Abstract
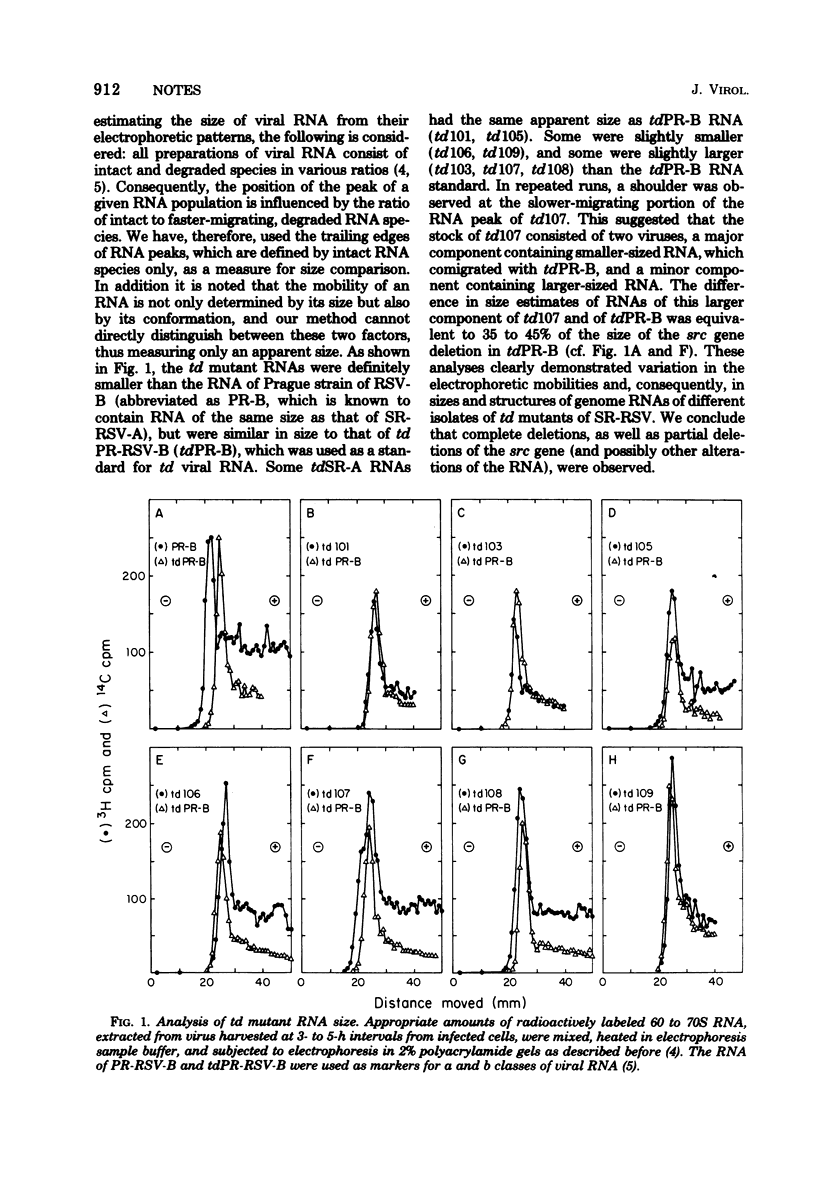
The RNAs of transformation-defective (td) deletion mutants of the Schmidt-Ruppin strain of Rous sarcoma virus were found to vary in size when compared by polyacrylamide gel electrophoresis. Three of seven td mutants appeared to recombine with a mutant of Rous sarcoma virus (Schmidt-Ruppin), which has a temperature-sensitive sarcoma (src) gene and is termed ts68, to give rise to recombinants with a reduced temperature sensitivity. The results suggested that different clones of td mutants exist: some in which the src gene appears to be deleted, and others in which the src gene is only partially deleted. A direct correlation between RNA size and the extent of src gene deletion measured by recombination was not obtained, possibly because the recombination assay could only detect src sequences homologous to the lesion(s) of ts68, whereas the electrophoretic analysis of the RNA measured src deletions as well as other possible alterations of the RNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker D., Kurth R., Critchley D., Friis R., Bauer H. Distinguishable transformation-defective phenotypes among temperature-sensitive mutants of Rous sarcoma virus. J Virol. 1977 Mar;21(3):1042–1055. doi: 10.1128/jvi.21.3.1042-1055.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A., MacCormick R., Martin G. S. Transformation-defective mutants of avian sarcoma viruses: the genetic relationship between conditional and nonconditional mutants. Virology. 1976 Mar;70(1):206–209. doi: 10.1016/0042-6822(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Kawai S., Wang L. H., Vogt P. K., Murphy H. M., Hanafusa H. RNA of replication-defective strains of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1569–1573. doi: 10.1073/pnas.72.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Graf T., Bauer H., Gelderblom H., Bolognesi D. P. Studies on the reproductive and cell-converting abilities of avian sarcoma viruses. Virology. 1971 Feb;43(2):427–441. doi: 10.1016/0042-6822(71)90315-1. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillova J., Hill M., Kalékine M. Inability of the nondefective Rous sarcoma provirus to generate, upon transfection, a transformation-defective virus. Virology. 1976 Oct 15;74(2):540–543. doi: 10.1016/0042-6822(76)90360-3. [DOI] [PubMed] [Google Scholar]
- Joho R. H., Billeter M. A., Weissmann C. Mapping of biological functions on RNA of avian tumor viruses: location of regions required for transformation and determination of host range. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4772–4776. doi: 10.1073/pnas.72.12.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Genetic recombination with avian tumor virus. Virology. 1972 Jul;49(1):37–44. doi: 10.1016/s0042-6822(72)80005-9. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Recombination between a temperature-sensitive mutant and a deletion mutant of Rous sarcoma virus. J Virol. 1976 Aug;19(2):389–397. doi: 10.1128/jvi.19.2.389-397.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Duesberg P. H. The a subunit in the RNA of transforming avian tumor viruses. I. Occurrence in different virus strains. II. Spontaneous loss resulting in nontransforming variants. Virology. 1972 Feb;47(2):494–497. doi: 10.1016/0042-6822(72)90287-5. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Fujita D. J., Padgett T., Varmus H. E., Bishop J. M. Detection and enumeration of transformation-defective strains of avian sarcoma virus with molecular hybridization. Virology. 1977 Feb;76(2):675–684. doi: 10.1016/0042-6822(77)90250-1. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Guntaka R. V., Varmus H. E., Bishop J. M. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Stone M. P., Smith R. E., Joklik W. K. 35S a and b RNA subunits of avian RNA tumor virus strains cloned and passaged in chick and duck cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):859–868. doi: 10.1101/sqb.1974.039.01.100. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. doi: 10.1016/0042-6822(71)90092-4. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



