Abstract
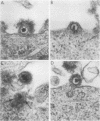
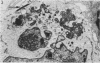
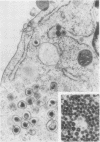
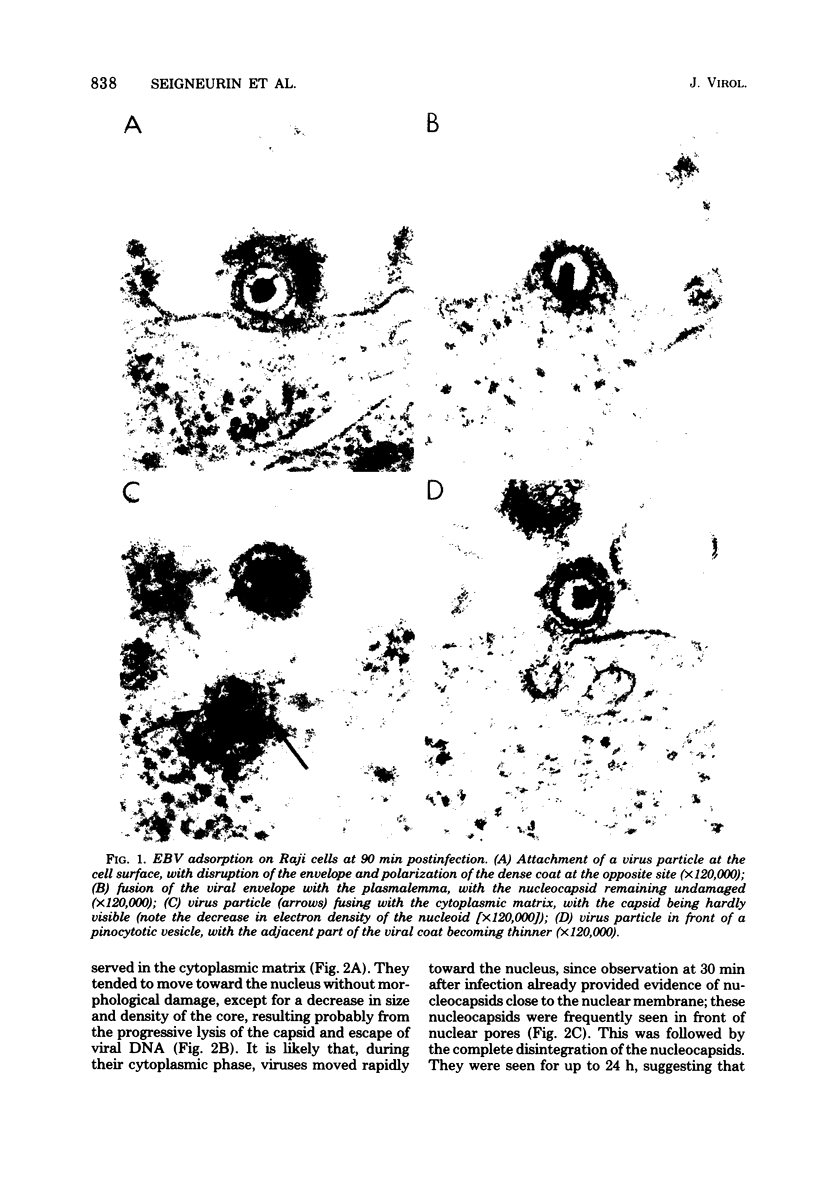
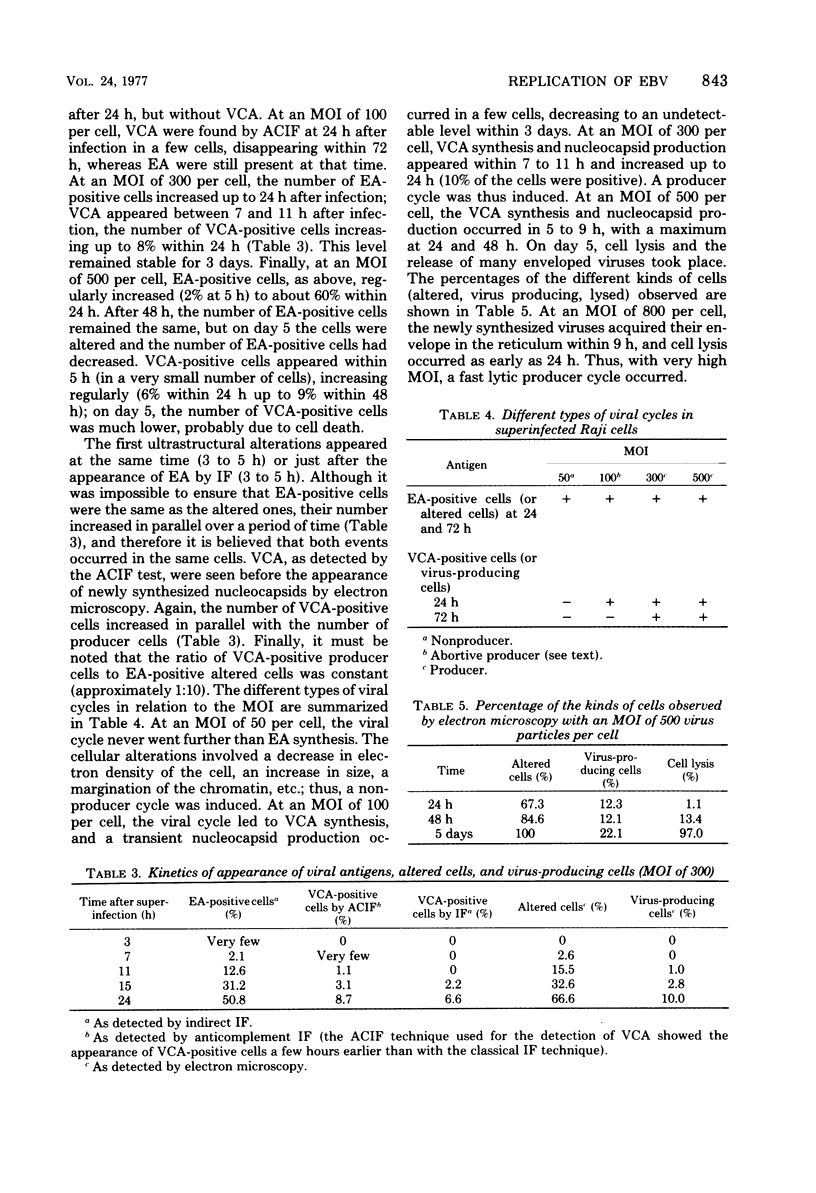
We have studied by means of electron microscopy and immunofluorescence the different steps of the replication of the P3HR1 strain of Epstein-Barr virus in Raji cells. The virus entered the cell by fusion of the viral envelope with the plasma membrane, followed by the disintegration of the capsid. In some cases, the migration of nucleocapsids toward the nuclear membrane was observed. The synthesis of new virions began as early as 7 h after infection (in the case of a high multiplicity of infection [MOI]-800 particles per cell) and took place in low-electron-density areas of the nucleus. A viral envelope was acquired by budding either through the nuclear membrane or more often through membranes of the Golgi apparatus or cytoplasmic vacuoles. Comparing immunofluorescence and electron microscopic data a good correlation was found between the presence of early antigen and ultrastructurally altered cells, as well as between the presence of viral capsid antigen and virus-producing cells. With different MOIs, different types of viral cycles were observed: at a low MOI (less than or equal to 50 particles per cell), a nonproducer cycle was induced, with early antigen synthesis only; at a higher MOI (100 particles per cell), a transient production of a small amount of virions was observed, and at a high MOI (greater than or equal to 300 particles per cell), a productive cycle was the rule.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedoya V., Rabson A. S., Grimley P. M. Growth in vitro of herpes simplex virus in human lymphoma cell lines. J Natl Cancer Inst. 1968 Sep;41(3):635–652. [PubMed] [Google Scholar]
- Bouroncle B. A., Clausen K. P., Darner E. M. Replication of herpes simplex virus in cultures of phytohemagglutinin-stimulated human lymphocytes. J Natl Cancer Inst. 1970 May;44(5):1065–1078. [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd The envelope of Herpesvirus. Prog Med Virol. 1969;11:16–45. [PubMed] [Google Scholar]
- Durr F. E., Monroe J. H., Schmitter R., Traul K. A., Hirshaut Y. Studies on the infectivity and cytopathology of Epstein-Barr virus in human lymphoblastoid cells. Int J Cancer. 1970 Nov 15;6(3):436–449. doi: 10.1002/ijc.2910060315. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., HENLE G., ACHONG B. G., BARR Y. M. MORPHOLOGICAL AND BIOLOGICAL STUDIES ON A VIRUS IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. J Exp Med. 1965 May 1;121:761–770. doi: 10.1084/jem.121.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G., Barr Y. M., Zajac B., Henle G., Henle W. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J Natl Cancer Inst. 1966 Oct;37(4):547–559. [PubMed] [Google Scholar]
- Ernberg I., Andersson-Anvret M., Klein G., Lundin L., Killanger D. Relationship between amount of Epstein-Barr virus-determined nuclear antigen per cell and number of EBV-DNA copies per cell. Nature. 1977 Mar 17;266(5599):269–271. doi: 10.1038/266269a0. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W., Klein G. Demonstration of two distinct components in the early antigen complex of Epstein-Barr virus-infected cells. Int J Cancer. 1971 Sep 15;8(2):272–282. doi: 10.1002/ijc.2910080212. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Furukawa T., Plotkin S., Koprowski H. Ultrastructural study on the sequence of human cytomegalovirus infection in human diploid cells. Arch Gesamte Virusforsch. 1973;40(3):311–324. doi: 10.1007/BF01242551. [DOI] [PubMed] [Google Scholar]
- Klein G., Dombos L., Gothoskar B. Sensitivity of Epstein-Barr virus (EBV) producer and non-producer human lymphoblastoid cell lines to superinfection with EB-virus. Int J Cancer. 1972 Jul 15;10(1):44–57. doi: 10.1002/ijc.2910100108. [DOI] [PubMed] [Google Scholar]
- Menezes J., Seigneurin J. M., Patel P., Bourkas A., Lenoir G. Presence of Epstein-Barr virus receptors, but absence of virus penetration, in cells of an Epstein-Barr virus genome-negative human lymphoblastoid T line (Molt 4). J Virol. 1977 Jun;22(3):816–821. doi: 10.1128/jvi.22.3.816-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Morgan C. Structure and development of viruses as observed in the electron microscope. XI. Entry and uncoating of herpes simplex virus. J Virol. 1971 Dec;8(6):910–918. doi: 10.1128/jvi.8.6.910-918.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J. H., Brandt P. M. Rapid semiquantitative method for screening large numbers of virus samples by negative staining electron microscopy. Appl Microbiol. 1970 Aug;20(2):259–262. doi: 10.1128/am.20.2.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. G., Achong B. G., Epstein M. A. Morphological observations on the replication of herpesvirus saimiri in monkey kidney cell cultures. J Gen Virol. 1976 Sep;32(3):461–470. doi: 10.1099/0022-1317-32-3-461. [DOI] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Toshima S., Takagi N., Minowada J., Moore G. E., Sandberg A. A. Electron microscopic and cytogenetic studies of cells derived from Burkitt's lymphoma. Cancer Res. 1967 Apr;27(4):753–759. [PubMed] [Google Scholar]
- Yajima Y., Nonoyama M. Mechanisms of infection with Epstein-Barr virus. I. Viral DNA replication and formation of noninfectious virus particles in superinfected Raji cells. J Virol. 1976 Jul;19(1):187–194. doi: 10.1128/jvi.19.1.187-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]