholar]
- [18].Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, et al.. Class IV semaphorin Sema4A enhances Tcell activation and interacts with Tim-2. Nature 2002; 419:629-33; PMID:12374982; http://dx.doi.org/ 10.1038/nature01037 [DOI] [PubMed] [Google Scholar]
- [19].Nakatsuji Y, Okuno T, Moriya M, Sugimoto T, Kinoshita M, Takamatsu H, Nojima S, Kimura T, Kang S, Ito D, et al.. Elevation of Sema4A Implicates Th Cell Skewing and the Efficacy of IFN-β Therapy in Multiple Sclerosis. J Immunol 2012; 188:4858-65; PMID:22491253; http://dx.doi.org/ 10.4049/jimmunol.1102023 [DOI] [PubMed] [Google Scholar]
- [20].Von Mutius E. Gene-environment interactions in asthma. J Allergy Clin Immunol 2009; 123:3-11; PMID:19130922; http://dx.doi.org/ 10.1016/j.jaci.2008.10.046 [DOI] [PubMed] [Google Scholar]
- [21].Zhang J, Paré PD, Sandford AJ. Recent advances in asthma genetics. Respir Res 2008; 9:4; PMID:18197984; http://dx.doi.org/ 10.1186/1465-9921-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol 2004; 22:789-815; PMID:15032597; http://dx.doi.org/ 10.1146/annurev.immunol.22.012703.104716 [DOI] [PubMed] [Google Scholar]
- [23].Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol 2010; 11:577-84; PMID:20562844; http://dx.doi.org/ 10.1038/ni.1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol 2010; 10:838-48; PMID:21060320; http://dx.doi.org/ 10.1038/nri2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nkyimbeng-Takwi EH, Shanks K, Smith E, Iyer A, Lipsky MM, Detolla LJ, Kikutani H, Keegan AD, Chapoval SP. Neuroimmune semaphorin 4A downregulates the severity of allergic response. Mucosal Immunol 2012; 5:409-19; PMID:22472774; http://dx.doi.org/ 10.1038/mi.2012.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morihana T, Goya S, Mizui M, Yasui T, Prasad DV, Kumanogoh A, Tamura M, Shikina T, Maeda Y, Iwamoto Y, et al.. An inhibitory role for Sema4A in antigen-specific allergic asthma. J Clin Immunol 2013; 33:200-9; PMID:23007237; http://dx.doi.org/ 10.1007/s10875-012-9798-5 [DOI] [PubMed] [Google Scholar]
- [27].Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008; 8:523-32; PMID:18566595; http://dx.doi.org/ 10.1038/nri2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003; 299:1057-61; PMID:12522256; http://dx.doi.org/ 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- [29].Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, et al.. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 2013; 501:252-6; PMID:23913274; http://dx.doi.org/ 10.1038/nature12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol 2003; 4:330-6; http://dx.doi.org/ 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- [31].Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299:1057-61; PMID:12522256; http://dx.doi.org/ 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- [32].Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. Schreiber. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 1994; 369:756-8; PMID:8008069; http://dx.doi.org/ 10.1038/369756a0 [DOI] [PubMed] [Google Scholar]
- [33].Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast. Cell 1994; 78:35-43; PMID:7518356; http://dx.doi.org/ 10.1016/0092-8674(94)90570-3 [DOI] [PubMed] [Google Scholar]
- [34].Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274-93; PMID:22500797; http://dx.doi.org/ 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ito D, Nojima S, Nishide M, Okuno T, Takamatsu H, Kang S, Kimura T, Yoshida Y, Morimoto K, Maeda Y, et al.. mTOR Complex Signaling through the SEMA4A-Plexin B2 Axis Is Required for Optimal Activation and Differentiation of CD8+ T Cells. J Immunol 2015; 195:934-43; PMID:26116513; http://dx.doi.org/ 10.4049/jimmunol.1403038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Meda C, Molla F, De Pizzol M, Regano D, Maione F, Capano S, Locati M, Mantovani A, Latini R, Bussolino F, et al.. Semaphorin 4A exerts a proangiogenic effect by enhancing vascular endothelial growth factor-A expression in macrophages. J Immunol 2012; 188:4081-92; PMID:22442441; http://dx.doi.org/ 10.4049/jimmunol.1101435 [DOI] [PubMed] [Google Scholar]
- [37].Lindor NM, Rabe K, Petersen GM, Haile R, Casey G, Baron J, Gallinger S, Bapat B, Aronson M, Hopper J, et al.. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA 2005; 293:1979-85; PMID:15855431; http://dx.doi.org/ 10.1001/jama.293.16.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lindor NM. Familial colorectal cancer type X: the other half of hereditary nonpolyposis colon cancer syndrome. Surg Oncol Clin N Am 2009; 18:637-45; PMID:19793571; http://dx.doi.org/ 10.1016/j.soc.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schulz E, Klampfl P, Holzapfel S, Janecke AR, Ulz P, Renner W, Kashofer K, Nojima S, Leitner A, Zebisch A, et al.. Germline variants in the SEMA4A gene predispose to familial colorectal cancer type X. Nat Commun 2014; 5:5191; PMID:25307848; http://dx.doi.org/ 10.1038/ncomms6191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pacione LR, Szego MJ, Ikeda S, Nishina PM, McInnes RR. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci 2003; 26:657-700; PMID:14527271; http://dx.doi.org/ 10.1146/annurev.neuro.26.041002.131416 [DOI] [PubMed] [Google Scholar]
- [41].Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet 2010; 11:273-84; PMID:20212494; http://dx.doi.org/ 10.1038/nrg2717 [DOI] [PubMed] [Google Scholar]
- [42].Sun H, Nathans J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. Implications for retinal disease. J Biol Chem 2001; 276:11766-74; PMID:11278627 [DOI] [PubMed] [Google Scholar]
- [43].Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007; 47:469-512; PMID:16968212; http://dx.doi.org/ 10.1146/annurev.pharmtox.47.120505.105225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gopalakrishnan MM, Grosch HW, Locatelli-Hoops S, Werth N, Smolenova E, Nettersheim M, Sandhoff K, Hasilik A. Purified recombinant human prosaposin forms oligomers that bind procathepsin D and affect its autoactivation. Biochem J 2004; 383:507-15; PMID:15255780; http://dx.doi.org/ 10.1042/BJ20040175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kishimoto Y, Hiraiwa M, O'Brien JS. Saposins: structure, function, distribution, and molecular genetics. J Lipid Res 1992; 33:1255-67; PMID:1402395 [PubMed] [Google Scholar]
- [46].Benes P, Vetvicka V, Fusek M. Cathepsin D–many functions of one aspartic protease. Crit Rev Oncol Hematol 2008; 68:12-28; PMID:18396408; http://dx.doi.org/ 10.1016/j.critrevonc.2008.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].O'Brien JS, Carson GS, Seo HC, Hiraiwa M, Kishimoto Y. Identification of prosaposin as a neurotrophic factor. Proc Natl Acad Sci U S A 1994; 91:9593-36; PMID:7937812; http://dx.doi.org/ 10.1073/pnas.91.20.9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Toyofuku T, Nojima S, Ishikawa T, Takamatsu H, Tsujimura T, Uemura A, Matsuda J, Seki T, Kumanogoh A. Endosomal sorting by Semaphorin 4A in retinal pigment epithelium supports photoreceptor survival. Genes Dev 2012; 26:816-29; PMID:22465952; http://dx.doi.org/ 10.1101/gad.184481.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lem J, Fain G. Constitutive opsin signaling: night blindness or retinal degeneration? Trends Mol Med 2004; 10:150-7; PMID:15059605; http://dx.doi.org/ 10.1016/j.molmed.2004.02.009 [DOI] [PubMed] [Google Scholar]
- [50].Abid A, Ismail M, Mehdi SQ, Khaliq S. Identification of novel mutations in the SEMA4A gene associated with retinal degenerative diseases. J Med Genet 2006; 43:378-81; PMID:16199541; http://dx.doi.org/ 10.1136/jmg.2005.035055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nojima S, Toyofuku T, Kamao H, Ishigami C, Kaneko J, Okuno T, Takamatsu H, Ito D, Kang S, Kimura T, et al.. A point mutation in Semaphorin 4A associates with defective endosomal sorting and causes retinal degeneration. Nat Commun 2013; 4:1406; PMID:23360997; http://dx.doi.org/ 10.1038/ncomms2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wang L, Song G, Zheng Y, Tan W, Pan J, Zhao Y, Chang X. Expression of Semaphorin 4A and its potential role in rheumatoid arthri. Arthritis Res Ther 2015; 17:227; PMID:26303122; http://dx.doi.org/ 10.1186/s13075-015-0734-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vadasz Z, Rainis T, Nakhleh A, Haj T, Bejar J, Halasz K, Toubi E. The Involvement of Immune Semaphorins in the Pathogenesis of Inflammatory Bowel Diseases (IBDs). PLoS One 2015; 10:e0125860; PMID:25978359; http://dx.doi.org/ 10.1371/journal.pone.0125860 [DOI] [PMC free article] [PubMed] [Google Scholar]