Abstract
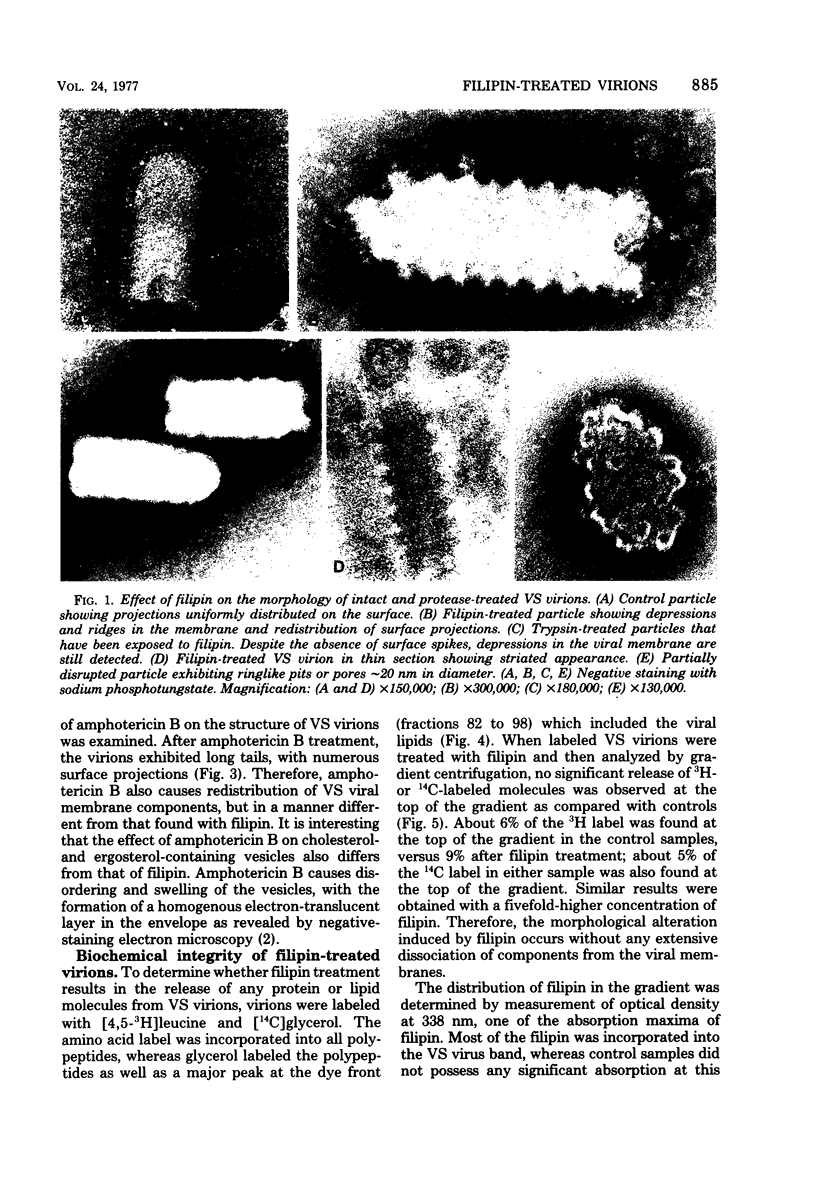
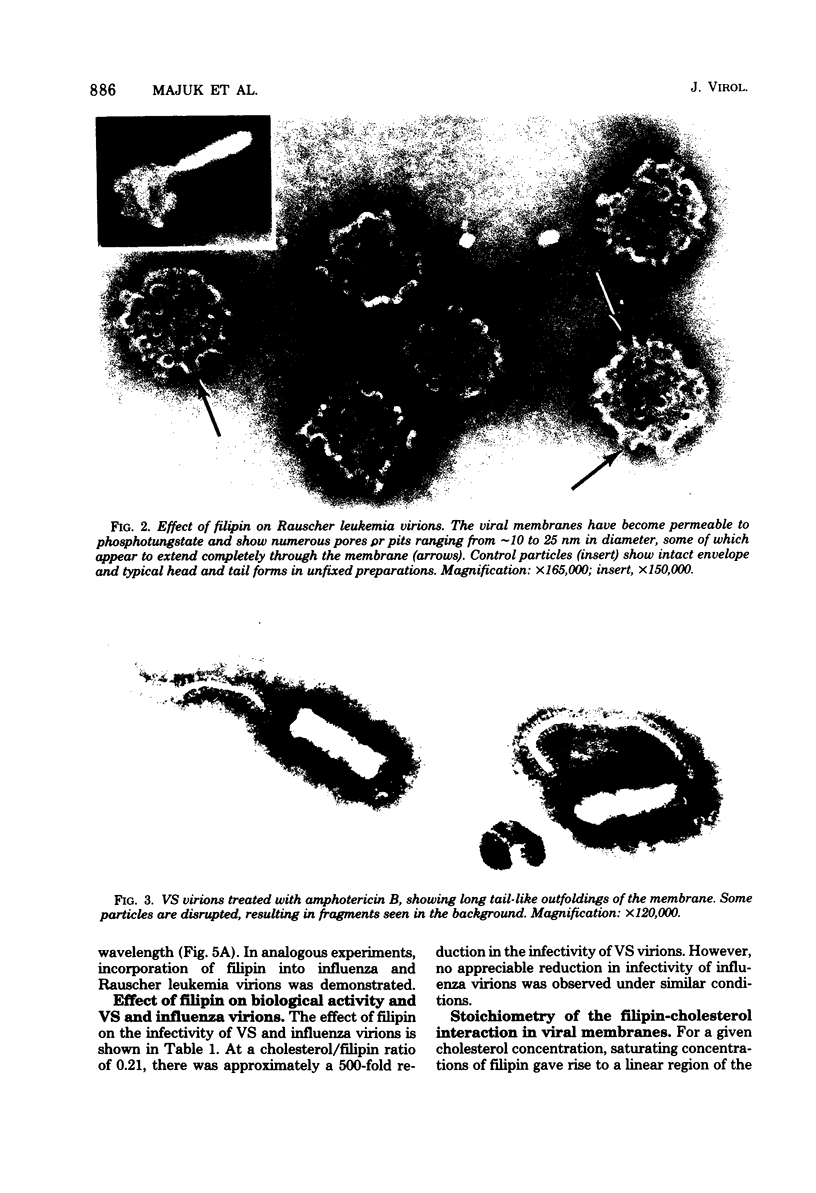
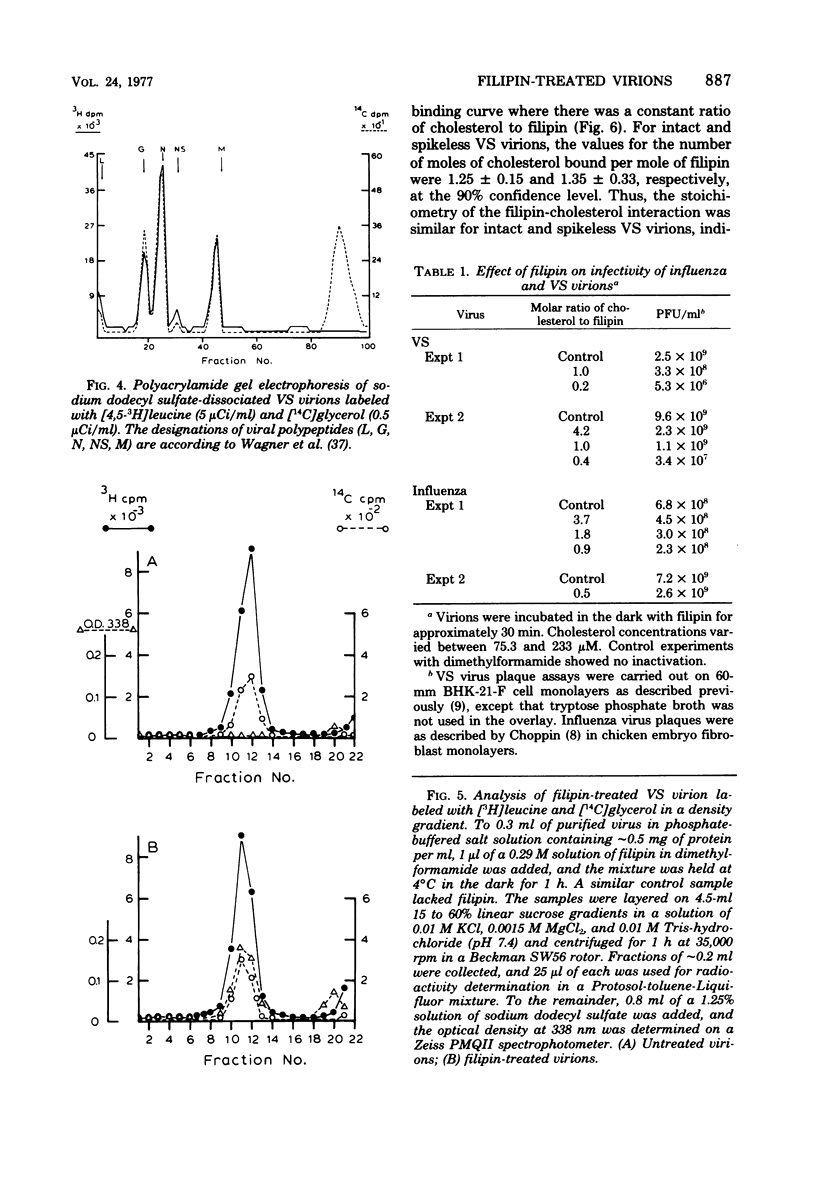
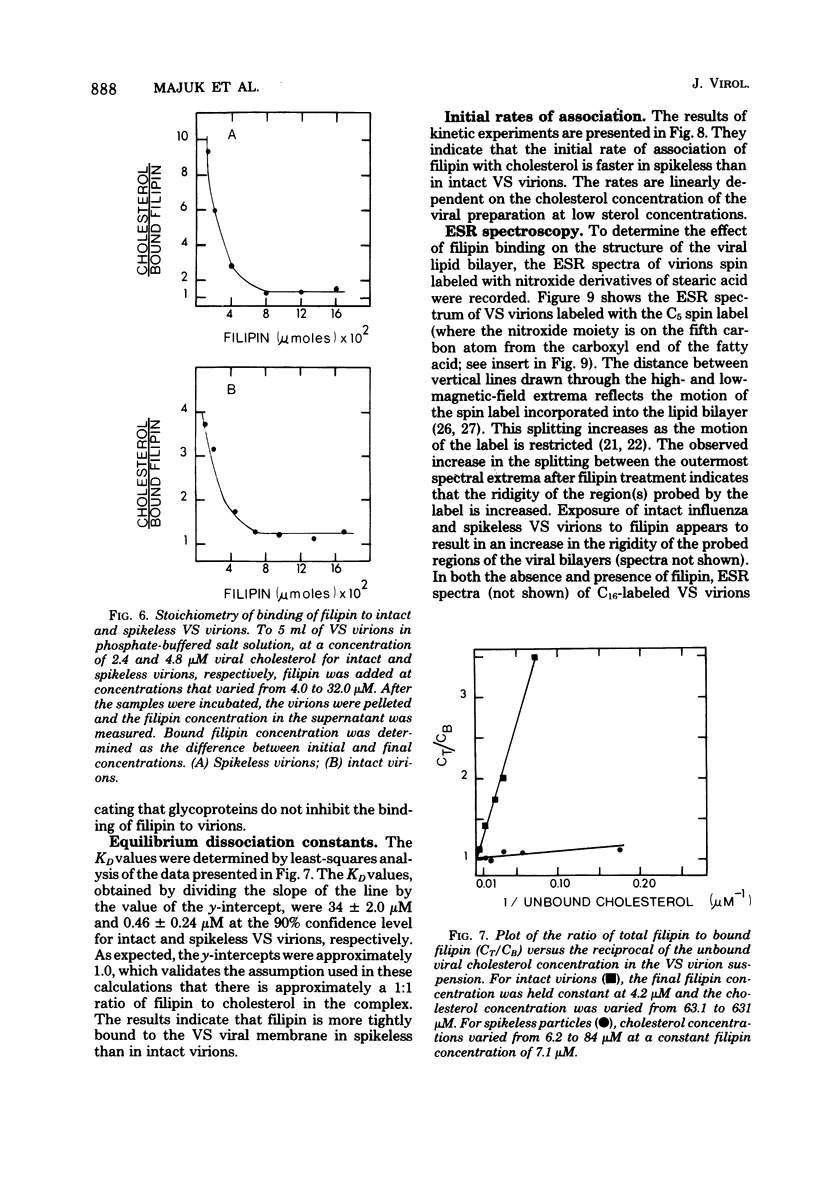
The interaction of the polyene antibiotic filipin with membrane-bound cholesterol in vesicular stomatitis (VS), influenza, and Rauscher leukemia virions was studied. Exposure of virions to filipin resulted in a series of depressions and ridges in the envelope of VS virions, with a periodicity of 15 to 20 nm perpendicular to the long axis of the particle; similar morphological alterations were observed in negatively stained preparations, in thin-sectioned virions, and in protease-treated virions that lack surface glycoproteins. This morphological effect was specific for filipin, since the envelopes of VS virions that had been treated with another polyene antibiotic, amphotericin B, exhibited markedly different morphology. Morphological alterations induced by filipin in influenza and Rauscher leukemia virions differed from those seen in VS virions. The infectivity of filipin-treated VS virions was reduced up to 500-fold, whereas influenza virions were resistant to filipin treatment. Incorporation of filipin into the virions was demonstrated, and no release of either lipids or proteins from virions was detected after filipin treatment. A stoichiometry of approximately 1 mol of bound filipin per mol of cholesterol was found in both intact and protease-treated VS virions. The equilibrium dissociation constant for filipin-cholesterol interaction was approximately 74-fold larger in intact than in protease-treated VS virions. The initial rate of association of filipin with cholesterol in intact virions was slower than that in protease-treated particles. The fluidity of lipids in VS viral membranes, as probed by a stearic acid derivative spin label, was markedly reduced when either intact or protease-treated virions were treated with filipin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E. The structure and function of amphotericin B-cholesterol pores in lipid bilayer membranes. Ann N Y Acad Sci. 1974 May 10;235(0):448–468. doi: 10.1111/j.1749-6632.1974.tb43283.x. [DOI] [PubMed] [Google Scholar]
- Bittman R., Chen W. C., Anderson O. R. Interaction of filipin 3 and amphotericin B with lecithin-sterol vesicles and cellular membranes. Spectral and electron microscope studies. Biochemistry. 1974 Mar 26;13(7):1364–1373. doi: 10.1021/bi00704a009. [DOI] [PubMed] [Google Scholar]
- Bittman R., Chen W. C., Blau L. Stopped-flow kinetic and equilibrium studies of filipin 3 binding to sterols. Biochemistry. 1974 Mar 26;13(7):1374–1379. doi: 10.1021/bi00704a010. [DOI] [PubMed] [Google Scholar]
- Bittman R., Majuk Z., Honig D. S., Compans R. W., Lenard J. Permeability properties of the membrane of vesicular stomatitis virions. Biochim Biophys Acta. 1976 Apr 16;433(1):63–74. doi: 10.1016/0005-2736(76)90178-4. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Klenk H. D., Choppin P. W. The proteins of the parainfluenza virus SV5. 1. Separation of virion polypeptides by polyacrylamide gel electrophoresis. Virology. 1969 Nov;39(3):460–466. doi: 10.1016/0042-6822(69)90094-4. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Compans R. W. Phenotypic mixing of envelope proteins of the parainfluenza virus SV5 and vesicular stomatitis virus. J Virol. 1970 May;5(5):609–616. doi: 10.1128/jvi.5.5.609-616.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Distinct carbohydrate components of influenza virus glycoproteins in smooth and rough cytoplasmic membranes. Virology. 1973 Oct;55(2):541–545. doi: 10.1016/0042-6822(73)90199-2. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A., Holz R. Aqueous pores created in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. Membranes. 1973;2:377–408. [PubMed] [Google Scholar]
- Flick C., Gelerinter E. Cholestane spin label study of filipin action on lipid planar multibilayers. Chem Phys Lipids. 1977 Jan;18(1):62–72. doi: 10.1016/0009-3084(77)90027-5. [DOI] [PubMed] [Google Scholar]
- Godici P. E., Landsberger F. R. The dynamic structure of lipid membranes. A 13C nuclear magnetic resonance study using spin labels. Biochemistry. 1974 Jan 15;13(2):362–368. doi: 10.1021/bi00699a022. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Jost P., Libertini L. J., Hebert V. C., Griffith O. H. Lipid spin labels in lecithin multilayers. A study of motion along fatty acid chains. J Mol Biol. 1971 Jul 14;59(1):77–98. doi: 10.1016/0022-2836(71)90414-1. [DOI] [PubMed] [Google Scholar]
- Katzenstein I. P., Spielvogel A. M., Norman A. W. Stoichiometry of interaction of the polyene antibiotic, filipin, with free and liposomal-bound cholesterol. J Antibiot (Tokyo) 1974 Dec;27(12):943–951. doi: 10.7164/antibiotics.27.943. [DOI] [PubMed] [Google Scholar]
- Kinsky S. C. Antibiotic interaction with model membranes. Annu Rev Pharmacol. 1970;10:119–142. doi: 10.1146/annurev.pa.10.040170.001003. [DOI] [PubMed] [Google Scholar]
- Kinsky S. C., Luse S. A., Zopf D., van Deenen L. L., Haxby J. Interaction of filipin and derivatives with erythrocyte membranes and lipid dispersions: electron microscopic observations. Biochim Biophys Acta. 1967;135(5):844–861. doi: 10.1016/0005-2736(67)90055-7. [DOI] [PubMed] [Google Scholar]
- Landsberger F. R., Compans R. W., Choppin P. W., Lenard J. Organization of the lipid phase in viral membranes. Effects of independent variation of the lipid and the protein composition. Biochemistry. 1973 Oct 23;12(22):4498–4502. doi: 10.1021/bi00746a030. [DOI] [PubMed] [Google Scholar]
- Landsberger F. R., Compans R. W. Effect of membrane protein on lipid bilayer structure: a spin-label electron spin resonance study of vesicular stomatitis virus. Biochemistry. 1976 Jun 1;15(11):2356–2360. doi: 10.1021/bi00656a017. [DOI] [PubMed] [Google Scholar]
- Landsberger F. R., Lenard J., Paxton J., Compans R. W. Spin-labeled electron spin resonance study of the lipid-containing membrane of influenza virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2579–2583. doi: 10.1073/pnas.68.10.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Tsai D. K., Compans R. W., Landsberger F. R. Observations on the membrane organization of standard and incomplete influenza grown in MDBK cells. Virology. 1976 Jun;71(2):389–394. doi: 10.1016/0042-6822(76)90366-4. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A. Glycoprotein fragment associated with vesicular stomatitis virus after proteolytic digestion. Virology. 1974 Dec;62(2):573–577. doi: 10.1016/0042-6822(74)90419-x. [DOI] [PubMed] [Google Scholar]
- Pinter A., Compans R. W. Sulfated components of enveloped viruses. J Virol. 1975 Oct;16(4):859–866. doi: 10.1128/jvi.16.4.859-866.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. doi: 10.1128/jvi.16.2.237-240.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Ultrastructure of membrane lesions in immune lysis, osmotic lysis and drug-induced lysis. Fed Proc. 1974 Oct;33(10):2116–2124. [PubMed] [Google Scholar]
- Tillack T. W., Kinsky S. C. A freeze-etch study of the effects of filipin on liposomes and human erythrocyte membranes. Biochim Biophys Acta. 1973 Sep 27;323(1):43–54. doi: 10.1016/0005-2736(73)90430-6. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., de Kruijff B., Gerritsen W. F., Demel R. A., van Deenen L. L., Ververgaert P. H. Freeze-etch electron microscopy of erythrocytes, Acholeplasma laidlawii cells and liposomal membranes after the action of filipin and amphotericin B. Biochim Biophys Acta. 1973 Jan 26;291(2):577–581. doi: 10.1016/0005-2736(73)90509-9. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Demel R. A. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim Biophys Acta. 1974 Feb 26;339(1):57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. I. Specificity of the membrane permeability changes induced by the polyene antibiotics. Biochim Biophys Acta. 1974 Feb 26;339(1):30–43. doi: 10.1016/0005-2736(74)90330-7. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., van Dijck P. W., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholesplasma laidlawii cells and lecithin liposomes. II. Temperature dependence of the polyene antibiotic-sterol complex formation. Biochim Biophys Acta. 1974 Feb 26;339(1):44–56. doi: 10.1016/0005-2736(74)90331-9. [DOI] [PubMed] [Google Scholar]





