ABSTRACT
Semaphorins are a large family of proteins characterized by sema domains and play a key role not only in the formation of neural circuits, but in the immune system, angiogenesis, tumor progression, and bone metabolism. To date, 15 semaphorins have been reported to be involved in the formation of the peripheral nervous system (PNS) in higher vertebrates. A number of experiments have revealed their functions in the PNS, where they act mainly as axonal guidance cues (as repellents or attractants). Semaphorins also play an important role in the migration of neurons and formation of sensory-motor connections in the PNS. This review summarizes recent knowledge regarding the functions of higher vertebrate semaphorins in the formation of the PNS.
KEYWORDS: axon guidance, chemoattraction, chemorepulsion, chick, higher vertebrates, mouse
Introduction
The ligands that contain sema domains are generically called semaphorins. Semaphorins are secreted or membrane-bound proteins, and their effects are mostly mediated by transemembrane proteins named plexins. Via plexins or alternative receptors, semaphorins are involved in a wide range of growth processes such as neural circuit formation, angiogenesis, and bone metabolism. To date, 20 semaphorins have been identified in higher vertebrates. By focusing on higher vertebrates (eg., mammals, birds, and reptiles), especially on the mouse and the chick, we will review the function of semaphorins in the formation of the peripheral nervous system (PNS) in higher vertebrates.
Semaphorins in higher vertebrates
Higher vertebrate semaphorins are divided into classes 3–7 subfamilies.1 Class 3 semaphorins are secreted proteins, and they are subdivided into 7 members (Sema3A–Sema3G). Class 4 semaphorins, also comprising 6 members (Sema4A–Sema4D, Sema4F, Sema4G), are transmembrane proteins known to be regulators of a variety of immune responses. Two class 5 semaphorins (Sema5A and Sema5B) contains 7 thrombospondin repeats. Class 6 semaphorins are transmembrane proteins. This class contains 4 members with alternatively spliced cytoplasmic regions (Sema6A–Sema6D; Sema6C has not been identified in the chick). The lone class 7 semaphorin (Sema7A) is a glycosylphosphatidylinositol (GPI)-anchored protein.
Semaphorin receptors in higher vertebrates
Plexins
Semaphorin signals in higher vertebrates are primarily transduced by 9 plexins which are subdivided into 4 type A plexins (PlexinA1–PlexinA4; PlexinA3 is not identified in the chick), 3 type B plexins (PlexinB1–PlexinB3), PlexinC1 and PlexinD1. Plexins are single-passed transmembrane receptors that are known to interact directly with small GTPases in the cytoplasm.2 Plexins bind directly to class 4–7 semaphorins and Sema3E to transduce signals intracellularly. Class 3 semaphorins except for Sema3E do not bind directly to plexins but to neuropilins. PlexinAs or PlexinD1 form a complex with neuropilins in which neuropilins act as a binding receptor and plexins as a signal transducer.
Neuropilins
Neuropilins are cell surface molecules that have a short intracellular domain. In higher vertebrates, 2 homologues–neuropilin-1 (Nrp1) and neuropilin-2 (Nrp2)–have been identified. Several class 3 semaphorins utilize neuropilins as a receptor for transducing their signals. Sema3A is known to bind to Nrp1 but not to Nrp2.3 Sema3A/Nrp1 signals are mediated by PlexinAs or PlexinD1. In contrast, Sema3F binds to Nrp2 with high affinity but to Nrp1 with low affinity.3,4 Sema3F signals are transduced by a complex of Nrp2 and PlexinAs. Other class 3 semaphorins, Sema3B and Sema3C bind to both neuropilins and can transduce signals through them. As mentioned above, unlike other class 3 semaphorins, Sema3E binds directly to PlexinD1, not to neuropilins. Interestingly, in addition to PlexinD1, Sema3E requires Nrp1 to induce axon attraction of subiculo-mammillary neurons in the central nervous system.5
Other receptors
Further studies have revealed that a member of immunoglobulin superfamily cell adhesion molecules L1CAM and neuron-glial related cell adhesion molecule (NrCAM) act as other co-receptors for class 3 semaphorins under certain circumstances.6,7 In addition, in vitro analyses have shown that heparan sulfate proteoglycans mediate Sema5A-induced attraction for midbrain axons as components of Sema5A receptors.8 In contrast, the attractive effect by Sema5A on growing axons can be converted into repulsion by chondroitin sulfate proteoglycans (CSPGs), suggesting that CSPGs are other components of Sema5A receptors.8 Interestingly, Sema7A-induced axon growth of olfactory bulb neurons is mediated by β1 integrins independent of the PlexinC1 receptor.9
The peripheral nervous system in higher vertebrates
In higher vertebrates, the peripheral nervous system (PNS) is divided into the somatic nervous system, which is composed of cranial and spinal nerves, and the autonomic nervous system. With the exception of the olfactory (n I) and optic (n II) nerves, which are considered to be parts of the central nervous system, the cranial nerves are parts of the PNS.10 Accordingly, the 10 remaining cranial nerves (n III–n XII) are considered to be included in the PNS in this review. The autonomic nervous system is composed of sympathetic and parasympathetic nervous systems.
An illustration of the PNS in higher vertebrate embryos briefly shows the pathways of cranial nerves (n III–n XII) and the location of their ganglia (Fig. 1). The oculomotor nerve (n III) projects as a tight motor fiber bundle from the mesencephalic flexure ventrally toward the ciliary ganglion and extraocular muscles. The trochlear nerve (n IV) exits the central nervous system at the dorsal hindbrain-midbrain (mesencephalon-metencephalon) junction and projects as motor fibers to the superior oblique muscle of the eye. The trigeminal nerve (n V) is the largest and most complex nerve among cranial nerves. It contains motor and sensory fibers. The ophthalmic branch (V1) of the trigeminal nerve projects to the frontal part of the head. The maxillary branch (V2) projects to the maxillary process. The mandibular branch (V3) projects to the first pharyngeal arch (mandibular arch). The abducens nerve (n IV) is derived from the basal plate of the hindbrain (metencephalon) and projects as motor fibers to the lateral rectus muscle. The facial nerve (n VII) projects to the second pharyngeal arch. It contains motor and sensory fibers. The pathways of this nerve are variable. The vestibulocochlear nerve (n VIII) is derived from the otic placode, which forms the otic vesicle. The glossopharyngeal nerve (n IX) projects to the third pharyngeal arch (the third pharyngeal arch is not shown in Figure 1 because it is behind the second pharyngeal arch). The vagus nerve (n X) is the longest cranial nerve. Its motor and sensory fiber elements are derived from the basal plate of the hindbrain (myelencephalon) and cranial neural crest cells, respectively. The vagus nerve has a large ganglion called the inferior (nodose) ganglion. Most of the accessory nerve fibers (n XI) grow out of neurons located in the 1st–5th cervical spinal cord. They are motor fibers and pass rostrally between the dorsal and ventral roots. The hypoglossal nerve (n XII) is also comprised of motor fibers and is derived from the basal plate of the hindbrain (myelencephalon).
Figure 1.
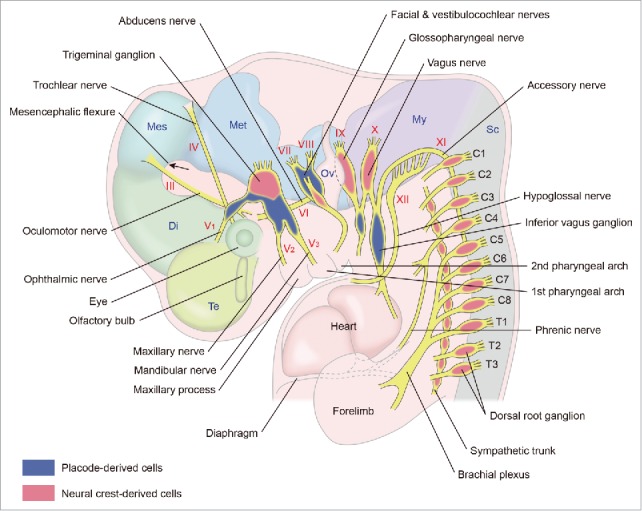
A schematic lateral view of the peripheral nervous system in a higher vertebrate embryo at the early stages. The 3rd–12th cranial nerves with their ganglia, spinal nerves with dorsal root ganglia and sympathetic trunk with its ganglia are shown. C1–C8 and T1–T3 indicate 1st–8th cervical and 1st–3rd thoracic spinal nerves, respectively. Di: diencephalon, Mes: mesencephalon, Met: metencephalon, My: myelencephalon, Ov: otic vesicle, Sc: spinal cord, Te: telencephalon.
Figure 1 also shows that fibers of the 5th–8th cervical spinal nerves and of the 1st thoracic spinal nerve converge to form the brachial plexus at the proximal region of the forelimb. In contrast, the sympathetic branches from the sympathetic ganglia in the thoracic 2nd–4th segments of the spinal cord project to the cardiac plexus (not shown in Fig. 1).
Discovery of semaphorins
Semaphorins have been implicated in the formation of the PNS from the time they were first identified. Sema3A is the first semaphorin whose role in the PNS has been closely examined. Sema3A was initially discovered to have an ability to collapse growth cones of dorsal root ganglion (DRG) neurons and to repel their axons in vitro.11 After this discovery, several studies using in vitro assays have revealed that other PNS axons, such as sympathetic and spinal motor axons, are also repelled by Sema3A. Subsequently, other higher vertebrate semaphorins have been examined by the same in vitro assays and/or genetic experiments using knock-out mice or RNAi. In this review, we will focus on several semaphorins (Sema3A, Sema3F, Sema6A, Sema6C and Sema6D), as mutant mice with these semaphorins have varied phenotypes with respect to the formation of the PNS. Furthermore, we will show additional data of both the in vitro and in vivo functions of other semaphorins and their receptors in Tables 1 and 2, respectively.
Table 1.
In vitro function of semaphorins and their receptors in the PNS of higher vertebrates.
| Semaphorins | Receptors* | In vitro function in the PNS of higher vertebrates | Refs. |
|---|---|---|---|
| Sema3A | PlexinA1–A4, PlexinD1, Nrp1, L1CAM | collapses DRG growth cones / repels DRG axons | 11,15 |
| collapses spinal motor growth cones / repels spinal motor axons | 13,19,52 | ||
| collapses sympathetic growth cones / repels sympathetic axons | 22,23 | ||
| collapses growth cones of oculomotor axons (III) | 25 | ||
| collapses trigeminal (sensory) growth cones / repels trigeminal (sensory) axons (V) | 27,29,53 | ||
| repels trigeminal (motor) axons (V) | 19 | ||
| repels abducens motor axons (VI) | 19 | ||
| collapses geniculate (sensory) growth cones / repels geniculate (sensory) axons (VII) | 27,28,29 | ||
| repels facial (motor) axons (VII) | 19 | ||
| repels glossopharyngeal (motor) axons (IX) | 19 | ||
| repels vagus (sensory) axons (X) | 29 | ||
| avoids the neural crest cell migration | 31 | ||
| Sema3B | Nrp1/2, NrCAM | repels sympathetic axons | 16,23 |
| Sema3C | PlexinA1, A2, PlexinD1, Nrp1/2 | repels sympathetic axons | 16,23 |
| collapses growth cones of oculomotor axons (III) | 25 | ||
| Sema3E | PlexinD1, Nrp1 | collapses DRG growth cones / repels DRG axons | 54,55 |
| Sema3F | PlexinA1–A4, Nrp1/2, NrCAM | repels sympathetic axons | 4,36 |
| Sema3G | Nrp2 | repels sympathetic axons | 56 |
| Sema4D | PlexinB1, B2, PlexinC1 | promotes DRG axonal growth | 57 |
| Sema4F | nd | mediates the interaction between Schwann cells and DRG axons | 58 |
| Sema5A | PlexinA1, A3**, PlexinB3, CSPG, HSPG | attracts DRG axons | 59 |
| Sema5B | PlexinA1, A3** | collapses DRG growth cones / inhibits DRG axonal growth | 60,61 |
| Sema6A | PlexinA1, A2, A4 | collapses sympathetic growth cones / repels sympathetic axons | 45,46 |
| regulates dendritic growth of LMC motor neurons | 42 | ||
| Sema6B | PlexinA2, A4 | inhibits sympathetic axonal growth | 45 |
| Sema6C** | PlexinA1 | collapses growth cones of DRG axons | 49,62 |
| Sema6D | PlexinA1 | collapses growth cones of DRG axons | 49 |
| Sema7A | PlexinC1, β1 integrins | promotes DRG axonal growth | 9 |
nd, not determined
These data are referred from refs. 2, 12, 35, 41, 63, 64
**
No homologue has been identified in the chick.
NrCAM: neuron-glial related cell adhesion molecule, CSPG: chondroitin sulfate proteoglycan, HSPG: heparan sulfate proteoglycan
Table 2.
In vivo function of semaphorins in the PNS of higher vertebrates.
| Semaphorins and their receptors | PNS phenotypes of mutant mice | Refs. |
|---|---|---|
| Sema3A | defects in the projection of DRG TrkA-positive afferents within the spinal cord | 17 |
| defects of segmental DRG formation | 32,33 | |
| defects in spinal motor fiber growth and guidance | 20 | |
| defects in sympathetic innervation and abnormal morphogenesis of the sympathetic trunk | 24,34 | |
| normal projections of the oculomotor nerve (III) | 26 | |
| premature projections of the trigeminal (V) and geniculate (VII) nerves to pharyngeal arches | 27 | |
| defects in sensory projections of vestibular ganglion neurons (VIII) | 30 | |
| disorganization of the trigeminal (V), facial (VII), glossopharyngeal (IX), vagus (X) and accessory nerves (XI) | 26 | |
| Sema3C | no abnormality in projections of cranial nerves and the sympathetic nervous system | 65 |
| Sema3E | disorganization of specific sensory-motor connections in the spinal cord | 66,67 |
| Sema3F | defects in the projection of LMCm neurons in the limb | 20 |
| severe defasciculation of the oculomotor nerve (III) | 39 | |
| absence of projections of the trochlear nerve (IV) | 39 | |
| disorganization of segmental migration patterns of NCCs | 40 | |
| Sema5A | no abnormality in DRG and spinal motor fibers, and in cranial nerves | 68 |
| Sema6A | ectopic migration of spinal motor neurons | 43 |
| Sema6D | aberrant projections of proprioceptive DRG neurons in the dorsal spinal cord | 48 |
| Nrp1 | defects in the projection of DRG TrkA-positive afferents within the spinal cord | 18 |
| defects of segmental DRG formation | 32,33 | |
| defects in spinal motor fiber growth and guidance | 20 | |
| ectopic positions of sympathetic neurons | 34 | |
| normal projections of the oculomotor nerve (III) | 69 | |
| defasciculation of the trigeminal (V), facial (VII), glossopharyngeal (IX) and vagus (X) nerves | 69 | |
| defects in the projection of the vestibulocochlear nerve (VIII) | 18 | |
| defasciculation of the hypoglossal nerve (XII) | 70 | |
| Nrp2 | defects in the projection of LMCm neurons in the limb | 20 |
| ectopic migration of spinal motor neurons | 43 | |
| no defects in the morphology of the sympathetic trunk | 38 | |
| aberrant projections of the oculomotor (III) and trochlear (IV) nerves similar to those in Sema3F mutants | 38,39,71 | |
| defasciculation of ophthalmic branches of the trigeminal nerve (V) | 71 | |
| partial defasciculation of the facial nerve (VII) | 38 | |
| normal projections of the vestibulocochlear (VIII) and vagus (X) nerves | 38 | |
| disorganization of segmental migration patterns of NCCs | 40 | |
| PlexinA3* | defasciculation of ophthalmic branches of the trigeminal nerve (V) | 72 |
| PlexinA4 | defects in DRG and sympathetic fibers | 45 |
| aberrant projections in sympathetic fibers | 45 | |
| defasciculation of the trigeminal (V), facial (VII), glossopharyngeal (IX) and vagus (X) nerves | 45 | |
| PlexinD1 | disorganization of specific sensory-motor connections in the spinal cord | 66,67 |
*
No homologue has been identified in the chick.
Sema3A in the formation of the PNS
Receptors for Sema3A are PlexinA1–PlexinA4 and PlexinD1 via co-receptor Nrp1 and L1CAM.6,12
DRG fibers
At the early stages before DRG fibers arrive at the dorsal spinal cord, Sema3A is expressed in the notochord, the myotome and a subset of spinal motor neurons (medial motor column [MMC] neurons; Fig. 2A).13 In vitro assays have shown that both myotome- and notochord-derived Sema3A strongly repel early DRG axons to block the aberrant innervation toward the myotome and the notochord (Fig. 2A).14
Figure 2.
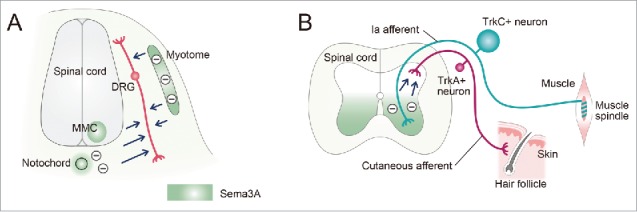
Both central and peripheral projections of DRG fibers are guided by Sema3A. Transverse views through the spinal cord showing DRG fibers and their target tissues. (A) Before DRG fibers reach the dorsal spinal cord, the myotome, medial motor column (MMC) motor neurons, notochord and perinotochordal mesenchyme exert Sema3A-induced repulsion (−) against DRG fibers (peppermint green). (B) After DRG fibers reach the dorsal spinal cord, small-diameter tyrosine receptor kinase A (TrkA)-positive neurons (nociceptive neurons, red) terminate their cutaneous afferents in the superficial layers of the dorsal spinal cord (laminae I and II) due to Sema3A-induced repulsion (−) from the ventral spinal cord (peppermint green). In contrast, large-diameter TrkC-positive neurons (proprioceptive neurons, blue) project their Ia afferents ventrally into the deeper laminae of the spinal cord; muscle spindle Ia afferents terminate in the intermediate laminae and the ventral horn because they are not repelled by Sema3A.
After DRG fibers have reached the dorsal spinal cord, Sema3A expression spreads over the ventral spinal cord (Fig. 2B). In vitro assays have also shown that tropomyosin receptor kinase A (TrkA)-positive DRG fibers (cutaneous afferents) are inhibited by Sema3A derived from the ventral spinal cord, suggesting that Sema3A may serve as a barrier to stop further projection of TrkA-positive DRG fibers into the ventral spinal cord (Fig. 2B).15,16 In contrast, TrkC-positive DRG fibers (Ia afferents) do not respond to Sema3A-induced repulsion, and they can project into the ventral spinal cord without blocking by Sema3A (Fig. 2B). Consistent with these results, a subset of TrkA-positive DRG fibers in Sema3A mutants extend into the ventral region of the spinal cord.17 Similar defects in the projection of TrkA-positive DRG fibers are observed in Nrp1sema- mutants (In Nrp1sema- mutants, the expression of the Nrp1 protein is normal but Sema/Nrp1 signaling is abolished).18
Spinal motor fibers
In vitro studies have revealed that spinal motor axons at embryonic stages are repelled by Sema3A.13,19 At early stages at the brachial or lumbar level, Sema3A is expressed in the notochord, the perinotochordal mesenchyme, the presumptive epaxial muscle (myotome), MMC neurons and the limb (Fig. 3A).20,21 In wild-type embryos, fibers of lateral motor column (LMC) neurons reach the base of the limb in a fasciculated manner, but do not enter the limb (Fig. 3A).20 In Sema3A-deficient mice, fibers of LMC neurons show defasciculation when they extend to the limb, indicating that the Sema3A signal derived from the myotome and notochord regulate fasciculation of fibers of LMC neurons.20 Furthermore, fibers of LMC neurons aberrantly enter the limb in advance, thus showing that the Sema3A signal also determines the timing of growth of these fibers to the limb.20
Figure 3.
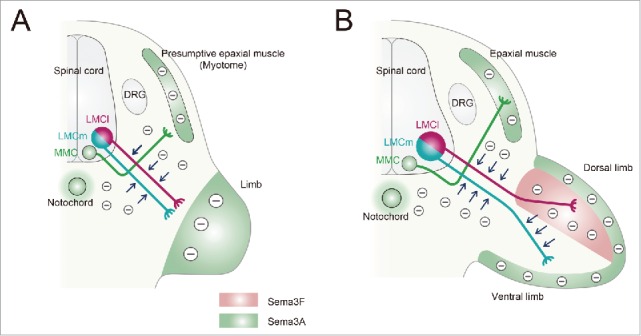
Spinal motor fibers are guided by Sema3A and Sema3F. A schematic transverse section through the spinal cord and limb bud at the brachial/lumbar levels summarizing spinal motor columns and Sema3A/3F for axonal targeting. (A) Lateral motor column (LMC) motor neurons are divided into 2 divisions: medial (m, blue) and lateral (l, red). In the early stages, both LMCm and LMCl motor neurons extend fibers straight toward the limb and pause before further growth in response to surround-repulsion by Sema3A (−): Sema3A secreted by the limb, presumptive epaxial muscle (myotome), perinotochordal mesenchyme and notochord (peppermint green). medial motor column (MMC) motor neurons expressing Sema3A are located medially and send fibers (green) to the epaxial muscle because intrinsic Sema3A proteins reduce the availability of Nrp1 receptors and modulate the sensitivity of MMC fibers to Sema3A. This modulation allows MMC fibers to extend dorsally toward the myotome. (B) At later stages, the expression of Sema3A in the limb is decreased, and the dorsal part of the limb begins to express Sema3F (pink). LMCl neurons express Nrp1 but not the Sema3F receptor Nrp2 and enter the dorsal limb because of their insensitivity to Sema3F. In contrast, LMCm neurons express both Nrp1 and Nrp2 and are constrained in the ventral limb by Sema3F-induced repulsion (−).
At later stages, Sema3A expression within the limb becomes weaker (Fig. 3B).20,21 In wild-type embryos, the fibers of both lateral and medial LMC (LMCl and LMCm, respectively) neurons diverge after entering the limb. LMCl fibers project toward the dorsal limb and LMCm fibers toward the ventral limb (Fig. 3B).20 In Sema3A-deficient mice, both fibers grow aberrantly in the dorso-ventral direction of the limb. These findings suggest that Sema3A signaling controls the dorso-ventral choice for the limb innervation by a subpopulation of spinal motor neurons.20 Furthermore, intrinsic Sema3A in MMC motor neurons is also involved in the pathway formation of their fibers.21 Loss- and gain-of-function experiments in chick embryos show that intrinsic Sema3A in MMC neurons modulates their axonal responses to environmental Sema3A, allowing MMC fibers to grow and project into the myotome/epaxial muscle regardless of the presence of Sema3A in these tissues (Fig. 3A and 3B).
Sympathetic fibers
Sema3A has been shown not only to collapse the growth cones of sympathetic axons but to repel sympathetic axons.22,23 Sema3A is synthesized by Purkinje fibers in the developing heart and displays an inverse distribution pattern with sympathetic fibers.24 A detailed examination of Sema3A mutants has shown that Sema3A plays a crucial role in the cardiac innervation by sympathetic fibers. Sema3A mutants lack this innervation, with this sympathetic dysfunction leading to sinus bradycardia. Overexpression of Sema3A in the heart also leads to reduced sympathetic innervation and sudden death.24 Taken together, these findings indicate that the appropriate expression of Sema3A in the heart is required for the normal innervation pattern of sympathetic fibers to control the heart rate.
Cranial nerves
As mentioned above, the oculomotor nerve (n III) projects into the extraocular muscles. Sema3A is expressed in developing extraocular muscles and has been shown to collapse growth cones of oculomotor axons.25 RNAi knockdown experiments on Sema3A receptor PlexinA1 in oculomotor neurons have induced guidance defects of oculomotor fibers. However, the trajectories of the oculomotor nerve are normal in Sema3A mutant embryos.26 This may be due to the fact that other PlexinA1 ligands, such as Sema3C expressed in the extraocular muscles, might prevent oculomotor fibers from projecting aberrantly and help guide their stereotypical projection.25
The V3 branch of the trigeminal nerve (n V) and fibers of geniculate ganglia (n VII) project to the first and second pharyngeal arches, respectively (see Fig. 1). In vitro studies have revealed that trigeminal and geniculate ganglion axons are repelled by Sema3A derived from pharyngeal arches.27,28 Consistent with these in vitro results, trigeminal and geniculate sensory fibers innervate the pharyngeal arches prematurely in the absence of Sema3A.27
Regarding other peripheral nerves, the glossopharyngeal (n IX) and vagus (n X) nerves are highly defasciculated in Sema3A mutants.26 Based on the fact that axons corresponding to these cranial nerves are shown to be repelled by Sema3A in culture experiments (see Table 1),19,29 defasciculation of these nerves may be caused by the repulsive activities of Sema3A secreted from the surrounding mesenchymal tissues. Of note, a previous study has reported that vestibular ganglion axons (n VIII) are not repelled by Sema3A.29 However, the expression patterns of Sema3A, Nrp1 and PlexinA1/3 strongly suggest the involvement of Sema3A signaling in the formation of vestibular circuits.30 Indeed, analyses of Sema3A-deficient mutant embryos have shown that Sema3A signaling is required for the proper afferent projection of vestibular ganglia.30
In addition, the 5th–12th cranial motor neurons exclude their fibers from the floor plate of the hindbrain during development. Guthrie's group has clearly demonstrated that the motor axons of trigeminal (n V), abducens (n VI), facial (n VII) and glossopharyngeal (n IX) nerves are repelled by Sema3A derived from the floor plate of the hindbrain.19 Thus, these cranial motor fibers may be guided in the proper direction partially by Sema3A.
Neural crest cell migration
Neural crest cells (NCCs) are known to migrate from the dorsal neural tube to specific peripheral districts. NCCs are precursors of DRG neurons which migrate into the anterior sclerotome. Sema3A is expressed in the posterior halves of sclerotomes, and Nrp1 is expressed in NCCs in the trunk.31 Detailed analyses demonstrate that Sema3A expressed in posterior sclerotomes plays an important role not only in guiding NCC migration through anterior sclerotomes by preventing NCCs from entering posterior sclerotomes but in the segmental neurogenesis of DRG neurons.32,33 NCCs are also a precursor for sympathetic neurons. In both Sema3A and Nrp1 mutants, many sympathetic neuron precursors are located at ectopic positions.34 Culture experiments have also shown that Sema3A suppresses the cell migration of sympathetic neurons.34 Thus, Sema3A expressed around the migratory path of NCCs/sympathetic neuron precursors further plays a crucial role in regulating the migration and arrest of NCCs and consequently contributes to the proper innervation of sympathetic fibers.
Sema3F in the formation of the PNS
Receptors for Sema3F are PlexinA1–PlexinA4, functioning together with co-receptors Nrp1/2 and NrCAM.12,35
Sympathetic fibers
Sema3F exerts repulsive activity toward sympathetic but not DRG axons.4,36 Additionally, close in vitro examinations using knockout mouse tissues have shown that Sema3F/Nrp2 signaling in sympathetic axons is mediated principally by PlexinA3 and partially by PlexinA4.37 However, Nrp2-deficient mice are observed to display no gross defects in the morphology of their sympathetic trunk.38 This result is consistent with the fact that Sema3F is absent in the migratory path of sympathetic neuron precursors and in the pathway of sympathetic fibers.
Spinal motor fibers
As mentioned above, LMCl motor fibers project into the dorsal limb, whereas LMCm motor ones project into the ventral limb at later stages (Fig. 3B). The dorsal part of the limb begins to express Sema3F at later stages (Fig. 3B).20 As LMCm neurons express the Sema3F receptor Nrp2, Sema3F blocks the projection of LMCm fibers to the dorsal limb and guides them to the ventral limb (Fig. 3B).20 Conversely, the fibers of LMCl neurons that do not express Nrp2 enter the dorsal limb because they are not affected by Sema3F-induced repulsion (Fig. 3B).20 Thus, Sema3F/Nrp2 signaling plays a critical role in guiding LMC motor fibers to the limb.
Cranial nerves
The oculomotor (n III) and trochlear (n IV) motor neurons express the Sema3F receptor Nrp2 during early prenatal stages.39 Interestingly, Sema3F expression is abundant in the caudal midbrain and the rostral hindbrain, but is absolutely absent in the hindbrain-midbrain junction where the trochlear nerve extends toward the eye.38 Taking into account the fact that the trochlear motor axons are repelled by Sema3F in vitro,38 these findings suggest that Sema3F expression flanking the path of the trochlear nerve may prevent trochlear fibers from projecting aberrantly and serve to promote their fasciculation. Consistent with this speculation, the trajectories of the trochlear nerve are absent in the absence of Sema3F. This phenotype in mutants further indicates an indispensable role for Sema3F in the normal development of the trochlear nerve.38,39 In contrast, the trajectories of the oculomotor nerve are severe defasciculated, but they still project into their normal target fields in Sema3F mutants.39
Neural crest cell migration
Sema3F and Nrp2 are expressed in the posterior sclerotome and in NCCs in the trunk, respectively.40 As is the case in Sema3A, Sema3F/Nrp2 signaling plays an important role in guiding segmental NCC migration through sclerotomes.40 However, this signaling is not required for segmental neurogenesis of DRG neurons.40
Sema6A
Receptors of Sema6A are PlexinA1, PlexinA2 and PlexinA4.12,41 Sema6A expression overlaps with the expression of PlexinA4 and an effecter molecule FARP-1 (FERM, RhoGEF and pleckstrin domain-containing protein 1) in LMC neurons of the spinal cord at the forelimb level.42 Both in vitro and in vivo analyses have revealed that Sema6A/PlexinA4 signaling regulates dendritic growth of LMC neurons in the spinal cord via FARP-1.42
In the developing spinal cord, boundary cap cells located at the motor exit points express Sema6A.41,43 On the other hand, the Sema6A receptors PlexinA1 and PlexinA2 are expressed in spinal motor neurons. In ovo experiments by RNAi in chicks have shown that the loss of Sema6A in boundary cap cells and the loss of PlexinA2 on spinal motor neurons result in the ectopic migration of spinal motor neurons in the spinal cord. Thus, Sema6A/PlexinA2 signaling regulates the location of immature spinal motor neurons within the spinal cord.43 Furthermore, the loss of Sema6A in boundary cap cells or the loss of PlexinA1 on spinal motor neurons pushes spinal motor neurons out of the spinal cord along the ventral roots, resulting in failure to form the dorsal roots properly.41 These results suggest that Sema6A produced by boundary cap cells functions as a separator between the central and peripheral nervous systems by gatekeeping the dorsal root entry and spinal motor axon exit sites.
Sema6A and PlexinA4 are co-expressed in DRG neurons, whereas sympathetic neurons express PlexinA4, but not Sema6A.44 In vitro studies have demonstrated that Sema6A can not only collapse the growth cones of sympathetic neurons, but also repel sympathetic axons via PlexinA4.45,46 In contrast, DRG neurons show little response to Sema6A-induced repulsive activities.46 This discrepancy may be due to the cis interaction between Sema6A and PlexinA4. Haklai-Topper et al.44 have shown that the cis interaction between Sema6A and PlexinA4 perturbs the cis-trans binding between trans Sema6A and cis PlexinA4. Namely, the DRG axonal response to extrinsic Sema6A might be diminished by intrinsic Sema6A.
Sema6C and Sema6D
The receptor of Sema6C and Sema6D is PlexinA1.12 Both Sema6C and Sema6D are expressed in the embryonic dorsal spinal cord.47 Leslie et al.48 have examined proprioceptive projections of DRG fibers in the absence of Sema6C and Sema6D. Sema6C-deficient mice show no obvious defects. In contrast, Sema6D-deficient mice show defects in the trajectories of the proprioceptive fibers similar to those in PlexinA1-deficient mice. Sema6C/Sema6D double-deficient mice are defective in the trajectories of the proprioceptive fibers to the same degree as Sema6D mutants.48 Taken together, the findings from analyses of these mutant phenotypes clearly show that Sema6D is a ligand for PlexinA1 in the dorsal spinal cord. Based on the fact that Sema6D is known to collapse growth cones of DRG axons,49 the ectopic placement of proprioceptive fibers in Sema6D mutants might be due to the loss of Sema6D repulsion in the dorsal spinal cord.
Conclusions
In this review, we regarded the relationship between semaphorins and plexins as a one-to-one correspondence. However, previous observations on plexinA1 and plexinB1 mutants strongly suggest that among their family members plexins play some redundant roles during development.50,51 To date, little is known about these functional redundancies, but future studies will reveal in more detail the redundant roles of individual plexins. At present, whether or not the epigenetic regulation of semaphorin genes plays a role in the axon guidance events or in the formation of the PNS during the fetal period remains unclear. We hope that novel findings regarding epigenetic regulation will emerge from future studies, thereby helping to further clarify the mechanism of the pathogenesis of neurodegenerative diseases caused by fetal developmental abnormalities.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants no. JP15K08148 and no. JP15H02765 from the program Grants-in-Aid for Scientific Research of the MEXT, Japan to T.M.
References
- [1].Semaphorin Nomenclature Committee . Unified nomenclature for the semaphorins/collapsins. Cell 1999; 97:551-2; PMID:10367884; http://dx.doi.org/ 10.1016/S0092-8674(00)80766-7 [DOI] [PubMed] [Google Scholar]
- [2].Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer 2008; 8:632-45; PMID:18580951; http://dx.doi.org/ 10.1038/nrc2404 [DOI] [PubMed] [Google Scholar]
- [3].Chen H, Chédotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 1997; 19:547-59; PMID:9331348; http://dx.doi.org/ 10.1016/S0896-6273(00)80371-2 [DOI] [PubMed] [Google Scholar]
- [4].Giger RJ, Urquhart ER, Gillespie SKH, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron 1998; 21:1079-92; PMID:9856463; http://dx.doi.org/ 10.1016/S0896-6273(00)80625-X [DOI] [PubMed] [Google Scholar]
- [5].Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, Segu L, Buhot MC, Jessell TM, Henderson CE et al.. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron 2007; 56:807-22; PMID:18054858; http://dx.doi.org/ 10.1016/j.neuron.2007.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Castellani V, Chédotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 2000; 27:237-49; PMID:10985345; http://dx.doi.org/ 10.1016/S0896-6273(00)00033-7 [DOI] [PubMed] [Google Scholar]
- [7].Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Püschel AW et al.. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron 2005; 48:63-75; PMID:16202709; http://dx.doi.org/ 10.1016/j.neuron.2005.10.024 [DOI] [PubMed] [Google Scholar]
- [8].Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ et al.. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 2004; 44:961-75; PMID:15603739; http://dx.doi.org/ 10.1016/j.neuron.2004.12.002 [DOI] [PubMed] [Google Scholar]
- [9].Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 2003; 424:398-405; PMID:12879062; http://dx.doi.org/ 10.1038/nature01790 [DOI] [PubMed] [Google Scholar]
- [10].Gould DJ, Fix JD. Neuroanatomy. 5th rev. ed. Gould DJ: Wolters Kluwer; 2014; 368 [Google Scholar]
- [11].Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 1993; 75:217-27; PMID:8402908; http://dx.doi.org/ 10.1016/0092-8674(93)80064-L [DOI] [PubMed] [Google Scholar]
- [12].Masuda T, Taniguchi M. Congenital diseases and semaphorin signaling: overview to date of the evidence linking them. Congenit Anom 2015; 55:26-30; http://dx.doi.org/ 10.1111/cga.12095 [DOI] [PubMed] [Google Scholar]
- [13].Masuda T, Sakuma C, Taniguchi M, Kanemoto A, Yoshizawa M, Satomi K, Tanaka H, Takeuchi K, Ueda S, Yaginuma H et al.. Development of the dorsal ramus of the spinal nerve in the chick embryo: A close relationship between development and expression of guidance cues. Brain Res 2012; 1480:30-40; PMID:22981415; http://dx.doi.org/ 10.1016/j.brainres.2012.08.055 [DOI] [PubMed] [Google Scholar]
- [14].Masuda T, Tsuji H, Taniguchi M, Yagi T, Tessier-Lavigne M, Fujisawa H, Okado N, Shiga T. Differential non-target-derived repulsive signals play a critical role in shaping initial axonal growth of dorsal root ganglion neurons. Dev Biol 2003; 254:289-302; PMID:12591248; http://dx.doi.org/ 10.1016/S0012-1606(02)00087-8 [DOI] [PubMed] [Google Scholar]
- [15].Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron 1995; 14:949-59; PMID:7748562; http://dx.doi.org/ 10.1016/0896-6273(95)90333-X [DOI] [PubMed] [Google Scholar]
- [16].Püschel AW, Adams RH, Betz H. The sensory innervation of the mouse spinal cord may be patterned by differential expression of and differential responsiveness to semaphorins. Mol Cell Neurosci 1996; 7:419-31; PMID:8812066; http://dx.doi.org/ 10.1006/mcne.1996.0030 [DOI] [PubMed] [Google Scholar]
- [17].Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 1996; 383:525-8; PMID:8849723; http://dx.doi.org/ 10.1038/383525a0 [DOI] [PubMed] [Google Scholar]
- [18].Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell 2003; 5:45-57; PMID:12852851; http://dx.doi.org/ 10.1016/S1534-5807(03)00169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Varela-Echavarría A, Tucker A, Püschel AW, Guthrie S. Motor axon subpopulations respond differentially to the chemorepellents netrin-1 and semaphorin D. Neuron 1997; 18:193-207; http://dx.doi.org/ 10.1016/S0896-6273(00)80261-5 [DOI] [PubMed] [Google Scholar]
- [20].Huber AB, Kania A, Tran TS, Gu C, De Marco Garcia N, Lieberam I, Johnson D, Jessell TM, Ginty DD, Kolodkin AL. Distinct roles for secreted semaphorin signaling in spinal motor axon guidance. Neuron 2005; 48:949-64; PMID:16364899; http://dx.doi.org/ 10.1016/j.neuron.2005.12.003 [DOI] [PubMed] [Google Scholar]
- [21].Moret F, Renaudot C, Bozon M, Castellani V. Semaphorin and neuropilin co-expression in motoneurons sets axon sensitivity to environmental semaphorin sources during motor axon pathfinding. Development 2007; 134:4491-501; PMID:18039974; http://dx.doi.org/ 10.1242/dev.011452 [DOI] [PubMed] [Google Scholar]
- [22].Koppel AM, Feiner L, Kobayashi H, Raper JA. A 70 amino acid region within the semaphorin domain activates specific cellular response of semaphorin family members. Neuron 1997; 19:531-7; PMID:9331346; http://dx.doi.org/ 10.1016/S0896-6273(00)80369-4 [DOI] [PubMed] [Google Scholar]
- [23].Adams RH, Lohrum M, Klostermann A, Betz H, Püschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J 1997; 16:6077-86; PMID:9321387; http://dx.doi.org/ 10.1093/emboj/16.20.6077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ieda M, Kanazawa H, Kimura K, Hattori F, Ieda Y, Taniguchi M, Lee JK, Matsumura K, Tomita Y, Miyoshi S et al.. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat Med 2007; 13:604-12; PMID:17417650; http://dx.doi.org/ 10.1038/nm1570 [DOI] [PubMed] [Google Scholar]
- [25].Ferrario JE, Baskaran P, Clark C, Hendry A, Lerner O, Hintze M, Allen J, Chilton JK, Guthrie S. Axon guidance in the developing ocular motor system and Duane retraction syndrome depends on Semaphorin signaling via alpha2-chimaerin. Proc Natl Acad Sci USA 2012; 109:14669-74; PMID:22912401; http://dx.doi.org/ 10.1073/pnas.1116481109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron 1997; 19:519-30; PMID:9331345; http://dx.doi.org/ 10.1016/S0896-6273(00)80368-2 [DOI] [PubMed] [Google Scholar]
- [27].Rochlin MW, O'Connor R, Giger RJ, Verhaagen J, Farbman AI. Comparison of neurotrophin and repellent sensitivities of early embryonic geniculate and trigeminal axons. J Comp Neurol 2000; 422:579-93; PMID:10861527; http://dx.doi.org/ 10.1002/1096-9861(20000710)422:4%3c579::AID-CNE7%3e3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- [28].Vilbig R, Cosmano J, Giger R, Rochlin MW. Distinct roles for Sema3A, Sema3F, and an unidentified trophic factor in controlling the advance of geniculate axons to gustatory lingual epithelium. J Neurocytol 2004; 33:591-606; PMID:16217616; http://dx.doi.org/ 10.1007/s11068-005-3329-8 [DOI] [PubMed] [Google Scholar]
- [29].Kobayashi H, Koppel AM, Luo Y, Raper JA. A role for collapsin-1 in olfactory and cranial sensory axon guidance. J Neurosci 1997; 17:8339-52; PMID:9334408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Katayama K, Imai F, Suto F, Yoshida Y. Deletion of Sema3a or plexinA1/plexinA3 causes defects in sensory afferent projections of statoacoustic ganglion neurons. PLoS One 2013; 8:e72512; PMID:23991118; http://dx.doi.org/ 10.1371/journal.pone.0072512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eickholt BJ, Mackenzie SL, Graham A, Walsh FS, Doherty P. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrain regions. Development 1999; 126:2181-9; PMID:10207143 [DOI] [PubMed] [Google Scholar]
- [32].Roffers-Agarwal J, Gammill LS. Neuropilin receptors guide distinct phases of sensory and motor neuronal segmentation. Development 2009; 136:1879-1888; PMID:19403658; http://dx.doi.org/ 10.1242/dev.032920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schwarz Q, Maden CH, Davidson K, Ruhrberg C. Neuropilin-mediated neural crest cell guidance is essential to organise sensory neurons into segmented dorsal root ganglia. Development 2009; 136:1785-9; PMID:19386662; http://dx.doi.org/ 10.1242/dev.034322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kawasaki T, Bekku Y, Suto F, Kitsukawa T, Taniguchi M, Nagatsu I, Nagatsu T, Itoh K, Yagi T, Fujisawa H. Requirement of neuropilin 1-mediated Sema3A signals in patterning of the sympathetic nervous system. Development 2002; 129:671-80; PMID:11830568 [DOI] [PubMed] [Google Scholar]
- [35].Sharma A, Verhaagen J, Harvey AR. Receptor complexes for each of the Class 3 Semaphorins. Front Cell Neurosci 2012; 6:28; PMID:22783168; http://dx.doi.org/ 10.3389/fncel.2012.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron 1998; 21:1283-90; PMID:9883722; http://dx.doi.org/ 10.1016/S0896-6273(00)80648-0 [DOI] [PubMed] [Google Scholar]
- [37].Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M. Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron 2005; 45:513-23; PMID:15721238; http://dx.doi.org/ 10.1016/j.neuron.2005.01.013 [DOI] [PubMed] [Google Scholar]
- [38].Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, Pickering S, Simmons D, Rastan S, Walsh FS et al.. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron 2000; 25:29-41; PMID:10707970; http://dx.doi.org/ 10.1016/S0896-6273(00)80869-7 [DOI] [PubMed] [Google Scholar]
- [39].Sahay A, Molliver ME, Ginty DD, Kolodkin AL. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci 2003; 23:6671-80; PMID:12890759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gammill LS, Gonzalez C, Gu C, Bronner-Fraser M. Guidance of trunk neural crest migration requires neuropilin 2/semaphorin 3F signaling. Development 2006; 133:99-106; PMID:16319111; http://dx.doi.org/ 10.1242/dev.02187 [DOI] [PubMed] [Google Scholar]
- [41].Mauti O, Domanitskaya E, Andermatt I, Sadhu R, Stoeckli ET. Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Dev 2007; 2:28; PMID:18088409; http://dx.doi.org/ 10.1186/1749-8104-2-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhuang B, Su YS, Sockanathan S. FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron 2009; 61:359-72; PMID:19217374; http://dx.doi.org/ 10.1016/j.neuron.2008.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J. Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural Dev 2007; 2:21; PMID:17971221; http://dx.doi.org/ 10.1186/1749-8104-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Haklai-Topper L, Mlechkovich G, Savariego D, Gokhman I, Yaron A. Cis interaction between Semaphorin6A and Plexin-A4 modulates the repulsive response to Sema6A. EMBO J 2010; 29:2635-45; PMID:20606624; http://dx.doi.org/ 10.1038/emboj.2010.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T et al.. Plexin-A4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci 2005; 25:3628-37; PMID:15814794; http://dx.doi.org/ 10.1523/JNEUROSCI.4480-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xu XM, Fisher DA, Zhou L, White FA, Ng S, Snider WD, Luo Y. The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J Neurosci 2000; 20:2638-48; PMID:10729344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yoshida Y, Han B, Mendelsohn M, Jessell TM. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron 2006; 52:775-88; PMID:17145500; http://dx.doi.org/ 10.1016/j.neuron.2006.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Leslie JR, Imai F, Fukuhara K, Takegahara N, Rizvi TA, Friedel RH, Wang F, Kumanogoh A, Yoshida Y. Ectopic myelinating oligodendrocytes in the dorsal spinal cord as a consequence of altered semaphorin 6D signaling inhibit synapse formation. Development 2011; 138:4085-95; PMID:21831918; http://dx.doi.org/ 10.1242/dev.066076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Qu X, Wei H, Zhai Y, Que H, Chen Q, Tang F, Wu Y, Xing G, Zhu Y, Liu S et al.. Identification, characterization, and functional study of the two novel human members of the semaphorin gene family. J Biol Chem 2002; 277:35574-85; PMID:12110693; http://dx.doi.org/ 10.1074/jbc.M206451200 [DOI] [PubMed] [Google Scholar]
- [50].Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K et al.. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol 2006; 8:615-22; PMID:16715077; http://dx.doi.org/ 10.1038/ncb1416 [DOI] [PubMed] [Google Scholar]
- [51].Fazzari P, Penachioni J, Gianola S, Rossi F, Eickholt BJ, Maina F, Alexopoulou L, Sottile A, Comoglio PM, Flavell RA et al.. Plexin-B1 plays a redundant role during mouse development and in tumour angiogenesis. BMC Dev Biol 2007; 7:55; PMID:17519029; http://dx.doi.org/ 10.1186/1471-213X-7-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shepherd IT, Luo Y, Lefcort F, Reichardt LF, Raper JA. A sensory axon repellent secreted from ventral spinal cord explants is neutralized by antibodies raised against collapsin-1. Development 1997; 124:1377-85; PMID:9118808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lwigale PY, Bronner-Fraser M. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev Biol 2007; 306:750-9; PMID:17499699; http://dx.doi.org/ 10.1016/j.ydbio.2007.04.012 [DOI] [PubMed] [Google Scholar]
- [54].Miyazaki N, Furuyama T, Amasaki M, Sugimoto H, Sakai T, Takeda N, Kubo T, Inagaki S. Mouse semaphorin H inhibits neurite outgrowth from sensory neurons. Neurosci Res 1999; 33:269-74; PMID:10401979; http://dx.doi.org/ 10.1016/S0168-0102(99)00015-2 [DOI] [PubMed] [Google Scholar]
- [55].Miyazaki N, Furuyama T, Sakai T, Fujioka S, Mori T, Ohoka Y, Takeda N, Kubo T, Inagaki S. Developmental localization of semaphorin H messenger RNA acting as a collapsing factor on sensory axons in the mouse brain. Neuroscience 1999; 93:401-8; PMID:10430503; http://dx.doi.org/ 10.1016/S0306-4522(99)00134-7 [DOI] [PubMed] [Google Scholar]
- [56].Taniguchi M, Masuda T, Fukaya M, Kataoka H, Mishina M, Yaginuma H, Watanabe M, Shimizu T. Identification and characterization of a novel member of murine semaphorin family. Genes Cells 2005; 10:785-92; PMID:16098142; http://dx.doi.org/ 10.1111/j.1365-2443.2005.00877.x [DOI] [PubMed] [Google Scholar]
- [57].Masuda K, Furuyama T, Takahara M, Fujioka S, Kurinami H, Inagaki S. Sema4D stimulates axonal outgrowth of embryonic DRG sensory neurones. Genes Cells 2004; 9:821-9; PMID:15330859; http://dx.doi.org/ 10.1111/j.1365-2443.2004.00766.x [DOI] [PubMed] [Google Scholar]
- [58].Parrinello S, Noon LA, Harrisingh MC, Wingfield Digby P, Rosenberg LH, Cremona CA, Echave P, Flanagan AM, Parada LF, Lloyd AC. NF1 loss disrupts Schwann cell-axonal interactions: a novel role for semaphorin 4F. Genes Dev 2008; 22:3335-48; PMID:19056885; http://dx.doi.org/ 10.1101/gad.490608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Masuda T, Sakuma C, Yaginuma H, Taniguchi M. Attractive and permissive activities of semaphorin 5A toward dorsal root ganglion axons in higher vertebrate embryos. Cell Adh Migr 2014; 8:603-6; PMID:25622099; http://dx.doi.org/ 10.4161/19336918.2014.972770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Browne K, Wang W, Liu RQ, Piva M, O'Connor TP. Transmembrane semaphorin5B is proteolytically processed into a repulsive neural guidance cue. J Neurochem 2012; 123:135-46; PMID:22817385; http://dx.doi.org/ 10.1111/j.1471-4159.2012.07885.x [DOI] [PubMed] [Google Scholar]
- [61].Liu RQ, Wang W, Legg A, Abramyan J, O'Connor TP. Semaphorin 5B is a repellent cue for sensory afferents projecting into the developing spinal cord. Development 2014; 141:1940-9; PMID:24718987; http://dx.doi.org/ 10.1242/dev.103630 [DOI] [PubMed] [Google Scholar]
- [62].Kikuchi K, Chédotal A, Hanafusa H, Ujimasa Y, de Castro F, Goodman CS, Kimura T. Cloning and characterization of a novel class VI semaphorin, semaphorin Y. Mol Cell Neurosci 1999; 13:9-23; PMID:10049528; http://dx.doi.org/ 10.1006/mcne.1998.0732 [DOI] [PubMed] [Google Scholar]
- [63].Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chédotal A, Peachey NS et al.. Class 5 transmembrane semaphorins control selective mammalian retinal lamination and function. Neuron 2011; 71:460-73; PMID:21835343; http://dx.doi.org/ 10.1016/j.neuron.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhou Y, Gunput RAF, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci 2008; 33:161-70; PMID:18374575; http://dx.doi.org/ 10.1016/j.tibs.2008.01.006 [DOI] [PubMed] [Google Scholar]
- [65].Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, Mombaerts P, Epstein JA, Raper JA. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 2001; 128:3061-70; PMID:11688556 [DOI] [PubMed] [Google Scholar]
- [66].Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature 2009; 459:842-6; PMID:19421194; http://dx.doi.org/ 10.1038/nature08000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fukuhara K, Imai F, Ladle DR, Katayama K, Leslie JR, Arber S, Jessell TM, Yoshida Y. Specificity of monosynaptic sensory-motor connections imposed by repellent Sema3E-PlexinD1 signaling. Cell Rep 2013; 5:748-58; PMID:24210822; http://dx.doi.org/ 10.1016/j.celrep.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fiore R, Rahim B, Christoffels VM, Moorman AF, Püschel AW. Inactivation of the Sema5a gene results in embryonic lethality and defective remodeling of the cranial vascular system. Mol Cell Biol 2005; 25:2310-9; PMID:15743826; http://dx.doi.org/ 10.1128/MCB.25.6.2310-2319.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 1997; 19:995-1005; PMID:9390514; http://dx.doi.org/ 10.1016/S0896-6273(00)80392-X [DOI] [PubMed] [Google Scholar]
- [70].Huettl RE, Huber AB. Cranial nerve fasciculation and Schwann cell migration are impaired after loss of Npn-1. Dev Biol 2011; 359:230-41; PMID:21925156; http://dx.doi.org/ 10.1016/j.ydbio.2011.08.019 [DOI] [PubMed] [Google Scholar]
- [71].Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, Lowenstein DH, Skarnes WC, Chédotal A, Tessier-Lavigne M. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron 2000; 25:43-56; PMID:10707971; http://dx.doi.org/ 10.1016/S0896-6273(00)80870-3 [DOI] [PubMed] [Google Scholar]
- [72].Cheng HJ, Bagri A, Yaron A, Stein E, Pleasure SJ, Tessier-Lavigne M. Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron 2001; 32:249-63; PMID:11683995; http://dx.doi.org/ 10.1016/S0896-6273(01)00478-0 [DOI] [PubMed] [Google Scholar]


