Abstract
Background
Phenotype of prostate cancer at diagnosis has changed through the years. We aim to evaluate the impact of year of surgery on clinical, pathologic and oncologic outcomes of high-risk prostate cancer patients.
Patients and methods
We evaluated 1,033 clinically high-risk patients, defined as the presence of at least one of the following risk factors: pre-operative prostate specific antigen (PSA) level >20 ng/ml, and/or clinical stage ≥T3, and/or biopsy Gleason score ≥8. Patients were treated between 1990 and 2013 at a single Institution. Year-per-year trends of clinical and pathologic characteristics were examined. Multivariable Cox regression analysis was used to test the relationship between year of surgery and oncologic outcomes.
Results
We observed a decrease over time in the proportion of high-risk patients with a pre-operative PSA level >20 ng/ml or clinical stage cT3. An opposite trend was seen for biopsy Gleason score ≥8. We observed a considerable increase in the median number of lymph nodes removed that was associated with an increased rate of LNI. At multivariable Cox regression analysis, year of surgery was associated with a reduced risk of biochemical recurrence (HR per 5-year: 0.90; 95% CI: 0.84–0.96; p=0.01) and distant metastasis (HR per 5-year: 0.91; 95% CI: 0.83–0.99; p=0.039), after adjusting for age, pre-operative PSA, pathologic stage, lymph node invasion, surgical margin status, and pathological Gleason score.
Conclusions
In this single center study, an increased diagnosis of localized and less extensive high-grade prostate cancer was observed over the last two decades. High-risk patients selected for radical prostatectomy showed better cancer control over time. Better definitions of what constitutes high-risk prostate cancer among contemporary patients are needed.
Keywords: prostate cancer, high-risk, radical prostatectomy, stage migration, cancer recurrence
Introduction
High-risk prostate cancer was originally defined more than 15 years ago [1] as the presence of at least one of the following risk factors: high pre-operative prostate specific antigen (PSA) level, and/or advanced clinical stage, and/or biopsy Gleason Score ≥8. This definition is still in wide use, and is part of treatment algorithms such as that of the National Comprehensive Cancer Network (NCCN) and the European Association of Urology (EAU) guidelines [2,3].
Historically, surgical treatment of high-risk prostate cancer was discouraged in this patient population [4,5], mainly because systemic spread of the disease was considered inevitable [6,7]. Radiotherapy and androgen deprivation therapy were more typically used for the initial management of high-risk prostate cancer. In contrast, surgery plays a major role in the management of contemporary high-risk patients. Indeed, radical prostatectomy combined with an extended pelvic lymph node dissection emerged as a valid strategy, providing important pathologic information and achieving good oncologic outcomes either alone or within the context of a multi-modal therapeutic approach [8-10].
Due to the increasing use of radical prostatectomy for high-risk patients, and the introduction of active surveillance for low risk patients, a stage migration towards more aggressive prostate cancer was recently reported for patients treated with radical prostatectomy, both in European and North-American series [11-13]. Moreover, there is evidence that the phenotype of prostate cancer at diagnosis has changed through the years due to PSA screening and changes in Gleason grading [14,15]. In this study, we aim to determine whether tumor characteristics have changed over time in the high-risk population and whether this has resulted in a change in oncologic outcomes.
Mterials and methods
Patient population
After Institutional Review Board approval, we identified 7884 consecutive prostate cancer patients treated with radical prostatectomy and pelvic lymph node dissection at our Institution between 1990 and 2013. Only patients with complete clinical and pathologic data who did not receive neo-adjuvant therapies were eligible. Of 5238 evaluable patients, 1033 met the criteria for high-risk disease, with at least one of the following risk factors according to the NCCN and the EAU guidelines [2,3]: PSA level >20 ng/ml, and/or clinical stage cT3, and/or biopsy Gleason Score ≥8. Of these, 124 (12%) patients met the criteria for very high-risk disease identified by the 2015 NCCN guidelines [2]: T3b-T4, primary Gleason pattern 5, or >4 cores with Gleason score 8-10. All patients were pre-operatively staged with bone scan and abdominopelvic computerized tomography.
Surgical technique
All patients were treated with radical prostatectomy preceded by extended pelvic lymph node dissection, regardless of tumor characteristics. Surgical procedures were performed by 15 different surgeons, who used standardized techniques [16] and applied the same anatomic template during pelvic lymph node dissection, as previously described [17].
Pathologic evaluation
All radical prostatectomy specimens were embedded in paraffin, cut at 3 mm, and stained with haematoxylin-eosin. Pelvic lymph node specimens were submitted for pathologic evaluation in multiple packages. Fat tissue containing lymph nodes were fixed in 10% buffered formalin. For each anatomic group, the number of nodes, the size of the largest node and any gross features were described.
Adjuvant treatments
Adjuvant therapy indications were based on the clinical judgment of each treating physician, according to patient and cancer characteristics. Additional treatments were considered as adjuvant therapies if administered within 3 months after surgery, regardless of post-operative PSA value. Adjuvant treatments consisted of radiation therapy and/or androgen deprivation therapy. Specifically, 358 patients (35%) received adjuvant radiation therapy, that was applied using the previously described technique [18]. Conversely, androgen deprivation therapy consisted of maximal androgen blockade, or luteinizing hormone-releasing hormone agonist alone, or bicalutamide in monotherapy. Adjuvant hormonal therapy was intended to be used lifelong. However, given the retrospective nature of the cohort, it is uncertain whether patients discontinued treatment after a period of androgen deprivation therapy. Overall, 219 patients (21%) received adjuvant hormonal therapy, alone or in combination with adjuvant radiation therapy.
Variable definition
We included age at surgery, preoperative PSA level, clinical stage, and biopsy Gleason score as clinical data, while pathologic data consisted of pathologic stage, pathologic Gleason score, surgical margins status, lymph node invasion (LNI), specimen confined disease, number of total lymph nodes removed and number of positive lymph nodes.
Pre-operative PSA level (AxSYM PSA assay; Abbott Laboratories, Abbott Park, IL, USA) was measured before digital rectal examination and transrectal ultrasound. Clinical stage was assigned by the attending urologist and was categorized as cT1, cT2, and cT3, while pathologic stage was categorized as pT2, pT3a, and pT3b/pT4. Both biopsy and pathologic Gleason score were reported as ≤6, 7, and ≥8. Positive surgical margin was defined as tumor extension to the inked surface of the radical prostatectomy specimen, while LNI was defined as involvement of one or more dissected pelvic lymph node. Finally, specimen confined disease was defined as pathologic stage pT2 / pT3a, negative surgical margins, and absence of LNI.
Outcome definition
The aim of this study was to evaluate changes in clinical and pathologic features among high-risk prostate cancer patients over time. Furthermore, we aimed to address the relationship between year of surgery and oncologic outcomes (namely, biochemical recurrence, distant metastasis, and cancer specific mortality). Biochemical recurrence was defined as a PSA value of 0.2 ng/ml or greater after surgery. Men who developed distant metastasis after radical prostatectomy and died from an unknown cause were assumed to have died from prostate cancer.
Statistics
Statistical analyses consisted of three main steps. First, Kernel-weighted local polynomial smoothing (lpoly) methods were used to observe the variation of clinico-pathological characteristics, and administration of adjuvant treatments in the study period. Changes over time were analyzed using either logistic or linear regression.
Second, locally weighted scatter plot smoothing (lowess) methods were used to examine the relation between year of surgery and oncologic outcomes (namely, biochemical recurrence rate at 3 years, distant metastasis rate at 5 years, and cancer specific mortality rate at 10 years). Changes over time were analyzed using Cox regression.
Third, multivariable Cox regression analysis was used to test the relationship between year of surgery and oncologic outcomes. Covariates consisted of patient age, pre-operative PSA, pathologic stage (≤pT3a vs. ≥pT3b), lymph node invasion (no vs. yes), positive surgical margins (no vs. yes), and pathological Gleason Score (≤6 vs. 7 vs. ≥8).
All statistical analyses were performed using Stata (StataCorp LP, College Station, TX, USA) version 12.0, with a 2-sided significance level set at p < 0.05.
Results
Descriptive characteristics of the study population are summarized in Table 1. The total number of radical prostatectomies performed yearly in high-risk patients considerably increased over the course of the study, from 5 procedures in 1990 to more than 100 procedures in 2011. However, the proportion of high-risk patients over the cohort of men surgically treated at our institution remains stable among time. Additionally, 124 (12%) patients met the criteria for very high-risk disease identified by the 2015 NCCN guidelines [2]. That proportion did not significantly change from the first half (n=25, 11%) to the second half of the study period (n=99, 12%; p=0.2).
Table 1.
Descriptive characteristics of 1,033 high risk prostate cancer patients treated with radical prostatectomy and extended pelvic lymph node dissection at a single tertiary referral center between 1990 and 2013. All numbers are medians (inter-quartile range) and frequencies (proportions). P values test the hypothesis that variables did not change over time.
| Variables | 1990-1994 (n=68; 7%) |
1995-1999 (n=98; 9%) |
2000-2004 (n=200; 19%) |
2005-2009 (n=378; 37%) |
2010-2013 (n=289; 28%) |
p value |
|---|---|---|---|---|---|---|
|
| ||||||
|
High-risk patients over total
number of cases |
68 (18%) | 98 (19%) | 200 (20%) | 378 (18%) | 289 (17%) | 0.1 |
|
| ||||||
| Age, yr | 69 (63, 72) | 66 (61, 71) | 67 (62, 72) | 66 (61, 71) | 67 (62, 71) | 0.2 |
|
| ||||||
| PSA, ng/ml | 21.0 (9.6, 30.1) | 22.3 (13.3, 36.0) | 20.6 (9.1, 31.8) | 10.0 (6.4, 25.0) | 8.9 (5.3, 21.0) | <0.0001 |
|
| ||||||
| Clinical stage | <0.0001 | |||||
| cT1 | 5 (7%) | 10 (10%) | 68 (33%) | 85 (22%) | 77 (26%) | |
| cT2 | 14 (21%) | 27 (28%) | 53 (27%) | 85 (22%) | 68 (24%) | |
| cT3 | 49 (72%) | 61 (62%) | 79 (40%) | 208 (56%) | 144 (50%) | |
|
| ||||||
| Biopsy Gleason score | <0.0001 | |||||
| ≤6 | 48 (71%) | 61 (62%) | 65 (33%) | 113 (30%) | 57 (20%) | |
| 7 | 14 (20%) | 22 (22%) | 57 (28%) | 100 (26%) | 85 (29%) | |
| ≥8 | 6 (9%) | 15 (16%) | 78 (39%) | 165 (44%) | 147 (51%) | |
|
| ||||||
| Pathological stage | 0.001 | |||||
| pT2 | 26 (38%) | 37 (38%) | 60 (30%) | 155 (41%) | 104 (36%) | |
| pT3a | 10 (15%) | 10 (10%) | 47 (23%) | 96 (25%) | 76 (26%) | |
| pT3b/pT4 | 32 (47%) | 51 (52%) | 93 (47%) | 127 (34%) | 109 (38%) | |
|
| ||||||
| Pathological Gleason score | <0.0001 | |||||
| ≤6 | 34 (50%) | 36 (37%) | 32 (16%) | 49 (13%) | 26 (9%) | |
| 7 | 20 (29%) | 43 (44%) | 91 (46%) | 174 (46%) | 134 (46%) | |
| ≥8 | 14 (21%) | 19 (19%) | 77 (38%) | 155 (41%) | 129 (45%) | |
|
| ||||||
| Positive surgical margins | 5 (7%) | 21 (21%) | 87 (44%) | 157 (42%) | 108 (37%) | <0.0001 |
|
| ||||||
| LN removed | 16 (12, 22) | 13 (9, 18) | 14 (10, 18) | 19 (14, 25) | 18 (13, 25) | <0.0001 |
|
| ||||||
| LNI | 19 (28%) | 29 (30%) | 69 (34%) | 115 (30%) | 114 (39%) | 0.045 |
|
| ||||||
| Specimen confined disease | 32 (47%) | 37 (38%) | 78 (39%) | 185 (49%) | 134 (46%) | 0.1 |
IQR = interquartile range; PSA = prostate specific antigen; LNI = lymph node invasion; LN = lymph nodes.
In the first step of our analyses, we examined the changes in clinical and pathologic cancer features over time. All the estimates for outcomes changes were reported per 5-year and were listed in Table 2.
Table 2.
Estimates for outcomes changes per 5-year interval in year at surgery. Results are presented in terms of odds ratio for binary outcomes and coefficients for continuous outcomes.
| Outcome | Estimate | 95% CI | p value |
|---|---|---|---|
| 1 high risk factor at diagnosis | 1.11 | 0.98, 1.26 | 0.11 |
| 2 high risk factors at diagnosis | 0.90 | 0.79, 1.02 | 0.10 |
| 3 high risk factors at diagnosis | 0.98 | 0.73, 1.32 | 0.9 |
| PSA > 20 ng/ml | 0.64 | 0.57, 0.73 | <0.001 |
| Clinical stage cT3 | 0.90 | 0.80, 1.01 | 0.07 |
| Biopsy Gleason score ≥ 8 | 1.65 | 1.45, 1.88 | <0.001 |
| Pathologic stage ≥ pT3b | 0.82 | 0.73, 0.92 | 0.001 |
| Pathologic Gleason score ≥ 8 | 1.41 | 1.24, 1.60 | <0.001 |
| Positive surgical margins | 1.34 | 1.18, 1.52 | <0.001 |
| Lymph node invasion | 1.12 | 0.99, 1.26 | 0.08 |
| Number of nodes removed | 2.01 | 1.44, 2.58 | <0.001 |
| Number of positive nodes | 0.54 | 0.12, 0.96 | 0.01 |
| Specimen confined disease | 1.08 | 0.97, 1.22 | 0.17 |
| Adjuvant radiation therapy | 0.51 | 0.36, 0.73 | <0.001 |
| Adjuvant hormonal therapy | 0.24 | 0.15, 0.38 | <0.001 |
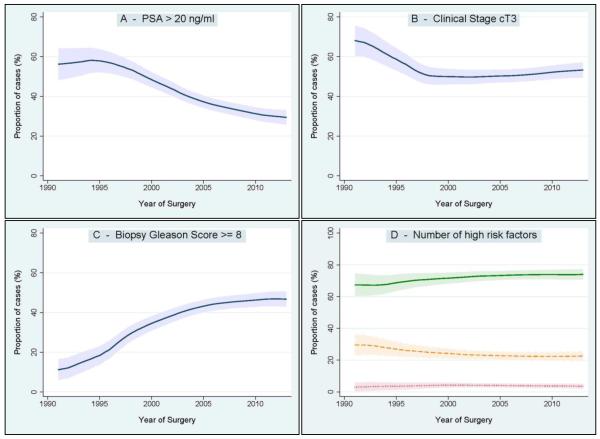
With respect to changes in clinical characteristics (Figure 1), we observed a significant decrease of patients diagnosed with a pre-operative PSA level >20 ng/ml. An opposite trend was seen for biopsy Gleason Score ≥8. The rate of clinical stage cT3 decreased between 1990 and 2000, and remained virtually stable thereafter. The number of high-risk factors at diagnosis (PSA >20 ng/ml, and/or clinical stage cT3, and/or biopsy Gleason Score ≥8) slightly changed between 1990 and 2013. Specifically, we observed a modest increase of patients with a single high-risk factor over time that was not statistically significant. Conversely, the rate of patients presenting with all three high-risk factors at diagnosis was relatively constant.
Figure 1.
Changes of clinical characteristics over the study period. Figures represent the proportion of patients diagnosed with PSA >20ng/ml (Figure 1A), clinical stage cT3 (Figure 1B) and biopsy Gleason score ≥8 (Figure 1C). Figure 1D represents the proportion of patients classified according to the number of high-risk factors at diagnosis. Green solid line: 1 high-risk factor. Orange dashed line: 2 high-risk factors. Red dotted line: 3 high-risk factors. Shaded area: 95% confidence interval.
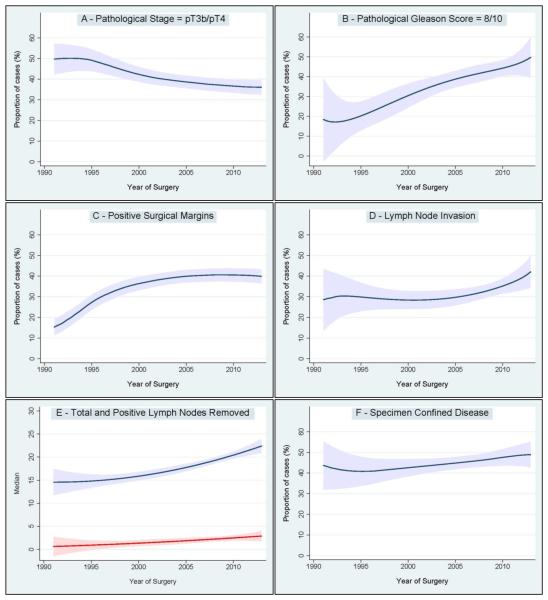
Changes in pathologic characteristics between 1990 and 2013 are shown in Figure 2. The rate of pathological stage pT3b/pT4 significantly decreased, while the rate of pathological Gleason Score ≥8 increased dramatically. On the other hand, the rate of positive surgical margins increased between 1990 and 2000, and remained virtually stable thereafter. Moreover, we observed a considerable increase in the median number of lymph nodes removed that was associated with both an increase in the median number of positive lymph nodes removed and an increase rate of LNI. Finally, we evaluated the variation of favorable pathologic outcome over time, which was defined as specimen confined disease (namely, pathological stage pT2/pT3a, negative surgical margins, and absence of LNI). We found a slight increase in the rate of specimen confined disease, which was not statistically significant.
Figure 2.
Changes of pathologic characteristics over the study period. Figure 2A: pathologic stage ≥pT3b. Figure 2B: pathologic Gleason score ≥8. Figure 2C: positive surgical margins. Figure 2D: lymph node invasion. Figure 2E: number of total (blue line) and positive (red line) lymph nodes removed. Figure 2F: specimen confined disease. Shaded area: 95% confidence interval.
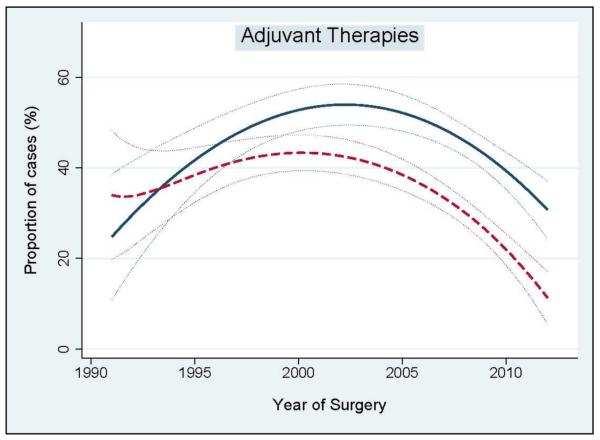
Figure 3 illustrates the administration of adjuvant treatments over time. The number of patients that received adjuvant radiation therapy increased between 1990 and 2005, and significantly decreased after 2005. Similarly, the use of androgen deprivation therapy increased between 1990 and 2000, and dramatically decreased after 2005.
Figure 3.
Adjuvant treatments administration over the study period. Blue solid line: adjuvant radiation therapy. Red dashed line: adjuvant hormonal therapy. Dotted lines: 95% confidence interval.
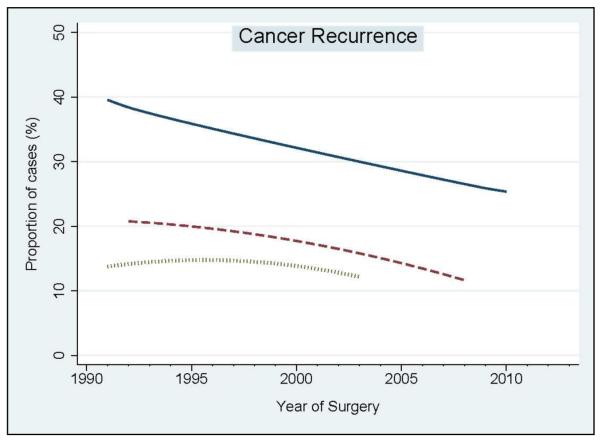
In the second step of our analyses, we assessed the relationship between year of surgery and oncologic outcomes (namely, biochemical recurrence rate at 3 years, distant metastasis rate at 5 years, and cancer specific mortality rate at 10 years) (Figure 4). We observed a significant decrease of biochemical recurrence rate at 3 years after surgery (hazard ratio [HR] per 5-year: 0.91; 95% CI: 0.86–0.95; p<0.001). Similarly, the decreased rate of distant metastasis at 5 years after surgery was statistically significant (HR per 5-year: 0.94; 95% CI: 0.88–0.99; p=0.01). Cancer specific mortality at 10 years also decreased between 1990 and 2003, although changes over time were not statistically significant (HR per 5-year: 0.86; 95% CI: 0.63–1.17; p=0.3).
Figure 4.
Year – per – year trend analysis of oncologic outcomes over the study period. Blue solid line: Biochemical recurrence rate at 3 years after surgery. Red dashed line: Distant metastasis rate at 5 years after surgery. Green dotted line: Cancer specific mortality rate at 10 years after surgery.
In the third step of our analyses, we assessed the relationship between year of surgery and oncologic outcomes using multivariable Cox regression analyses (Table 3). After adjusting for age, pre-operative PSA, pathologic stage, lymph node invasion, surgical margin status, and pathological Gleason score, year of surgery emerged as a significant predictor of biochemical recurrence (HR per 5-year: 0.90; 95% CI: 0.84–0.96; p=0.01) and distant metastasis (HR per 5-year: 0.91; 95% CI: 0.83–0.99; p=0.039). Conversely, year of surgery was not significantly associated with cancer specific mortality (HR per 5-year: 0.97; 95% CI: 0. 88–1.06; p=0.4).
Table 3.
Multivariable Cox regression analysis assessing the relationship between year of surgery and oncologic outcomes (namely, biochemical recurrence, distant metastasis, and cancer specific mortality) in 1,033 high risk prostate cancer patients treated with radical prostatectomy and extended pelvic lymph node dissection between 1990 and 2013 at a single institution. Models were adjusted for age at surgery, pre-operative prostate specific antigen, pathologic stage (≤pT3a vs. ≥pT3b), lymph node invasion (no vs. yes), positive surgical margins (no vs. yes), and pathologic Gleason score (≤6 vs. 7 vs. ≥8).
| Oncologic Outcomes | HR | 95% CI | p value |
|---|---|---|---|
| Biochemical Recurrence | 0.90 | 0.84, 0.96 | 0.01 |
| Distant Metastasis | 0.91 | 0.83, 0.99 | 0.039 |
| Cancer Specific Mortality | 0.97 | 0.88, 1.06 | 0.4 |
HR = hazard ratio per 5-year interval at surgery; CI = confidence interval.
Discussion
In this study we evaluated changes of clinical and pathologic tumor characteristics over time, as well as the impact of year of surgery on oncologic outcomes in high-risk prostate cancer patients. Several results of our study deserve attention. First, we found that high-risk patients treated with radical prostatectomy showed a lower PSA level at diagnosis, as well as a lower rate of locally advanced disease and a higher rate of high grade prostate cancer over time. Changes in PSA level and clinical stage at diagnosis were somewhat expected for the introduction of PSA screening programs, as similar results were shown in a recent population-based study [19]. Moreover, such results confirmed previous studies that showed a stage migration towards more aggressive prostate cancer for patients treated with radical prostatectomy [11-13].
Second, we observed a slight increase in the proportion of patients with specimen confined disease at final pathology. That outcome combines pathologic stage pT2 / pT3a with the absence of both positive surgical margins and LNI. Interestingly, the decreased rate of locally advance disease at final pathology was associated with a concomitant increase in the proportion of patients with LNI that reached a very high rate in the last years (approximately 40%). Compared to previous series, such LNI rate was significantly higher and was likely related to the increased number of lymph nodes removed over time. Specifically, Yossepowitch et al. found an increased rate of LNI in high-risk patients compared to low / intermediate risk categories (23% vs. 7%) [6]. Similarly, Walz et al. showed a LNI rate of 11% in a multi-institutional series of high-risk patients [20]. These findings vary considerably from our own, highlighting the role of extended pelvic lymph node dissection for accurate staging. Indeed, there is evidence that the higher the number of dissected LNs at time of surgery, the higher the likelihood of identifying metastatic disease [21]. Therefore, the increase of LNI rate in high-risk patients at our Institution may be related to an increased sensitivity of pelvic lymph node dissection secondary to better surgical technique. However, more extensive indications to surgical treatment over time may explain the increase of pN1 disease as well. Conversely, the rate of positive surgical margins remained virtually stable after 2000 (approximately 40%). This finding was in line with previous multi-institutional studies that showed a similar rate of positive surgical margins in high-risk patients [22,23].
Third, the use of adjuvant therapies largely decreased after 2005. This result was probably due to the developing opinion that not all men with pT3 stage or pT2 and positive surgical margins benefit from adjuvant radiation therapy. This is supported by the long-term results of two randomized trials (the EORTC 22911 and the ARO 96-02) where, despite lower biochemical recurrence on adjuvant radiation, there was no affect on clinical progression nor cancer specific and overall survival [24,25]. On the contrary, the 13 years results of the SWOG trial showed that postoperative irradiation did improve metastasis-free survival and overall survival in T3N0M0 patients [26]. Therefore, the correct post-operative management of patients with advanced and / or aggressive disease still remains a matter of debate, as the selection of salvage rather than adjuvant radiation therapy still represents a complex issue.
Fourth, surgical treatment has provided better cancer control over the years. Indeed, we observed a decreased biochemical recurrence and distant metastasis rate over the study period. These results are even more important in the context of an increase of LNI and high-grade disease at final pathology, even if during the decades patients presented with more benign features at diagnosis (i.e. lower clinical stage and PSA). However, these findings may be highly influenced by patient selection for surgical treatment.
Fifth, we did not observe a significant reduction of cancer specific mortality rate over time. This can be explained by the fact that the follow-up of patient treated in the most recent years is not yet long enough to show any improvements in survival. However, the encouraging results concerning biochemical outcome and clinical relapse may translate to better cancer control in the future, especially with a longer follow-up.
Our study has important clinical implications for management of prostate cancer. The “high-risk” term appears nowadays anachronistic. In light of novel trials investigating systemic agents in a neo-adjuvant context before surgery, we are in need of a novel high-risk definition possibly including molecular markers to define truly high-risk disease at diagnosis. Moreover, improvements in diagnosis with judicious use of PSA, more extensive biopsy sampling and adjustments in Gleason grading, have allowed for diagnosis of aggressive disease at earlier stages. While stage migration may have certainly impacted on early diagnosis, increase in Gleason grading over time might not reflect a real shift towards more aggressive disease. Rather, it may only represent a change towards more accurate histological assessment by pathologists. In addition to stage migration, cancer control improvement may be due to several reasons, such as patient selection, refinements in surgical technique as well as increasing surgical expertise of each treating physician [27,28]. All together, these data strongly support a change in cancer features and outcomes over time in the high-risk setting, where better cancer control rates can be expected after surgery in more contemporary patients as compared to what reported in historical series. This is especially as improved cancer control was paralleled by a decreased use of adjuvant treatments. Therefore, our data suggest that surgery alone can guarantee good cancer control in a significant proportion of high-risk patients, while multi-modal approaches can be reserved only for patient sub-groups having more adverse pathologic features. As suggested by a recently published study [29], our findings reiterate the need for contemporary and novel definitions of high-risk disease that should exclude those patients with more favorable outcomes and focus on lethal prostate cancer. Additionally, patient risk is simply a continuous variable ranging from 0% to 100%, and the categorization into risk groups (e.g. low- vs. intermediate- vs. high-risk) assumes discontinuities in risk at specific cut-points, with all patients within a risk category at similar risk. This can be a reasonable assumption at low-risk, but can be highly misleading at high-risk. As an example, a patient with PSA 21 ng/ml, clinical stage T1c, Gleason score 7 (3+4) in 3 positive cores is considered at the same risk of a different patient with cT3 disease and Gleason score 9 (4+5) in 12 positive cores. Recently, the 2015 NCCN guidelines [2] identified a sub-group of patients as “very high-risk”: T3b-T4, primary Gleason pattern 5, or >4 cores with Gleason score 8-10. This is a good attempt to face the problem of categorization. However, contemporary definitions including imaging parameters and / or genomic markers are welcome to go beyond traditional definitions based on clinical characteristics, and to develop a personal risk evaluation for each single patient.
The single Institution nature of the current study allows for important strengths. First, the same surgical technique for radical prostatectomy and the same template for extended pelvic lymph node dissection were used through the years and by different surgeons, all of which have trained under the same master. Second, pathologic evaluation of all radical prostatectomy specimens was performed with the similar technique.
Despite its strengths, our study is not devoid of limitations. Specifically, patients treated at a single institution might not be representative of the overall population of high-risk patients. In particular, tertiary referral centers might attract the most challenging cases. Moreover, all these patients, despite their aggressive cancer features, were considered eligible for surgery, thus introducing another important possible patient selection bias. Second, an internal revision of Gleason grading either at biopsy or at radical prostatectomy was not performed, thus potentially limit the validity of changes in grade reported over time. Despite this limitation, it has to be acknowledged that all pathologists used the same standardized approach at the same high volume centre. Third, the MRI usage could represent a further reason for the increase of specimen confined disease over time. However, this retrospective study focused on a 20-years time span, and we were unable to test such hypothesis. Fourth, the lack of standardized indications for adjuvant treatments as well as the lack of standardized adjuvant treatment protocols represent a further limitation of our study that could impact on oncologic outcomes. Lastly, the length of follow-up limits the interpretations of oncologic outcomes, with special regards to long-term cancer specific survival.
Conclusions
In this single center study, an increased diagnosis of localized and less extensive high-grade prostate cancer was observed over the last two decades. In this context, high-risk patients selected for radical prostatectomy showed better cancer control over time. These results underline the need for a better definition of what constitutes high-risk prostate cancer among contemporary patients.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
grant: P30CA008748.
Footnotes
The authors declare no conflicts of interest in preparing this article.
References
- [1].D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- [2].NCCN Clinical Practice Guidelines in Oncology [Accessed 04/01/2015];Prostate cancer. 2015 2014:1–98. Available at http://www.nccn.org/professionals/physician_gls/pdf/prostatepdf. [Google Scholar]
- [3].Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, et al. EAU Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent—Update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- [4].Hodgson D, Warde P, Gospodarowicz M. The management of locally advanced prostate cancer. Urologic Oncology: Seminars and Original Investigations. 1998;4:3–12. doi: 10.1016/s1078-1439(98)00025-8. [DOI] [PubMed] [Google Scholar]
- [5].Loeb S, Schaeffer EM, Trock BJ, Epstein JI, Humphreys EB, Walsh PC. What are the outcomes of radical prostatectomy for high-risk prostate cancer? Urology. 2010;76:710–4. doi: 10.1016/j.urology.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yossepowitch O, Eggener SE, Bianco FJ, Jr, Carver BS, Serio A, Scardino PT, et al. Radical Prostatectomy for Clinically Localized, High Risk Prostate Cancer: Critical Analysis of Risk Assessment Methods. J Urol. 2007;178:493–9. doi: 10.1016/j.juro.2007.03.105. [DOI] [PubMed] [Google Scholar]
- [7].Alkhateeb S, Alibhai S, Fleshner N, Finelli A, Jewett M, Zlotta A, et al. Impact of positive surgical margins after radical prostatectomy differs by disease risk group. J Urol. 2010;183:145–50. doi: 10.1016/j.juro.2009.08.132. [DOI] [PubMed] [Google Scholar]
- [8].Gontero P, Spahn M, Tombal B, Bader P, Hsu C-Y, Marchioro G, et al. Is there a prostate-specific antigen upper limit for radical prostatectomy? BJU International. 2011;108:1093–100. doi: 10.1111/j.1464-410X.2011.10076.x. [DOI] [PubMed] [Google Scholar]
- [9].Bastian PJ, Gonzalgo ML, Aronson WJ, Terris MK, Kane CJ, Amling CL, et al. Clinical and pathologic outcome after radical prostatectomy for prostate cancer patients with a preoperative Gleason sum of 8 to 10. Cancer. 2006;107:1265–72. doi: 10.1002/cncr.22116. [DOI] [PubMed] [Google Scholar]
- [10].Yossepowitch O, Eggener SE, Serio AM, Carver BS, Bianco FJ, Jr, Scardino PT, et al. Secondary Therapy, Metastatic Progression, and Cancer-Specific Mortality in Men with Clinically High-Risk Prostate Cancer Treated with Radical Prostatectomy. Eur Urol. 2008;53:950–9. doi: 10.1016/j.eururo.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Budäus L, Spethmann J, Isbarn H, Schmitges J, Beesch L, Haese A, et al. Inverse stage migration in patients undergoing radical prostatectomy: results of 8916 European patients treated within the last decade. BJU International. 2011;108:1256–61. doi: 10.1111/j.1464-410X.2010.09982.x. [DOI] [PubMed] [Google Scholar]
- [12].Silberstein JL, Vickers AJ, Power NE, Fine SW, Scardino PT, Eastham JA, et al. Reverse stage shift at a tertiary care center. Cancer. 2011;117:4855–60. doi: 10.1002/cncr.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pierorazio PM, Ross AE, Han M, Epstein JI, Partin AW, Schaeffer EM. Evolution of the clinical presentation of men undergoing radical prostatectomy for high-risk prostate cancer. BJU International. 2011;109:988–93. doi: 10.1111/j.1464-410X.2011.10514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shao Y-H, Demissie K, Shih W, Mehta AR, Stein MN, Roberts CB, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101:1280–3. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Outcomes of localized prostate cancer following conservative management. Jama. 2009;302:1202–9. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Walsh PC. Preservation of sexual function in the surgical treatment of prostatic cancer--an anatomic surgical approach. Important Adv Oncol. 1988:161–70. [PubMed] [Google Scholar]
- [17].Abdollah F, Suardi N, Gallina A, Bianchi M, Tutolo M, Passoni N, et al. Extended pelvic lymph node dissection in prostate cancer: a 20-year audit in a single center. Ann Oncol. 2013;24:1459–66. doi: 10.1093/annonc/mdt120. [DOI] [PubMed] [Google Scholar]
- [18].Abdollah F, Suardi N, Cozzarini C, Gallina A, Capitanio U, Bianchi M, et al. Selecting the optimal candidate for adjuvant radiotherapy after radical prostatectomy for prostate cancer: a long-term survival analysis. Eur Urol. 2013;63:998–1008. doi: 10.1016/j.eururo.2012.10.036. [DOI] [PubMed] [Google Scholar]
- [19].Ohmann EL, Loeb S, Robinson D, Bill-Axelson A, Berglund A, Stattin P. Nationwide, population-based study of prostate cancer stage migration between and within clinical risk categories. Scand J Urol. 2014;48:426–35. doi: 10.3109/21681805.2014.892150. [DOI] [PubMed] [Google Scholar]
- [20].Walz J, Joniau S, Chun FK, Isbarn H, Jeldres C, Yossepowitch O, et al. Pathological results and rates of treatment failure in high-risk prostate cancer patients after radical prostatectomy. BJU International. 2010;107:765–70. doi: 10.1111/j.1464-410X.2010.09594.x. [DOI] [PubMed] [Google Scholar]
- [21].Abdollah F, Gandaglia G, Suardi N, Capitanio U, Salonia A, Nini A, et al. More Extensive Pelvic Lymph Node Dissection Improves Survival in Patients with Node-positive Prostate Cancer. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.05.011. [DOI] [PubMed] [Google Scholar]
- [22].Briganti A, Joniau S, Gontero P, Abdollah F, Passoni NM, Tombal B, et al. Identifying the Best Candidate for Radical Prostatectomy Among Patients with High-Risk Prostate Cancer. Eur Urol. 2012;61:584–92. doi: 10.1016/j.eururo.2011.11.043. [DOI] [PubMed] [Google Scholar]
- [23].Abern MR, Terris MK, Aronson WJ, Kane CJ, Amling CL, Cooperberg MR, et al. The impact of pathologic staging on the long-term oncologic outcomes of patients with clinically high-risk prostate cancer. Cancer. 2014;120:1656–62. doi: 10.1002/cncr.28647. [DOI] [PubMed] [Google Scholar]
- [24].Bolla M, Van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–27. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- [25].Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, et al. Adjuvant Radiotherapy Versus Wait-and-See After Radical Prostatectomy: 10-year Follow-up of the ARO 96-02/AUO AP 09/95 Trial. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.03.011. [DOI] [PubMed] [Google Scholar]
- [26].Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–62. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vickers AJ, Savage CJ, Hruza M, Tuerk I, Koenig P, Martínez-Piñeiro L, et al. The surgical learning curve for laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncology. 2009;10:475–80. doi: 10.1016/S1470-2045(09)70079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bianco FJ, Vickers AJ, Cronin AM, Klein EA, Eastham JA, Pontes JE, et al. Variations among experienced surgeons in cancer control after open radical prostatectomy. J Urol. 2010;183:977–82. doi: 10.1016/j.juro.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sundi D, Wang VM, Pierorazio PM, Han M, Bivalacqua TJ, Ball MW, et al. Very-high-risk localized prostate cancer: definition and outcomes. Prostate Cancer Prostatic Dis. 2013;17:57–63. doi: 10.1038/pcan.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]