Abstract
Objective
To comprehensively describe the use of dexmedetomidine in a single institutional series of adult ICU patients with severe TBI. We describe the dexmedetomidine dosage and infusion times, as well as the physiological parameters, neurological status, and daily narcotic requirements before, during, and after dexmedetomidine infusion.
Methods
We identified 85 adult patients with severe TBI who received dexmedetomidine infusions in the Trauma ICU at Vanderbilt University Medical Center between 2006 and 2010. Demographic, hemodynamic, narcotic use, and sedative use data were systematically obtained from the medical record and analyzed for changes associated with dexmedetomidine infusion.
Results
During infusion with dexmedetomidine, narcotic and sedative use decreased significantly (p<.001 and p<.05). Median MAP, SBP, DBP, and HR also decreased significantly during infusion when compared to pre-infusion values (p<.001). Despite the use of dexmedetomidine, RASS and GCS scores improved from pre-infusion to infusion time periods.
Conclusions
Our findings demonstrate that initiation of dexmedetomidine infusion is not associated with a decline in neurological functioning in adults with severe TBI. Although there was an observed decrease in hemodynamic parameters during infusion with dexmedetomidine, the change was not clinically significant, and the requirements for narcotics and additional sedatives were minimized.
Keywords: Traumatic brain injury, dexmedetomidine, sedation
Introduction
Dexmedetomidine is a selective α2-adrenergic agonist that is US Food and Drug Administration (FDA) approved for the sedation of intubated patients in an intensive care setting [1]. Dexmedetomidine causes a central decrease in sympathetic outflow from the locus ceruleus, a decrease in activity in spinal motor neurons and spinothalamic pathways, and results in decreases in heart rate and blood pressure without compromising respiratory drive [2]. There is evidence that it may reduce brain dysfunction and delirium, while also decreasing the duration of mechanical ventilation [3, 4].
Numerous studies have examined dexmedetomidine use in critically ill populations [4–14]. In a mechanically ventilated intensive care unit (ICU) population, patients receiving dexmedetomidine were more easily aroused, better able to communicate pain, and had a shorter time to extubation when compared to patients sedated with propofol or midazolam [5]. Dexmedetomidine has also been shown to reduce the requirement for opioids, benzodiazepines, and propofol in critical care settings [6–7].
The efficacy of dexmedetomidine for sedation in intubated ICU patients is well established; however, its use in patients with traumatic brain injury (TBI) has not been comprehensively described. In samples inclusive of but not limited to TBI, dexmedetomidine has been shown to be as effective as propofol in providing sedation [11] and does not negatively affect cerebral metabolism [11–13]. Dexmedetomidine’s short half-life, easy reversibility, and minimally depressive effects make it an appealing choice of sedative in patients with TBI [14, 15]; it may allow for more reliable neurological assessment [14]. A case report discusses the effective use of dexmedetomidine to treat paroxysmal sympathetic hyperactivity in an adult with severe TBI [16]. Additionally, animal models suggest that dexmedetomidine may have a neuroprotective effect after ischaemic brain injury [17–19].
The objective of this retrospective study is to comprehensively describe the clinical use of dexmedetomidine in a large, single-institution cohort of adult, ICU patients with severe TBI. Specifically, we describe the dexmedetomidine dosage and infusion times, as well as the physiological parameters, neurological status, and daily narcotic requirements before, during, and after dexmedetomidine infusion.
Methods
We conducted a retrospective review of adult patients with severe TBI who received dexmedetomidine infusions in the Trauma ICU at Vanderbilt University Medical Center between 2006 and 2010. We included patients who received any infusion of dexmedetomidine and had a head abbreviated injury score (AIS) ≥3, Glasgow Coma Scale score (GCS) ≤8, ICU length of stay (LOS) ≥96 hours, and age ≥18 years. We obtained patient data from the Vanderbilt University StarPanel electronic medical record system, the trauma registry of the American College of Surgeons database, and the Vanderbilt enterprise data warehouse. Drug infusion administration was extracted through an automated process (Appendix). The remainder of the data was extracted manually.
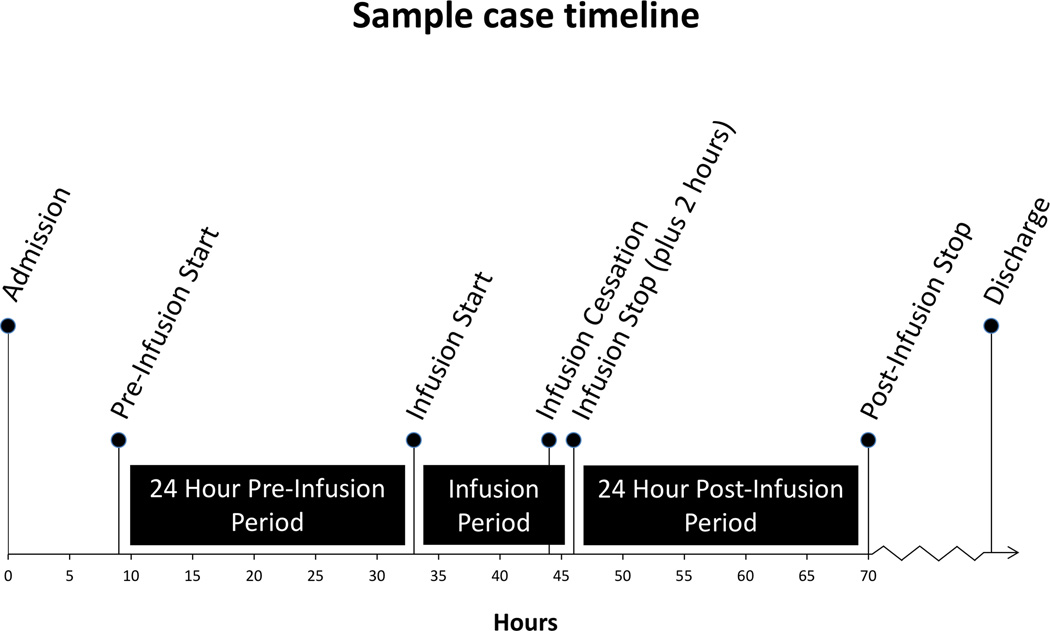
For each patient, the following demographic and injury data were collected from the trauma registry: sex, race, age, injury type, discharge disposition, days on ventilator, ICU LOS, hospital LOS, admission GCS, injury severity score (ISS), and head AIS. To assess patterns of dexmedetomidine usage, we computed the following: hours until infusion initiation, infusion duration, and median infusion rates in µg/kg/h (Appendix). To describe changes associated with dexmedetomidine infusion, we collected data from three time periods: pre-infusion, infusion, and post-infusion. We operationalized infusion times as follows (Figure 1): ‘pre-infusion’ was defined as either 24 hours prior to infusion start time or the number of hours since hospital admission, whichever was less; ‘infusion’ encompassed the actual duration of infusion (plus two hours to account for the terminal elimination half-life of dexmedetomidine [1]); and ‘post-infusion’ was the 24 hours following the ‘infusion’ period.
Figure 1.
Sample case timeline
To describe physiological changes, we recorded the median hourly systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) in each time period for each patient. Six patients received a second infusion of dexmedetomidine (i.e. infusion after 24-hour infusion-free interval); the data for this second infusion were not included in the study. To assess neurological changes, we recorded median hourly Richmond Agitation-Sedation Scale (RASS) and Glasgow Coma Scale (GCS) scores for each patient. Finally, we recorded the daily dosage of opioids (converted to fentanyl equivalents), propofol, and midazolam for each time period.
We performed descriptive statistics of sample characteristics for our patient cohort. We utilized the Friedman test, an omnibus, repeated measures analysis of variance by ranks to identify differences in the pre-infusion, infusion, and post-infusion hemodynamic, behavioural, and sedative and analgesic variables. Planned Wilcoxon signed-rank tests followed significant omnibus values.
Results
This study included 85 patients with severe TBI (Table 1). The median time from hospital admission to initiation of dexmedetomidine infusion was 63 hours (IQR: 44, 98), with a median infusion period of 32 hours (IQR: 18, 50). The median infusion dose of dexmedetomidine was 0.49 µg/kg/h (IQR: 0.38, 0.74).
Table 1.
Demographics, injury severity, and hospital course for 85 adult patients with severe TBI sedated with dexmedetomidine in the ICU
| Median | IQR | |
|---|---|---|
| Age (years) | 35 | 24, 51 |
| Head AIS score | 4 | 3, 5 |
| ISS score | 30 | 24, 38 |
| Admission GCS score | 3 | 3, 3 |
| Hospital LOS (days) | 15 | 11, 22 |
| ICU LOS (days) | 9 | 5, 13 |
| Time on ventilator (days) | 7 | 4, 13 |
| % | N | |
| Sex (male/female) | 86/14 | 73/12 |
| Race (Caucasian/Black/Hispanic) |
87/9/4 | 74/8/3 |
| Injury type (blunt/penetrating) | 95/5 | 81/4 |
| Discharge disposition | ||
| Death in hospital | 9 | 8 |
| Hospice | 2 | 2 |
| Nursing facility | 19 | 16 |
| Rehabilitation facility | 36 | 31 |
| Home | 29 | 25 |
| Other | 4 | 3 |
Abbreviations: TBI= Traumatic Brain Injury; ICU= Intensive Care Unit; IQR= Interquartile Range; AIS=Abbreviated Injury Score; ISS= Injury Severity Score; GCS=Glasgow Coma Scale; LOS=Length of Stay
SBP, DBP, MAP, and HR decreased during dexmedetomidine infusion and returned to baseline values after discontinuation of infusion (Table 2). From pre-infusion to infusion, SBP, DBP, and MAP decreased by 8.5, 5.5, and 7 mmHg, respectively. From infusion to post-infusion, SBP, DBP, and MAP increased by 13.5, 6.5, and 8 mmHg, respectively. These changes indicate a statistically significant decrease in blood pressure during the infusion period as compared to pre- and post-infusion periods (p<.001). Similarly, we observed a statistically significant (p<.001) change in median heart rate during infusion: median heart rate decreased by 2.5 beats/minute at the onset of infusion and increased by 6.5 during the post-infusion period. There was no significant difference between pre-infusion and post-infusion values. There were 24, 30, and 16 patients who experienced at least one episode of hypotension (i.e. SBP<90) during the pre-infusion, infusion, and post-infusion periods, respectively; this is not a statistically significant change in the number of patients experiencing hypotension.
Table 2.
Pre-infusion, infusion, and post-infusion median values for physiologic, behavioural, and drug variables related to 85 adult patients with severe TBI sedated with dexmedetomidine
| Variable | Pre-infusion period in median (IQR) |
Infusion period in median (IQR) |
Post-infusion period in median (IQR) |
|---|---|---|---|
| SBP (mmHg) |
129.5 (117, 141) | 121 (111, 131.5) | 134.5 (120, 144) |
| DBP (mmHg) |
67 (60, 75) | 61.5 (55.5, 67) | 68 (60, 76.5) |
| MAP (mmHg) |
84 (78, 93) | 77 (71, 84.5) | 85 (77, 93) |
| HR (beats/min) |
91.5 (83, 103) | 89 (73, 97) | 95.5 (85.5, 102) |
| RASS | −2 (−2, −1) | −1 (−2, −1) | −1 (−2, 0) |
| GCS | 8 (7, 10) | 9.5 (7.5, 10) | 10 (8, 11) |
| Fentanyl equivalents (mcg/hr) |
59.10 (33.30, 92.85) | 23.00 (11.33, 59.20) | 15.10 (5.33, 62.00) |
| Propofol (mcg/hr) |
20.48 (1.31, 35.48) | 0.00 (0.00, 5.36) | 0.00 (0.00, 14.88) |
| Midazolam (mg/hr) |
0.42 (0.00, 1.08) | 0.08 (0.00, 0.31) | 0.21 (0.00, 1.38) |
Note: Propofol dosage is converted to dosage requirement for a standard 70 kg patient.
GCS scores increased from pre-infusion to infusion to post-infusion (Table 2). From pre-infusion to infusion, the median GCS score increase from 8 to 9.5 was significant (p<.01). From infusion to post infusion, the median GCS score increase from 9.5 to 10 was significant (p<.01). RASS scores increased significantly from pre-infusion to infusion (p<.01) and from infusion to post-infusion (p<.05). From pre-infusion to infusion, median RASS score increased from -2 to -1. Although median RASS score did not change from infusion to post-infusion, the change in the distribution of RASS scores was significant. The observed increase in RASS scores indicates less sedation.
Daily dosages of propofol and midazolam were recorded (Table 2). Of the 85 patients, 51 received propofol and 35 received midazolam during the study period. Propofol infusion rate decreased from pre-infusion to infusion and remained lower during the post-infusion period. From pre-infusion to infusion, the decrease in median propofol infusion rate from 20.48 to 0.00 µg/kg/h was statistically significant (p<.001). The median propofol infusion rate during the post-infusion period was significantly lower than during pre-infusion (p<.05). Midazolam use decreased during infusion and increased from infusion to post-infusion. From pre-infusion to infusion, the decrease in median midazolam infusion rate from 0.42 to 0.08 mg/h was significant (p<.05). From infusion to post-infusion, the increase in median midazolam infusion rate from 0.08 to 0.21 mg/h was significant (p<.05) There was no significant difference in midazolam usage during the pre- and post-infusion periods.
Daily narcotic dosages for each patient were recorded, converted into micrograms of fentanyl, and summed to calculate median fentanyl equivalents (Table 2). Of the 85 patients, 78 received narcotics during the study period. Narcotic use decreased significantly during dexmedetomidine infusion compared to pre-infusion rates (p<.001). Narcotic use between infusion and post-infusion did not differ significantly.
Discussion
This large retrospective case series describes the analgesic and sedative requirements, neurological status, and physiologic changes in 85 patients with severe TBI that received a dexmedetomidine infusion. The results of the study did not demonstrate clinically significant changes in hemodynamics during the infusion period. Analgesic and sedative drug requirements were reduced during infusion and measures of sedation and neurological status improved throughout the study period.
Although median values of SBP, DBP, MAP, and HR all decreased during dexmedetomidine infusion, this decrease was not clinically significant. In a previous study, 26% of neurosurgical patients experienced hypotension during sedation with dexmedetomidine [8]. The current study found documented hypotensive episodes in 35% of patients with TBI during dexmedetomidine treatment; however, the number of patients experiencing hypotension was not significantly different before, during, and after infusions. A randomized controlled trial comparing sedation with dexmedetomidine versus midazolam did not find a difference in the proportion of mechanically-ventilated ICU patients experiencing hypotension [4]. Although our findings suggest a decrease in blood pressure during treatment with dexmedetomidine, the magnitude of the observed decrease is not clinically significant. In a continuously monitored ICU population with severe TBI, the risk of hypotension does not appear to contraindicate sedation with dexmedetomidine.
Midazolam and propofol usage were significantly lower during treatment with dexmedetomidine. This result is not surprising as dexmedetomidine has been used to effectively sedate patients without use of an adjunct sedative medication [4]. Additionally, analgesic requirements were lower during infusion than during the pre- and post-infusion periods. This suggests that preferred levels of sedation and patient comfort can be achieved with dexmedetomidine while decreasing the need for pain medication.
The observed increase in RASS score from -2 (light sedation) to -1 (drowsy) indicates patients were more easily aroused during dexmedetomidine infusion than during the pre-infusion period. Increased arousability was maintained in the post-infusion period. The median RASS score of -1 during the infusion time period indicates that patients can be cooperatively sedated with dexmedetomidine, and may be better able to communicate pain or changes in neurological status. A prior study found ICU patients better able to be aroused, cooperate, and communicate pain when sedated with dexmedetomidine versus propofol or midazolam [5]. In patients with TBI, the goal of sedation is to provide comfort without compromising the ability to administer serial neurological examinations. Our findings suggest that dexmedetomidine may facilitate more appropriate levels of sedation in patients with severe TBI.
The increase in GCS from pre- to infusion to post-infusion is consistent with a time-dependent improvement in the neurological functioning of patients with TBI. The retrospective nature of the study makes it unclear whether dexmedetomidine is causally related to improved GCS scores. A randomized controlled trial of a mixed ICU population without primary brain injury (e.g. no stroke, no TBI) found that patients treated with dexmedetomidine were less likely to experience delirium as compared to patients treated with midazolam [3]; however, the impact of dexmedetomidine on GCS remains unclear. Our findings do not demonstrate an association between initiation of dexmedetomidine infusion and decline in neurological functioning.
To date, this study of dexmedetomidine represents the largest group of patients with severe TBI, but there are several limitations due to its retrospective nature. Information about drug administration, RASS, GCS and hemodynamic values were obtained from manually entered sources. Despite our systematic extraction of data, problems with initial data entry may have impacted our findings. Additionally, we did not obtain information about the clinical decision to begin or end sedation with dexmedetomidine. Prior studies indicate that use of a loading dose impacts hemodynamic changes [4, 14]; loading doses were not consistently recorded in the medical record and are rarely used clinically at our institution. Finally, this study and all case series are limited by its lack of a control group. Observed changes across the study period cannot distinguish recovery due to treatment from recovery over time.
In an intensive care setting, patients with severe TBI present unique challenges. The need for frequent neurological examination and prolonged mechanical ventilation makes choice of sedative particularly important. Future prospective studies should evaluate the effectiveness of dexmedetomidine versus other sedatives, such as propofol and midazolam, in this unique population. A randomized controlled trial would minimize influences (e.g. recovery trajectory, pulmonary function, clinical judgment to begin dexmedetomidine) that we were unable to control in the present study. Also, future work should evaluate the effects of long-term (>24h) dexmedetomidine sedation in patients with severe TBI on coma recovery, ICU utilization (e.g., ventilator time, ICU days) and functional, cognitive, and mental health outcomes.
Acknowledgments
We thank M.S. Delozier for her help in the initial data extraction and verification.
The project utilized REDCap, which is supported by Clinical and Translational Science Award (CTSA) Grant (UL1 TR000448 from NCATS/NIH). Author MBP was supported by the Vanderbilt Faculty Scholars Research Program, AHRQ Health Services (5T32HS013833-08), and the Eastern Association for the Surgery of Trauma Foundation Research Scholarship. Author PPP is supported by NIH grants (R01AG027472, R01HL111111, R01AG035117).
Appendix: Computation of drug amounts via infusion
During the study period smart-pumps at Vanderbilt very sparsely recorded the rate/drug used for individual subjects for later reference, complicating the calculation of drug infused. Nurses also recorded drug rates via flowsheets in clinical drug units (mg/kg/h, mg/h, etc.) as well as the physical flow rate in mL/h at regular intervals. These flows depended upon the accuracy and consistency of nursing charts.
The following sources of information were used to compute drug amounts:
Nursing flowsheets of fluid intake (including drugs) were collected from copies housed in Vanderbilt’s Enterprise Data Warehouse (EDW).
Verified pharmacy orders, enabling drug concentration computation, were also collected from the EDW.
Patient weights were collected from operational copies of computerized physician order entry (CPOE) records, the pharmacy system, and the ICU admission registry, also housed in the EDW.
The following procedure was used to compute infusion amounts for each subject, via an internally developed web-database program, termed Panama, used by multiple research groups at Vanderbilt.
The flowsheet event information was compiled and each charted drug mapped to its RxNorm semantic clinical drug form (SCDF). For example, a nurse selection of ‘Precedex’ was mapped to dexmedetomidine Injectable Solution.
The concentration of pharmacy verified orders that mapped to the same RxNorm SCDF was computed, and organized temporally according to a drug concentration timeline for each subject.
For the flows in clinical drug units, the patient’s weight at the event time was incorporated if needed to a compute a rate in drug mass units/hour (e.g. mg/h)
For the flows in mL/h, the event time was compared to the drug concentration timeline to compute an initial drug mass rate/hour.
As a quality control measure, a set of rules determined the most clinically likely interpretation of the nurse flowsheet data to improve the drug mass rate/hour in a second pass. These rules depended upon the presence of either or both the clinical rate and the physical flow rate, preceding charted rates, following charted rates, as well as the normal flow rates for the drug.
The timeline of nursing flows was used to interpolate the flow rate vectors into a total drug infused via an area under the curve computation.
For each event, the investigators could override the program’s determinations and assert a finalized infusion amount. In this study, no overrides were made.
Footnotes
Presented at:
Local Poster Presentation at 31st Vanderbilt Annual Research Forum on April 18, 2013.
Poster Presentation at Neurotrauma 2013, the 31st Annual Symposium of the National Neurotrauma Society on August 4-7, 2013, Nashville, TN. Sun DZ, Wilson LD, McKenna JW, Hamblin SE, Humble SS, Pandharipande PP, Patel MB. Five-Year Dexmedetomidine (Precedex) Use for Sedation after Traumatic Brain Injury. Journal of Neurotrauma 2013; 30: A41-A42.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- 1.Full prescribing information for precedex. [cited 2012 Aug 1];US Food and Drug Administration. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021038s021lbl.pdf.
- 2.Mirski MA, Lewin JJ. Sedation and pain management in acute neurological disease. Seminars in Neurology. 2008;28(5):611–630. doi: 10.1055/s-0028-1105970. [DOI] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 4.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 5.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J. Dexmedetomidine for Long-Term Sedation Investigators. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 6.Martin E, Ramsay G, Mantz J, Sum-Ping STJ. The role of the α2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. Journal of Intensive Care Medicine. 2003;18(1):29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- 7.Triltsch AE, Welte M, von Homeyer P, Grosse J, Genähr A, Moshirzadeh M, Sidiropoulos A, Konertz W, Kox WJ, Spies CD. Bispectral index-guided sedation with dexmedetomidine in intensive care: A prospective, randomized, double blind, placebo-controlled phase II study. Critical Care Medicine. 2002;30(5):1007–1014. doi: 10.1097/00003246-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Venn RM, Hell J, Michael Grounds R. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4(5):302. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aryan HEMD, Box KW, Ibrahim D, Desiraju U, Ames CP. Safety and efficacy of dexmedetomidine in neurosurgical patients. Brain Injury. 2006;20(8):791–798. doi: 10.1080/02699050600789447. [DOI] [PubMed] [Google Scholar]
- 10.Grof TM, Grof TM, Bledsoe KA, Bledsoe KA. Evaluating the use of dexmedetomidine in neurocritical care patients. Neurocrit Care. 2010;12(3):356–361. doi: 10.1007/s12028-008-9156-x. [DOI] [PubMed] [Google Scholar]
- 11.James ML, Olson DM, Graffagnino C. A pilot study of cerebral and haemodynamic physiological changes during sedation with dexmedetomidine or propofol in patients with acute brain injury. searchproquestcomproxylibraryvanderbiltedu. 2012;40(6):949–957. doi: 10.1177/0310057X1204000605. [DOI] [PubMed] [Google Scholar]
- 12.Tarabrin O, Shcherbakov S, Gavrychenko D, Mazurenko G. Comparison of dexmedetomidine and propofol for sedation in patients with traumatic brain injury. Crit Care. 2014;18(Suppl 1):P416. [Google Scholar]
- 13.Wang X, Ji J, Fen L, Wang A. Effects of dexmedetomidine on cerebral blood flow in critically ill patients with or without traumatic brain injury: A prospective controlled trial. Brain Inj. 2013;27(13–14):1617–1622. doi: 10.3109/02699052.2013.831130. [DOI] [PubMed] [Google Scholar]
- 14.Tang JF, Chen PL, Tang EJ, May TA, Stiver SI. Dexmedetomidine controls agitation and facilitates reliable, serial neurological examinations in a non-intubated patient with traumatic brain injury. Neurocrit Care. 2011;15(1):175–181. doi: 10.1007/s12028-009-9315-8. [DOI] [PubMed] [Google Scholar]
- 15.Flower O, Hellings S. Sedation in traumatic brain injury. Emerg Med Int. 2012;2012:637171. doi: 10.1155/2012/637171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goddeau RP, Jr, Silverman SB, Sims JR. Dexmedetomidine for the treatment of paroxysmal autonomic instability with dystonia. Neurocrit Care. 2007;7(3):217–220. doi: 10.1007/s12028-007-0066-0. [DOI] [PubMed] [Google Scholar]
- 17.Maier C, Steinberg GK, Sun GH, Zhi GT, Maze M. Neuroprotection by the alpha 2-adrenoreceptor agonist dexmedetomidine in a focal model of cerebral ischemia. Anesthesiology. 1993;79(2):306–312. doi: 10.1097/00000542-199308000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Kuhmonen J, Haapalinna A, Sivenius J. Effects of dexmedetomidine after transient and permanent occlusion of the middle cerebral artery in the rat. J Neural Transm. 2001;108(3):261–271. doi: 10.1007/s007020170071. [DOI] [PubMed] [Google Scholar]
- 19.Nakano T, Okamoto H. Dexmedetomidine-induced cerebral hypoperfusion exacerbates ischemic brain injury in rats. J Anesth. 2009;23(3):378–384. doi: 10.1007/s00540-009-0777-9. [DOI] [PubMed] [Google Scholar]