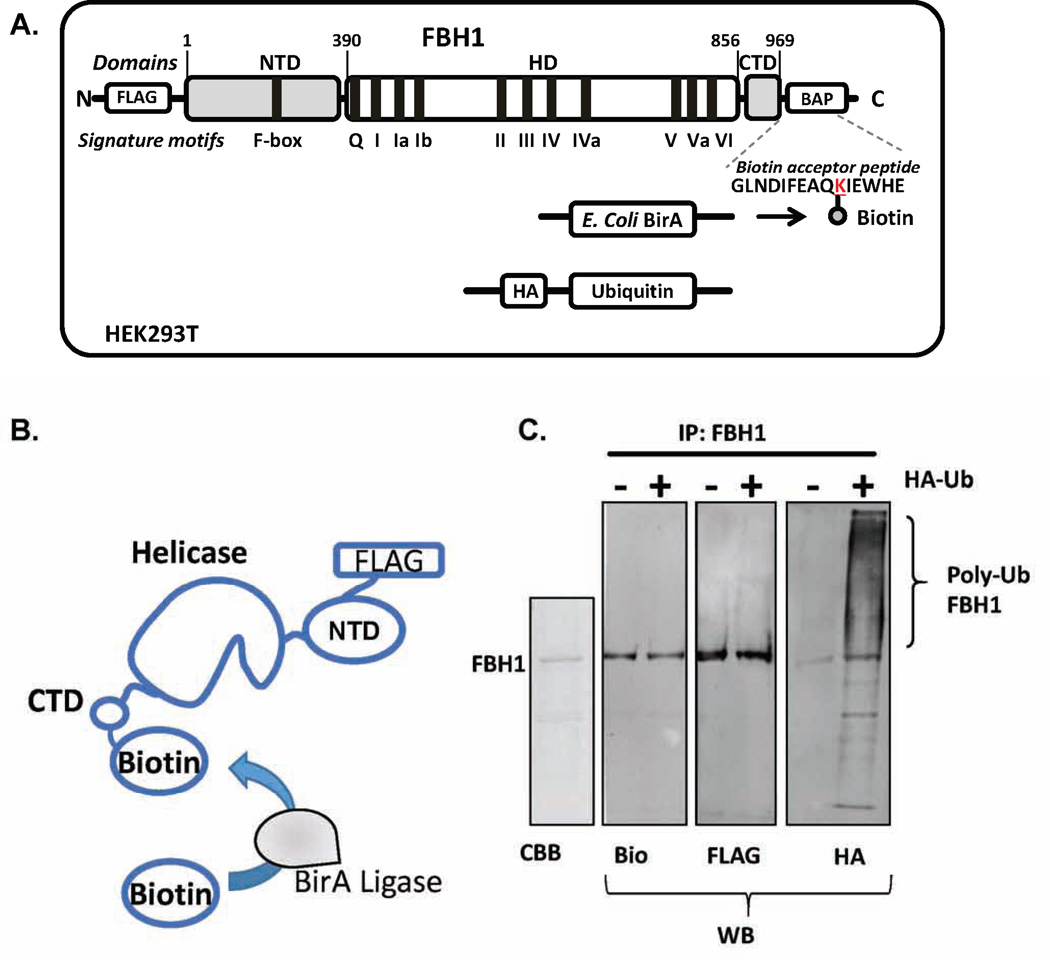
Figure 1. Production of dually labeled FBH1 for single-molecule TIRFM experiments.
(A) FBH1 was expressed and purified from HEK293 cells following transient transfection. FBH1 was cloned into a pcDNA3 vector. A FLAG tag was also incorporated on the N-terminus of the protein and a biotin acceptor peptide was added to the C-terminus. HEK293 cells were transfected with the FBH1 construct along with pcDNA3-BirA, which encoded the E. coli BirA ligase. To produce ubiquitylated FBH1, HEK293 cells were additionally transfected with a pcDNA3 vector that expressed HA-tagged ubiquitin. (B) BirA ligase covalently biotinylates the lysine residue within the biotin acceptor peptide with high efficiency and specificity in vivo, thereby producing biotinylated FBH1. (C) Coomassie staining (CBB) and western blot (WB) analysis of purified FBH1. The blot of FBH1 using anti-HA antibody (HA) showed that FBH1 is poly-ubiquitylated in vivo. The blot of FBH1 using anti-biotin (bio) and anti-FLAG (FLAG) antibodies are also shown. Modified from Ref.14.