Abstract
Background
Probiotic supplementation has been promoted for numerous health conditions, however safety in immunosuppressed patients is unknown. We evaluated bloodstream infections (BSIs) caused by common probiotic organisms in hematopoietic cell transplant recipients.
Methods
All blood culture (BC) results from a cohort of hematopoietic cell transplant recipients transplanted at Fred Hutchinson Cancer Research Center in Seattle, Washington, between 2002 and 2011 were reviewed. Patients with at least 1 positive BC for common probiotic organisms (Lactobacillus species, Bifidobacterium species, Streptococcus thermophiles, and Saccharomyces species) within 1 year post hematopoietic cell transplantation (HCT) were considered cases. Data were collected from center databases, which contain archived laboratory data, patient demographics, and clinical summaries.
Results
A total of 19/3796 (0.5%) patients developed a BSI from 1of these organisms within 1 year post HCT; no Bifidobacterium species or S. thermophilus were identified. Cases had a median age of 49 years (interquartile range [IQR]: 39–53), and the majority were allogeneic hematopoietic cell transplant recipients (14/19, 74%). Most positive BCs were Lactobacillus species (18/19) and occurred at a median of 84 days (IQR: 34–127) post transplant. The incidence rate of Lactobacillus bacteremia was 1.62 cases per 100,000 patient-days; the highest rate occurred within 100 days post transplant (3.3 per 100,000 patient-days). Eight patients (44%) were diagnosed with acute graft-versus-host disease of the gut prior to the development of bacteremia. No mortality was attributable to any of these infections.
Conclusion
Organisms frequently incorporated in available over-the-counter probiotics are infrequent causes of bacteremia after HCT. Studies evaluating the use of probiotics among high-risk patients are needed.
Keywords: probiotic, Lactobacillus, bacteremia, fungemia, hematopoietic cell transplant, over-the-counter
Probiotics are microorganisms taken as part of nutritional intake or oral supplementation that, in sufficient quantities, are purported to alter or restore balance to host gastrointestinal flora (1). Probiotic supplementation has been promoted for the treatment or prevention of various health conditions including Clostridium difficile infection, antibiotic-associated diarrhea, and inflammatory bowel disease (2–4), although, to date, high quality randomized clinical trials are of limited size and scope (4–7). At the same time, over-the-counter (OTC) access and increased marketing of probiotics have not only led to widespread availability but increasing use in the general population (8, 9). Although most probiotics are “generally regarded as safe” (8) and thus are not regulated by the U.S. Food and Drug Administration, a theoretical risk exists for gut translocation and bloodstream infection (BSI) (10, 11).
Because these OTC preparations are sold as food additives/supplements, and are increasingly available in pharmacies and grocery stores, the potential risk of these agents in high-risk populations needs to be better understood. Probiotics offer the potential to restore or replace “protective” gut organisms; however, data are limited on the safety or efficacy of probiotics in highly immunocompromised hosts, such as hematopoietic cell transplant recipients (12, 13).
We sought to describe the incidence and outcomes of BSIs from organisms common to OTC probiotic supplements in a cohort of hematopoietic cell transplant recipients, in order to explore the theoretical consequences of probiotics in hematopoietic cell transplantation (HCT), and to address the potential safety of future trials with probiotics.
Methods
Study design
We performed a retrospective 10 year cohort study of all patients who underwent HCT at The Fred Hutchinson Cancer Research Center (FHCRC) in Seattle, Washington between January 2, 2002 and December 31, 2011. Patients provided informed consent for review of their records for research purposes, and the FHCRC institutional review board approved all study activities.
Center-based infection prevention strategies and laboratory procedures/testing
All patients received standard viral, fungal, and Pneumocystis jirovecii prophylaxis, as well as ceftazidime or levofloxacin for neutropenic prophylaxis per protocol as described previously (14). Standard practice guidelines strongly encourage blood cultures (BCs) during acute febrile episodes, but all microbiologic cultures were conducted at physician discretion. Routine surveillance BCs are drawn once weekly, regardless of symptoms in outpatients (twice weekly while inpatient) in patients with graft-versus-host disease (GVHD) receiving daily prednisone doses of ≥1 mg/kg. Decisions regarding treatment of positive BCs, including antibiotic selection and central line removal, are made by the primary team often in conjunction with the infectious diseases (ID) service.
Patients undergoing transplantation are encouraged to follow an immunosuppressed patient diet (15) until 3 months post transplant (autologous recipients) or until cessation of immunosuppressive drugs (allogeneic recipients). Hematopoietic cell transplant recipients at the center have access to nutritional services as part of their pre- and post-transplant care, and caregivers/patients are encouraged to take a pre-transplant nutrition course, which provides education on safe eating habits, nutritional guidelines, and high-risk foods (e.g., blue-veined cheeses, smoked seafood); patients are not actively discouraged from eating foods that include live probiotic cultures (e.g., yogurt). While education is routine, adherence to dietary guidelines are not routinely evaluated. Importantly, no restrictions exist on where patients acquire, prepare, or consume their food. No current policies are aimed at screening for active probiotic use. Whether a patient was taking a probiotic supplement or not was unknown.
Definitions
A case was defined as a patient with at least 1 positive BC, from a set of aerobic and anaerobic cultures, for the organisms commonly used in probiotics (henceforth referred to as “probiotic organisms”) within 1 year post HCT. For the purposes of this study, common probiotic organisms found in available OTC preparations included Lactobacillus species, Bifidobacterium species, Streptococcus thermophiles, and Saccharomyces species. Patients with BCs growing any of these organisms prior to transplantation were excluded. Deaths within 1 year of first positive BC were reviewed by 2 ID physicians to determine whether the death was directly attributable to bacteremia/fungemia with the probiotic organism(s). Deaths were considered attributable if they occurred within 14 days of isolating a common probiotic organism in culture and if patients had compatible antecedent signs or symptoms. A third ID physician served as an adjudicator if the first 2 reviewers disagreed. Established consensus criteria were used in the diagnosis and grading of GVHD (16).
Data collection and statistical analysis
Archived laboratory data, clinical transplant summaries, and long-term follow-up databases were reviewed up to 1 year post HCT. Patient demographics, transplant details, outcomes, and hospitalization data were recorded; all dietary notes were reviewed in detail. In addition, antimicrobial therapy and rates of central venous catheter removals in response to bacteremia or fungemia with probiotic organisms were assessed. Where available, organism susceptibility was also documented.
Incidence rates were calculated by dividing the number of incident cases by the number of patient-days at risk. Patients were considered “at risk” from the first day post HCT until censorship (at 1 year, death, re-transplant, or loss to follow-up) or infection by probiotic organism; 95% confidence intervals (CI) were estimated based on a Poisson distribution. Data analyses were conducted using Stata version 14.1 (StataCorp; College Station, Texas USA).
Results
A total of 3799 patients underwent HCT in this cohort. Of these, 19/3796 (0.5%) had an organism commonly found in probiotic products isolated from BC; 3 patients were excluded from analysis because of detection of these organisms pre-transplant (all Lactobacillus species; 1 Lactobacillus casei and 2 unknown species). Affected patients had a median age of 49 years (interquartile range [IQR]: 39–53), and the majority of cases were allogeneic transplant recipients (14/19, 74%). Seven of the 14 allogeneic transplant recipients (50%) had documented acute GVHD of the gut prior to bacteremia. Most cases occurred in hospitalized patients (13/19 [68%]) who had been inpatients for a median of 36 days (IQR: 10–76) before their positive BC meeting the case definition.
The overall incidence rate for detection of a common probiotic organism in BCs was 1.62 cases per 100,000 patient-days. The highest incidence rate occurred in the first 100 days post transplant (3.3 per 100,000 patient-days) and accounted for 12/19 (63%) of cases. Cases occurred throughout the study period and did not appear to cluster per calendar year (data not shown). BCs identified a probiotic organism at a median of 91 days (IQR: 41–128) post transplant (Fig. 1).
Fig. 1.
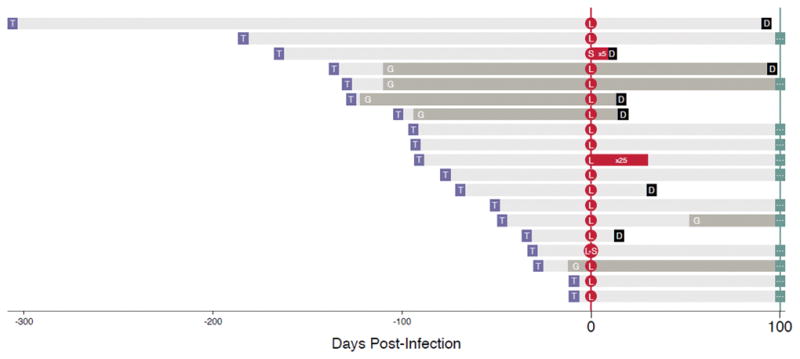
Timing of infection, graft-versus-host diseases (GVHDs) and mortality post hematopoietic cell transplantation (HCT) among patients with BSIs from common over-the-counter (OTC) organisms. T: Date of transplant ; G: Onset of GHVD, grey bar indicates ongoing GVHD; L: Date of first blood culture positive for Lactobacillus; S: Date of first blood culture positive for Saccharomyces; D: Death; red bar indicates period on going positive cultures; grey bar indicates ongoing GVHD; bluegrey ellipses: Censored.
The majority of patients developed bacteremia from Lactobacillus species (18/19 [95%]) (Table 1). Most of these isolates were not classified beyond the level of genus (1 L. casei, 1 Lactobacillus fermentus, 1 Lactobacillus rhamnosos, and 14 unknown species). One additional patient had fungemia due to Saccharomyces cerevisiae, while another had positive BCs for both Lactobacillus species (not further classified) and S. cerevisiae detected from the same BCs.
Table 1.
Patient demographics and outcomes of HCT recipients with probiotic bloodstream infections
| Patient | Organism | Age/ Gend er |
Transplant type |
Transplant day |
Positive cultures number |
Pre-existing gut GVHD |
Treatment | Reason for cultures |
Infection outcome |
Survival 100 days after infection |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lactobacillus | 71/M | Allogeneic | 9 | 1 | No | D,E,Lv | Surveillancea | Resolved | Survived |
| 2 | Lactobacillus | 51/M | Autologous | 9 | 1 | N/A | Cx, V | Neutropenic fever | Resolved | Survived |
| 3 | Lactobacillus | 41/M | Allogeneic | 28 | 1 | Yes (Grade 3) | V | Surveillancea | Resolvedb | Survived |
| 4 | Lactobacillus & Saccharomyces cerevisiae | 45/F | Autologous | 31 | 1 | N/A | None (L); Mf (S) | Surveillancea | Resolved* | Survived |
| 5 | Lactobacillus | 62/F | Autologous | 34 | 1 | N/A | Lv | Fever | Resolved | Died (day 15) |
| 6 | Lactobacillus | 49/M | Allogeneic | 47 | 1 | No | V | Surveillance | Resolvedb | Survived |
| 7 | Lactobacillus | 59/F | Autologous | 51 | 1 | N/A | Lv, V | Surveillance | Resolved | Survived |
| 8 | Lactobacillus | 42/M | Allogeneic | 69 | 1 | Yes (Grade 1) | I, V | Surveillance | Resolved | Died (day 32) |
| 9 | Lactobacillus | 47/F | Allogeneic | 77 | 1 | Yes (Grade 2) | V | Surveillance | Resolvedb | Survived |
| 10 | Lactobacillus | 4/M | Autologous | 91 | 25 | N/A | Cm | Unknown | Resolved | Survived |
| 11 | Lactobacillus | 23/F | Allogeneic | 93 | 1 | No | None | Neutropenic fever | Resolvedb | Survived |
| 12 | Lactobacillus | 9/M | Allogeneic | 94 | 1 | No | Cz, Cm, G, V | Unknown | Resolved | Survived |
| 13 | Lactobacillus | 50/M | Allogeneic | 102 | 1 | Yes (Grade 4) | Lz, Mp, P | Hypotension | Resolved | Died (day 17) |
| 14 | Lactobacillus | 52/M | Allogeneic | 127 | 1 | Yes (Grade 4) | Cz, Lv, Mz | Diarrhea | Resolved | Died (day 16) |
| 15 | Lactobacillus | 61/F | Allogeneic | 129 | 1 | Yes (Grade 3) | None | Surveillance | Resolvedb | Survived |
| 16 | Lactobacillus | 38/F | Allogeneic | 136 | 1 | No | Lv, V | Surveillancea | Resolved | Survived |
| 17 | S. cerevisiae | 32/M | Allogeneic | 165 | 5 | No | AB | Uncal herniation | Resolved | Died (day 11)c |
| 18 | Lactobacillus | 50/F | Allogeneic | 184 | 1 | Yes (Grade 1) | A | Surveillance | Resolved | Survived |
| 19 | Lactobacillus | 54/F | Allogeneic | 306 | 1 | No | V | Surveillancea | Resolvedb | Survived |
While on treatment for preexisting bloodstream infection.
On inappropriate therapy.
Occurred within 14 days of infection with probiotic organism.
Transplant Day: Time from transplant date to bloodstream infection.
Resolved: probiotic infection cleared without attributable complications.
Rx: therapy(ies) patient received to treat bacteremia/fungemia where A = augmentin, AB = amphotericin B, Cz = ceftazidime, Cx = ceftriaxone, Cm = clindamycin, D = daptomycin, E = ertapenem, G = gentamicin, I = Imipenem, Lv = Levofloxacin, Lz = linezolid, Mf = micafungin, Mp = meropenem, Mz = metronidazole, P = piperacillin, V = vancomycin.
HCT, hematopoietic cell transplantation; GVHD, graft-versus-host disease; M, male; F, female; N/A, not applicable.
Most patients (17/19 [90%]) had only a single positive culture. However, 1 patient experienced prolonged Lactobacillus bacteremia (25 consecutive cultures) and another had prolonged Saccharomyces fungemia (5 consecutive cultures). In the majority of cases (16/19, 84%), physicians treated patients for these positive cultures. Of those treated for Lactobacillus bacteremia, 4/15 (27%) were treated with vancomycin monotherapy, to which Lactobacillus is generally resistant; 3 patients were never treated for Lactobacillus bacteremia. The catheter was removed in 4/18 (22%) cases. Only 1 patient failed active antimicrobial therapy, with serial BCs positive for a total of 33 days until their central venous catheter was removed. In the 2 patients with S. cerevisiae fungemia, 1 received intravenous amphotericin B and the other micafungin.
Susceptibility testing was only obtained in 6 patients with Lactobacillus bacteremia (33%). Similar to standard resistance profiles, Lactobacillus species were resistant to vancomycin and susceptible to clindamycin, erythromycin, gentamicin, and penicillin in each of these cases. Resistance to imipenem-cilastin was noted in 1 of 2 tested.
Only 4/17 (24%) patients were noted to have symptomatic infection, with fever being the most common sign. The majority of cultures (65%) that grew a probiotic organism were ordered either for surveillance in the setting of high-dose steroids, or during treatment for an unrelated BSI, in the absence of new symptoms. Importantly, although 1 patient had sustained bacteremia for 25 days, no cases of other major infectious complications, such as endocarditis, occurred, and no patient deaths were deemed attributable to Lactobacillus bacteremia or Saccharomyces fungemia. One patient developed of S. cerevisiae fungemia and died within 14 days of their culture, in the setting of refractory candidemia and Escherichia coli bacteremia.
Discussion
In the current study, we examine the incidence and outcome of common organisms found in OTC probiotics detected from BCs in a large cohort of hematopoietic cell transplant recipients over the course of 10 years. Isolation of these probiotic organisms from BCs was infrequent (0.5%), and in those that did develop these events, no deaths were directly attributable to these organisms. The majority of cases were Lactobacillus species and occurred in allogeneic transplant recipients (72%), in whom one-half had a history of gut GVHD. Not surprisingly, most of these events occurred within the first 100 days post transplant, coinciding with what is typically the peak of immunosuppression as well as mucosal disruptions by chemotherapy and GVHD. Because patients in this cohort were not known to be taking probiotics, bacteremia and fungemia in this study likely resulted from multiple mechanisms unrelated to nutritional supplementation (e.g., spontaneous gut translocation in the setting of altered intestinal integrity and microbiota following HCT). These data suggest that organisms frequently incorporated in available nutritional and OTC probiotic supplements appear to be rare causes of BSIs and are associated with low attributable mortality after HCT.
The market for probiotics in the United States has expanded rapidly over the last decade (17, 18). While sales of foods with live active cultures such as yogurts and kefir continue to increase, many Americans consume probiotics in the form of OTC dietary supplements, with minimal regulation by the Food and Drug Administration and no specific governmental standards (8, 19). Commonly consumed probiotic organisms in food and supplements include Lactobacillus, Bifidobacterium, Saccharomyces, and Streptococcus species (17).
In other observational studies of probiotic supplementation, the risk of subsequent BSI have been relatively sanguine. One review of all Lactobacillus bacteremia in Finland from 1990–2000 — a time of rapid rise in probiotic consumption — showed no corresponding increase in Lactobacillus bacteremia (20). The same group went on to examine risk factors and outcomes for 89 patients with Lactobacillus bacteremia (21); most patients (82%) had severe comorbidities including gastrointestinal malignancy. Overall mortality was 26% at 1 month, although attributable mortality to Lactobacillus bacteremia could not be calculated. Another recent large study on the incidence of Lactobacillus bacteremia at a single hospital in Taiwan found 89 cases, with an annual incidence of 8.2 cases per 100,00 admissions (22). The majority of the patients tended to be older, with underlying unspecified malignancy in 64% of cases. Interestingly, 40% had polymicrobial bacteremia, most commonly with Candida or Bacteroides species.
Data are limited evaluating probiotic use in immunosuppressed patients. One retrospective review of inpatient prescriptions at a single center identified 400 patients over the course of 1 year who were prescribed combination of Lactobacillus acidophilus and Lactobacillus bulgaricus while hospitalized (23). Of these, 47% of patients were considered to have moderate to severe immunosuppression including neutropenia, acquired immunodeficiency syndrome, and solid organ transplant recipients. Interestingly, only 2 patients developed Lactobacillus bacteremia (blood isolates were not typed to confirm whether they matched the probiotic strain); both improved on broad-spectrum antibiotic therapy given for concurrent infections unrelated to Lactobacillus.
In a recent controlled randomized trial of Bifidobacterium breve supplementation was assessed in 42 pediatric patients with malignancy admitted for chemotherapy (24). Overall, fewer episodes of fever occurred in the treatment group, as well as less antibiotic use. The authors also profiled the stool using real-time polymerase chain reaction in a subset of patients and showed trends toward restoration of commensal anaerobes, normalization of stool pH, and improved concentrations of organic acids in the probiotic group.
However, supplementation is not without risk. In addition to bacteremia seen in the prior studies, 2 cases of S. cerevisiae fungemia related to dietary probiotic supplementation with this organism have also been reported in a solid organ transplant recipient and a patient with human immunodeficiency virus infection (25). Three additional cases among patients receiving Saccharomyces probiotic supplementation by nasogastric tube as an adjunctive treatment for C. difficile diarrhea have also been reported (26). Cases of Lactobacillus bacteremia after probiotic supplementation have been rarely reported (11, 27). Furthermore, the lack of standardization for such supplementations, methods of preparation (e.g., live vs. freeze-dried), and concerns about specificity and viability of organisms present (28), are other important issues that need further investigation.
While these results provide important background data for future studies, as with any retrospective study there are expected limitations. To better assess an association between bacteremia and probiotic consumption, it would be informative to know how many patients were actively taking probiotic supplementation. In the current study, we are unable to track probiotic consumption via supplements or foods with live active cultures. Because nutritional or probiotic supplementation of patients in this study was unknown, we cannot make firm conclusions regarding the mechanism of bacteremia. Importantly, many of these organisms are constituents of normal gut and genitourinary microbiota. Because many of the lactobacilli recovered in BC were not classified further, we are unable to report on the similarity of these isolates to common probiotic strains. We also cannot completely account for inter-practitioner variability; however, given that these are rare events, it seems unlikely that occasional deviations in practice would significantly affect our findings. Even with these limitations, these data suggest that these bacterial species were not associated with major complications among our cohort of highly immunosuppressed patients.
This is the first study, to our knowledge, to examine a large cohort of hematopoietic cell transplant recipients and report on incidence and outcomes of patients who had BSIs with organisms common to OTC probiotics. These results must be interpreted cautiously, as invasive infections with probiotic organisms were rare in this cohort. Many cases of bacteremia noted in this study were recovered from a single bottle, indicating contamination was a possible etiology in some cases. As most patients in this study were treated with antibiotics, such findings also suggest there may be additional opportunities for modifications in practice, particularly focused on antimicrobial stewardship. Importantly, clinical outcomes between those with single and multiple positive cultures were no different, and no clear indication that serious adverse events resulted from infection with these organisms, particularly Lactobacillus species.
Despite the theoretical benefits of probiotics to restore microbial diversity and outcompete the growth of pathogenic bacteria in the gut of hematopoietic cell transplant recipients, studies evaluating the safety of such preparations are limited. Our data could serve as a baseline incidence by which future probiotic trials could be compared in high-risk hematopoietic cell transplant recipients. Future randomized clinical trials, which would address the safety and efficacy of probiotics on the diversity of intestinal microbiota, are warranted.
Acknowledgments
Funding: M.B. was supported in part by K24HL HL093294.
Prior presentation: Data from this manuscript were presented in part at the American Society of Blood and Marrow Transplant Tandem Meetings in Grapevine, Texas USA, February 2014.
References
- 1.Sullivan Å, Nord CE. The place of probiotics in human intestinal infections. Int J Antimicrob Agents. 2002;20(5):313–319. doi: 10.1016/s0924-8579(02)00199-1. [DOI] [PubMed] [Google Scholar]
- 2.Goldin BR, Gorbach SL. Clinical Indications for probiotics: an overview. Clin Infect Dis. 2008;46(s2):S96–S100. doi: 10.1086/523333. [DOI] [PubMed] [Google Scholar]
- 3.Khailova L, Frank DN, Dominguez JA, et al. Probiotic administration reduces mortality and improves intestinal epithelial homeostasis in experimental sepsis. Anesthesiology. 2013;119(1):166–177. doi: 10.1097/ALN.0b013e318291c2fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston BC, Ma SSY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157(12):878–888. doi: 10.7326/0003-4819-157-12-201212180-00563. [DOI] [PubMed] [Google Scholar]
- 5.D’Souza AL, Rajkumar C, Cooke J, et al. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002;324(7350):1361. doi: 10.1136/bmj.324.7350.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shadnoush M, Hosseini RS, Khalilnezhad A, et al. Effects of probiotics on gut microbiota in patients with inflammatory bowel disease: a double-blind, placebo-controlled clinical trial. Korean J Gastroenterol. 2015;65(4):215–221. doi: 10.4166/kjg.2015.65.4.215. [DOI] [PubMed] [Google Scholar]
- 7.Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80–80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanderhoof JA, Young R. Probiotics in the United States. Clin Infect Dis. 2008;46(Suppl 2):S67–72. doi: 10.1086/523339. [DOI] [PubMed] [Google Scholar]
- 9.Stanton C, Gardiner G, Meehan H, et al. Market potential for probiotics. Am J Clin Nutr. 2001;73(2):476s–483s. doi: 10.1093/ajcn/73.2.476s. [DOI] [PubMed] [Google Scholar]
- 10.Meini S, Laureano R, Fani L, et al. Breakthrough Lactobacillus rhamnosus GG bacteremia associated with probiotic use in an adult patient with severe active ulcerative colitis: case report and review of the literature. Infection. 2015;43(6):777–781. doi: 10.1007/s15010-015-0798-2. [DOI] [PubMed] [Google Scholar]
- 11.Haghighat L, Crum-Cianflone NF. The potential risks of probiotics among HIV-infected persons: bacteraemia due to Lactobacillus acidophilus and review of the literature. Int J STD AIDS. 2015 Jun 30; doi: 10.1177/0956462415590725. pii: 0956462415590725. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Snydman DR. The safety of probiotics. Clin Infect Dis. 2008;46(Suppl 2):S104–S111. doi: 10.1086/523331. [DOI] [PubMed] [Google Scholar]
- 13.Ladas EJ, Bhatia M, Chen L, et al. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2016;51(2):262–266. doi: 10.1038/bmt.2015.275. [DOI] [PubMed] [Google Scholar]
- 14.Miles-Jay A, Butler-Wu S, Rowhani-Rahbar A, et al. Incidence rate of fluoroquinolone-resistant gram-negative rod bacteremia among allogeneic hematopoietic cell transplantation patients during an era of levofloxacin prophylaxis. Biol Blood Marrow Transplant. 2015;21(3):539–545. doi: 10.1016/j.bbmt.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 17.McFarland LV. From yaks to yogurt: the history, development, and current use of probiotics. Clin Infect Dis. 2015;60(Suppl 2):S85–S90. doi: 10.1093/cid/civ054. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopal N. The North American probiotics market. [Accessed November 11, 2015];Natural Products Insider.com. ( http://www.naturalproductsinsider.com/articles/2012/10/the-north-american-probiotics-market.aspx)
- 19.Saldanha LG. US Food and Drug Administration regulations governing label claims for food products, including probiotics. Clin Infect Dis. 2008;46(s2):S119–S121. doi: 10.1086/523328. [DOI] [PubMed] [Google Scholar]
- 20.Salminen MK, Tynkkynen S, Rautelin H, et al. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Dis. 2002;35(10):1155–1160. doi: 10.1086/342912. [DOI] [PubMed] [Google Scholar]
- 21.Salminen MK, Rautelin H, Tynkkynen S, et al. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin Infect Dis. 2004;38(1):62–69. doi: 10.1086/380455. [DOI] [PubMed] [Google Scholar]
- 22.Lee MR, Tsai CJ, Liang SK, Lin CK, Huang YT, Hsueh PR. Clinical characteristics of bacteraemia caused by Lactobacillus spp and antimicrobial susceptibilities of the isolates at a medical centre in Taiwan, 2000–2014. Inter J Antimicrob Agents. 2015;46(4):439–445. doi: 10.1016/j.ijantimicag.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Simkins J, Kaltsas A, Currie BP. Investigation of inpatient probiotic use at an academic medical center. Int J Infect Dis. 2013;17(5):e321–e324. doi: 10.1016/j.ijid.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Wada M, Nagata S, Saito M, et al. Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Support Care Cancer. 2010;18(6):751–759. doi: 10.1007/s00520-009-0711-6. [DOI] [PubMed] [Google Scholar]
- 25.Riquelme AJ, Calvo MA, Guzmán AM, et al. Saccharomyces cerevisiae fungemia after Saccharomyces boulardii treatment in immunocompromised patients. J Clin Gastroenterol. 2003;36(1):41–43. doi: 10.1097/00004836-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz P, Bouza E, Cuenca-Estrella M, et al. Saccharomyces cerevisiae fungemia: an emerging infectious disease. Clin Infect Dis. 2005;40(11):1625–1634. doi: 10.1086/429916. [DOI] [PubMed] [Google Scholar]
- 27.Land MH, Rouster-Stevens K, Woods CR, et al. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;115(1):178–181. doi: 10.1542/peds.2004-2137. [DOI] [PubMed] [Google Scholar]
- 28.Saxelin M. Probiotic Formulations and applications, the current probiotics market, and changes in the marketplace: a European perspective. Clin Infect Dis. 2008;46(s2):S76–S79. doi: 10.1086/523337. [DOI] [PubMed] [Google Scholar]


