Abstract
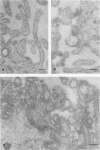
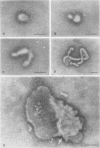
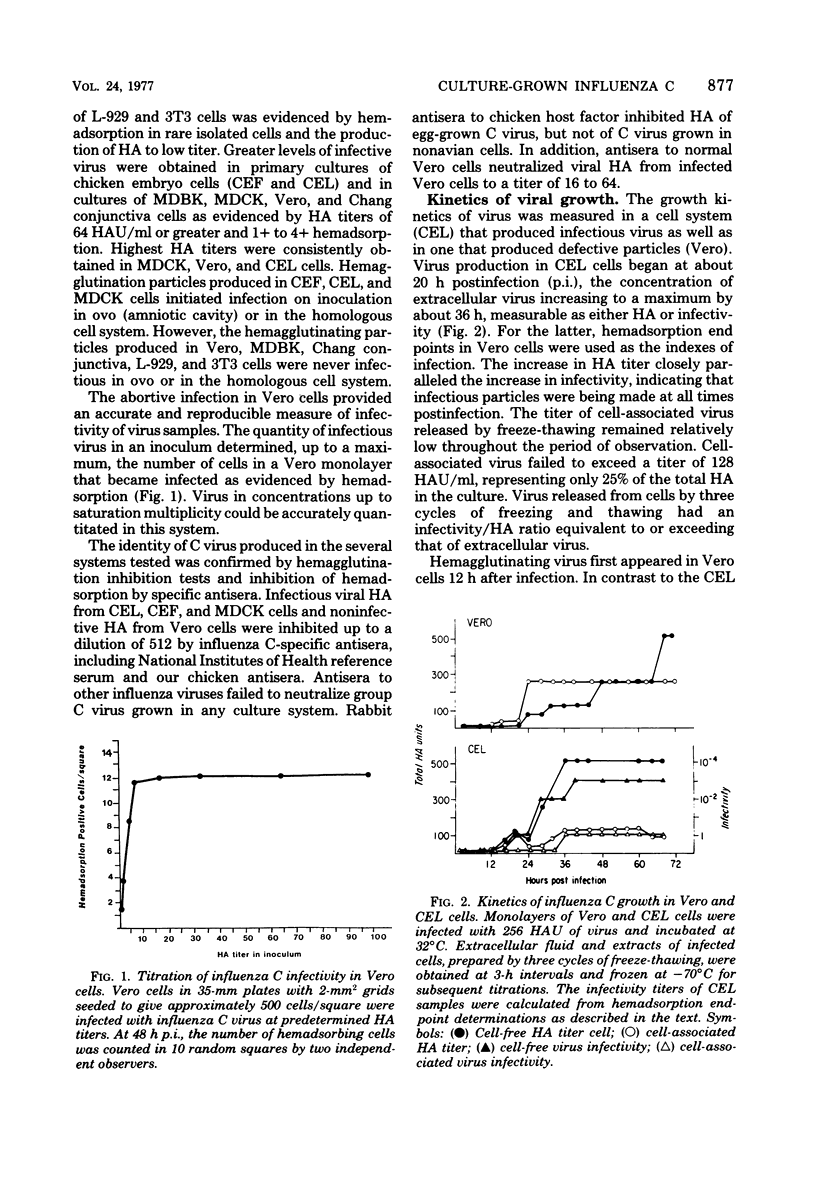
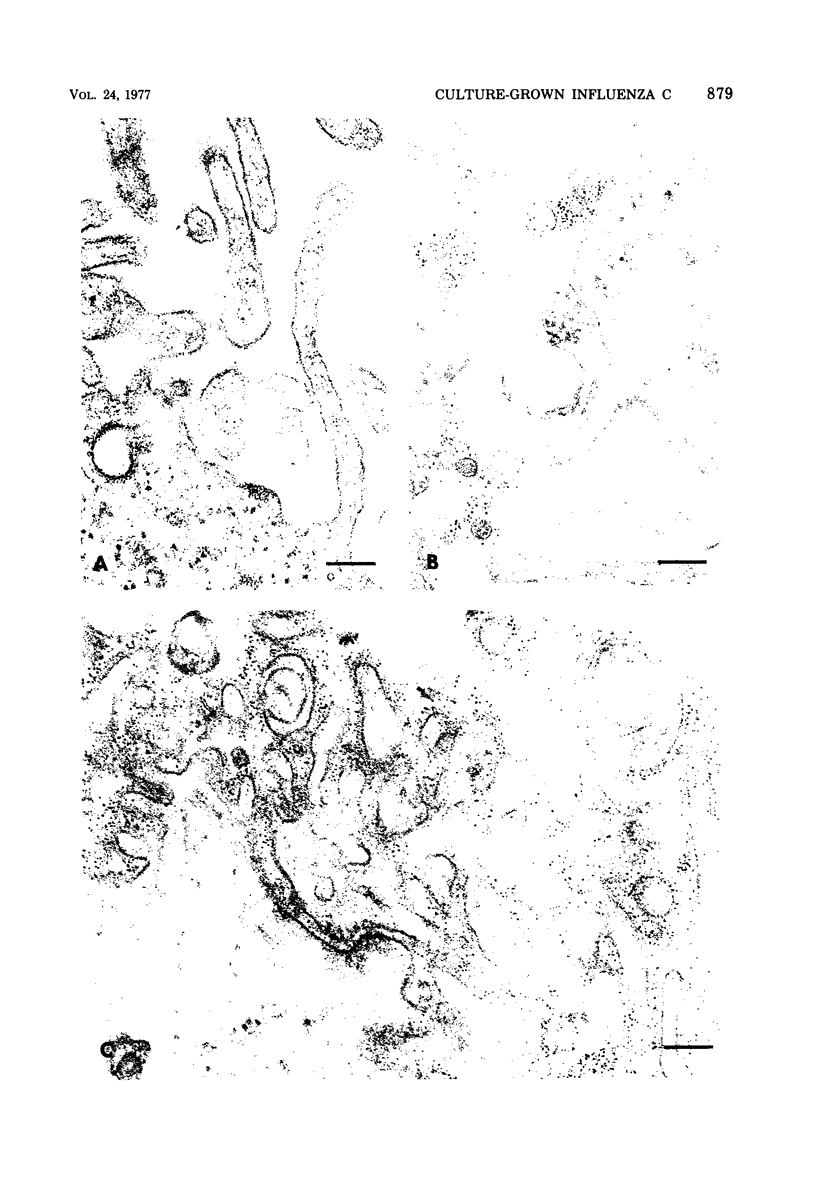
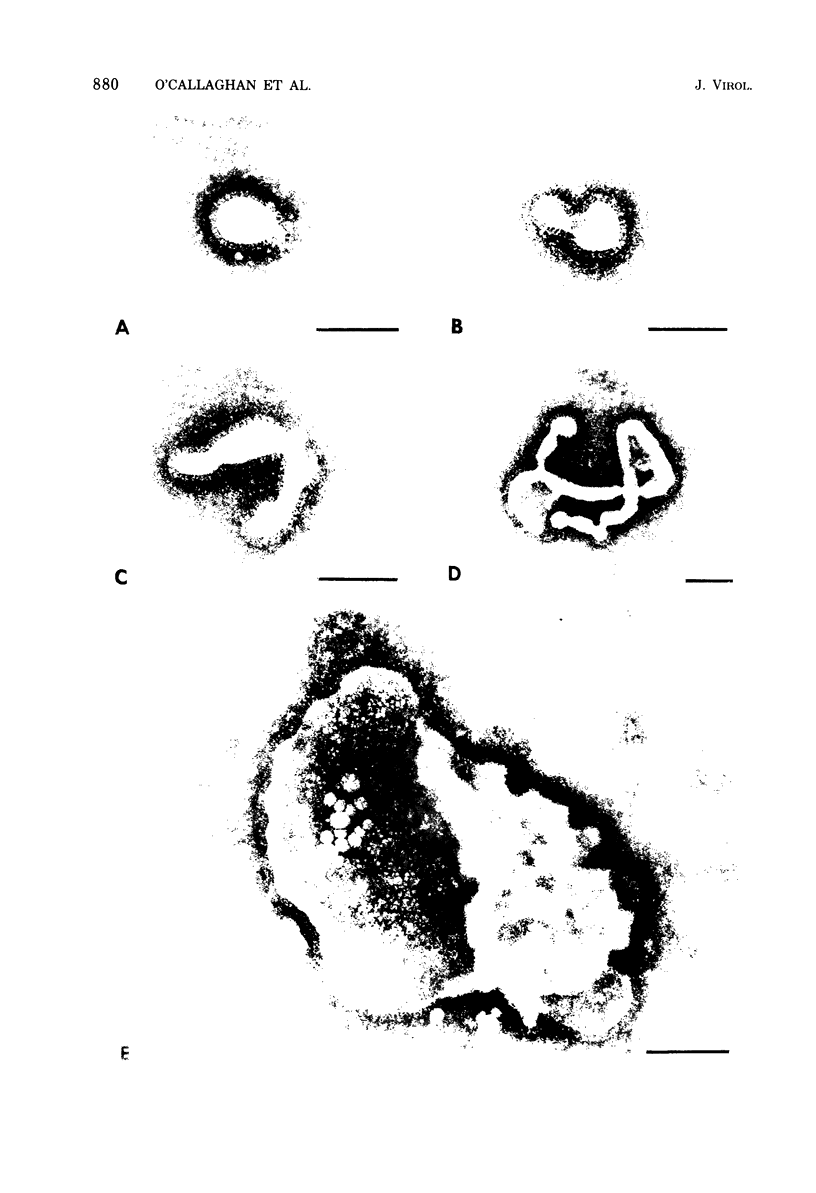
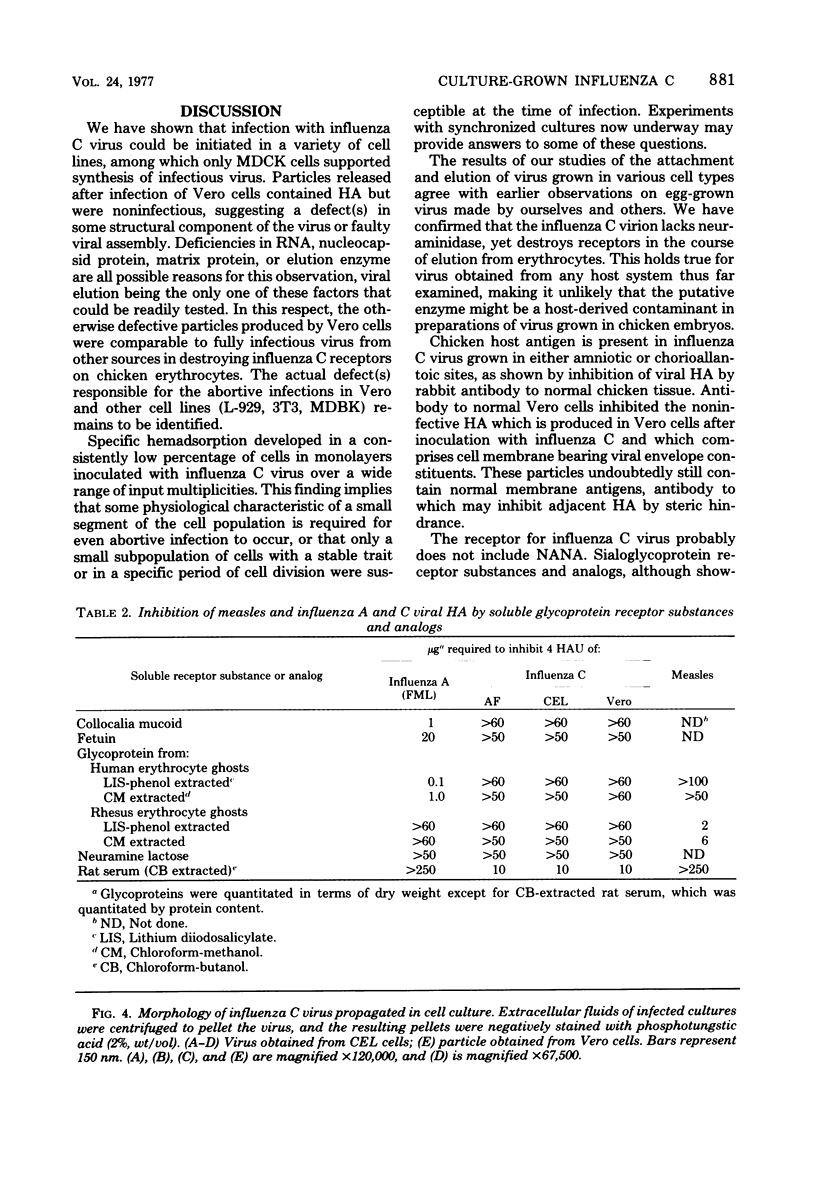
Influenza C virus was propagated successfully in primary chicken embryo lung (CEL) and fibroblast cells and in Madin-Darby canine kidney (MDCK) cells. In other cell lines, either no virus or only noninfectious hemagglutinin (HA) was produced. In productively infected cells (CEL), HA and infectious virus appeared by 24 h and reached a maximum by 36 to 48 h, cell-associated virus remaining at a constant low level. Infected Vero cells produced noninfective HA by 24 h which also remained predominantly cell associated until 60 to 72 h, when the cells disintegrated. Viral antigen was demonstrable on membranes of both CEL- and Vero-infected cells at 24 h; Vero cells yielded membrane vesicles containing HA, but none of the spherical or filamentous viral particles synthesized in CEL cells. Influenza C virus produced in cell culture or in eggs differed in several important respects from A and B viruses and from Newcastle diseases virus. All influenza C preparations, regardless of infectivity or source, lacked detectable neuraminidase activity, yet retained the ability specifically to inactivate receptors only for influenza C. Influenza C HA was not inhibited by soluble glycoproteins highly active against HA of A virus. A rat serum glycoprotein uniquely inhibited influenza C by binding to the surface components of virious.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostolov K., Flewett T. H. Further observations on the structure of influenza viruses A and C. J Gen Virol. 1969 Apr;4(3):365–370. doi: 10.1099/0022-1317-4-3-365. [DOI] [PubMed] [Google Scholar]
- Cherry J. D., Taylor-Robinson D. Large-quantity production of chicken embryo tracheal organ cultures and use in virus and mycoplasma studies. Appl Microbiol. 1970 Apr;19(4):658–662. doi: 10.1128/am.19.4.658-662.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Bishop D. H., Meier-Ewert H. Structural components of influenza C virions. J Virol. 1977 Feb;21(2):658–665. doi: 10.1128/jvi.21.2.658-665.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward J. E., Harter D. H., Hsu K. C., Morgan C. Electron microscopic study of development of vesicular stomatitis virus using ferritin-labelled antibodies. J Gen Virol. 1971 Oct;13(1):27–34. doi: 10.1099/0022-1317-13-1-27. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Portner A., Kingsbury D. W. Sendai virus replication: an ultrastructural comparison of productive and abortive infections in avian cells. J Gen Virol. 1970 Dec;9(3):169–177. doi: 10.1099/0022-1317-9-3-169. [DOI] [PubMed] [Google Scholar]
- FRANCIS T., Jr, QUILLIGAN J. J., Jr, MINUSE E. Identification of another epidemic respiratory disease. Science. 1950 Oct 27;112(2913):495–497. doi: 10.1126/science.112.2913.495. [DOI] [PubMed] [Google Scholar]
- HANA L., STYK B. Some properties of influenza C virus haemagglutination inhibitor from rat serum. Acta Virol. 1959;3(Suppl):85–90. [PubMed] [Google Scholar]
- HIRST G. K. The relationship of the receptors of a new strain of virus to those of the mumps-NDV-influenza group. J Exp Med. 1950 Feb;91(2):177–184. doi: 10.1084/jem.91.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWE C., LEE L. T., ROSE H. M. Collocalia mucoid: a substrate for myxovirus neuraminidase. Arch Biochem Biophys. 1961 Dec;95:512–520. doi: 10.1016/0003-9861(61)90184-9. [DOI] [PubMed] [Google Scholar]
- Hamaguchi H., Cleve H. Solubilization of human erythrocyte membrane glycoproteins and separation of the MN glycoprotein from a glycoprotein with I, S, and A activity. Biochim Biophys Acta. 1972 Sep 29;278(2):271–280. doi: 10.1016/0005-2795(72)90232-2. [DOI] [PubMed] [Google Scholar]
- Howe C., Lee L. T., Harboe A., Haukenes G. Immunochemical study of influenza virus and associated host tissue components. J Immunol. 1967 Mar;98(3):543–557. [PubMed] [Google Scholar]
- Kendal A. P. A comparison of "influenza C" with prototype myxoviruses: receptor-destroycing activity (neuraminidase) and structural polypeptides. Virology. 1975 May;65(1):87–99. doi: 10.1016/0042-6822(75)90009-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Kilbourne E. D. RNAs of influenza A, B, and C viruses. J Virol. 1976 May;18(2):738–744. doi: 10.1128/jvi.18.2.738-744.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]