Abstract
A previous systematic review reported that topical NSAIDs were effective in relieving pain in chronic conditions like osteoarthritis and tendinitis. More trials, a better understanding of trial quality and bias, and a reclassification of certain drugs necessitate a new review.
Studies were identified by searching electronic databases, and writing to manufacturers. We identified randomised, double blind trials comparing topical NSAID with either placebo or another active treatment, in adults with chronic pain. The primary outcome was a reduction in pain of approximately 50% at two weeks, and secondary outcomes were local and systemic adverse events and adverse event-related withdrawals. Relative benefit and number-needed-to-treat (NNT), and relative harm and number-needed-to-harm (NNH) were calculated, and the effects of trial quality, validity and size, outcome reported, and condition treated, were examined by sensitivity analyses.
Twelve new trials were added to 13 trials from a previous review. Fourteen double blind placebo-controlled trials had information from almost 1,500 patients. Topical NSAID was significantly better than placebo with relative benefit 1.9 (95% confidence interval 1.7 to 2.2), NNT 4.6 (95% confidence interval 3.8 to 5.9). Results were not affected by trial quality, validity or size, outcome reported, or condition treated. Three trials with 764 patients comparing a topical with an oral NSAID found no difference in efficacy. Local adverse events (6%), systemic adverse events (3%), or the numbers withdrawing due to an adverse event were the same for topical NSAID and placebo.
Topical NSAIDs were effective and safe in treating chronic musculoskeletal conditions for two weeks. Larger and longer trials are necessary to fully elucidate the place of topical NSAIDs in clinical practice.
Background
A systematic review of topical NSAIDs reported that they were effective for relieving pain in both acute and chronic conditions [1]. Number-needed-to-treat (NNT), the number of patients that need to be treated for one to benefit from a particular drug, who would not have benefited from placebo, was used to estimate efficacy. In chronic conditions, NNT for topical NSAIDs at two weeks was 3.1 (2.7 to 3.8).
There are three reasons why an updated review of topical NSAIDs in chronic pain is needed. First, we have a better appreciation of factors that can introduce bias [2-4], and would not now accept trials that were not double blind, or were very small. Second, topical salicylate and benzydamine are no longer classed as topical NSAIDs [5]. Thirdly, there are now more trials. We believed that updating the review would improve efficacy estimates for topical NSAIDs, with a prior intent to determine efficacy for individual drugs.
Methods
Searching
Relevant studies were sought regardless of publication language, type, date or status. Studies included in the previous review were considered for inclusion, and the Cochrane Library, MEDLINE and PreMedline, EMBASE and PubMed, were searched for relevant studies published since the last review, for the years 1996 to April 2003. The search strategy included "application: topical" together with "cream", "gel" etc, together with generic names of NSAIDs, and proprietary preparations of topical treatment in which the principal active ingredient was an NSAID [6,7] (additional file 1:search strategy). Reference lists of retrieved articles were also searched. We wrote to 20 pharmaceutical companies in the UK, 66 in continental Europe, and two in North America, known to manufacture topical NSAIDs, asking if they could supply papers.
Selection
We identified reports of randomised, double blind, active or placebo-controlled trials in which treatments were given to adult patients with moderate to severe chronic pain resulting from musculoskeletal or other painful disorders. We excluded treatments for mouth or eye diseases. At least ten patients had to be randomised to a treatment group and application of treatment had to be at least once daily. Outcomes closest to two weeks (but at least seven days) were extracted. Longer outcomes were also accepted when available.
Quality and validity assessment
Trial quality was assessed using a validated three-item scale with a maximum quality score of five [8]. Included studies had to score at least two points, one for randomisation and one for blinding. A sixteen-point scale was used to assess trial validity [9]. Quality and validity assessments were made independently by at least two reviewers and verified by one other reviewer. Disputes were settled by discussion between all reviewers.
Outcomes
We defined our own outcome of clinical success, representing approximately a 50% reduction in pain [1]. This was either the number of patients with a "good" or "excellent" global assessment of treatment, or "none" or "slight" pain on rest or movement (or comparable wording) measured on a categorical scale. A hierarchy of outcomes was used to extract efficacy information [1], shown below in order of preference:
1) number of patients with a 50% or more reduction in pain
2) patient reported global assessment of treatment
3) pain on movement
4) pain on rest or spontaneous pain
5) physician or investigator global assessment of treatment
In addition, the number of patients showing undefined "improvement" was also accepted. All of these outcomes were grouped together as a "success", and categories 1–4 were used as preferred outcomes in the sensitivity analysis.
Secondary outcomes were extracted from included papers that reported them. These were the number of patients (i) reporting one or more local adverse event (itching, stinging, rash), (ii) reporting one or more systemic adverse event (iii) withdrawing from trials due to adverse events.
Quantitative data synthesis
The number of patients randomised into each treatment group (intention to treat) was used in the efficacy analysis. Information was pooled for the number of patients in each trial approximating at least 50% pain relief, or similar measure, for both topical NSAID and control. These were used to calculate NNT with a 95% confidence interval (CI) [10]. Relative benefit and relative risk estimates with 95% CIs were calculated using the fixed effects model [11]. A statistically significant benefit of topical NSAID over control was assumed when the lower limit of the 95% confidence interval (CI) of the relative benefit was greater than one. A statistically significant benefit of control over active treatment was assumed when the upper limit of the 95% CI was less than one. Homogeneity of trials was assessed visually [12-14]. Number-needed-to-harm (NNH) and relative risk were calculated in the same way as for NNTs and relative benefit. All calculations were performed using Microsoft Excel X for the Macintosh and RevMan 4.2. In sensitivity analyses the z test was used [15]. QUOROM guidelines were followed [16].
Sensitivity analysis
Our prior intention was to perform sensitivity analyses on pooled outcomes using the z test [15] for quality score (2 versus 3 or more), validity score (8 or less versus 9 or more), trial size (less than 40 patients per group versus more than 40 patients per group), reported outcome (higher versus lower preference), drug, and condition treated (knee osteoarthritis versus other musculoskeletal). At least three studies had to be available in each category before information was pooled.
Results
Study characteristics
Ten out of the 20 UK companies, and two out of the 66 continental European companies replied to our request for studies. Only three companies supplied useful material, either published studies or bibliographies. None provided unpublished material.
Searches identified 60 target papers, but 35 were excluded; 23 studies failed to meet the inclusion criteria and 12 had no useable data. Twenty-four of these 60 target papers were included in the previous review. We included 13 of those in this review, and excluded 11; seven were not double blind, two compared a salicylate with placebo or oral analgesics, one did not have daily application, and one had insufficient data (additional file 2: excluded studies, additional file 3: QUOROM flow diagram).
Twenty-five trials therefore met the selection criteria, 12 of which were additional trials. Fifteen trials had only placebo controls [17-31], seven only active controls [32-38], and three had both placebo and active controls [39-41]. Of the 10 active controlled trials, four compared a topical NSAID with a different topical NSAID, three compared a topical NSAID with a different oral NSAID, and one each compared a topical NSAID with a homeopathic gel, a topical rubefacient, and topical trinitroglycerin (GTN). Details of all included studies with outcomes and quality and validity scores are in additional files 4 (Outcome details of placebo-controlled trials) and additional files 5 (Outcome details of active-controlled trials).
Patients were generally over 40 years old, predominantly with musculoskeletal disorders, and with baseline pain of moderate to severe intensity. Fourteen studies examined general musculoskeletal conditions, and eleven examined osteoarthritis (9 studies of the knee, one of finger joints, and one of mixed sites). Five studies in osteoarthritis specified use of a standard scale (ACR, Kellgren and Lawrence, ISK) to assess the severity of disease, four specified that the disease was radiologically confirmed, one specified that patients had "well documented mild osteoarthritis", and one made no statement.
Quality scores were high, with 16/18 placebo controlled and 9/10 active controlled trials scoring 3 or more points out of a maximum of 5. Validity scores were also high, with 14/18 placebo controlled and 8/10 active controlled trials scoring 9 or more out of a maximum of 16 (additional files 4 and 5).
Placebo controlled trials
Dichotomous information was available to pool from 14 placebo controlled trials for efficacy, from 16 placebo controlled trials for local adverse events, 17 placebo controlled trials for systemic adverse events, and from 11 placebo controlled trials for adverse event related withdrawals.
Efficacy
Fourteen trials (1,502 patients) provided data on efficacy. Topical NSAIDs were significantly better than placebo (Table 1). The mean placebo response rate was 26% ranging from 7% to 78%. The mean treatment response rate was 48% ranging from 2% to 90% (Figure 1). The NNT was 4.6 (95% CI 3.8 to 5.9) for one patient to experience improvement in chronic musculoskeletal pain at two weeks with topical NSAIDs, compared with placebo.
Table 1.
Summary data and sensitivity analyses for placebo controlled trials
| Number of | Success/total | |||||
| Trial characteristic | Trials | Patients | Treatment | Placebo | RB (95% CI) | NNT (95% CI) |
| All trials | 14 | 1502 | 371/771 | 193/731 | 1.9 (1.7 to 2.2) | 4.6 (3.8 to 5.9) |
| Quality and validity | ||||||
| Quality score 3 to 5 | 11 | 1312 | 294/678 | 144/634 | 2.0 (1.7 to 2.4) | 4.8 (3.9 to 6.4) |
| Quality score 2 | 3 | 190 | 77/93 | 49/97 | 1.6 (1.3 to 2.0) | 3.1 (2.2 to 5.1) |
| Validity score ≥ 9 and quality score ≥ 3 | 10 | 1197 | 247/622 | 98/575 | 2.4 (2.0 to 3.0) | 4.4 (3.6 to 5.6) |
| Trial group size | ||||||
| ≤ 50 patients | 6 | 343 | 95/172 | 54/171 | 1.8 (1.4 to 2.3) | 4.2 (3.0 to 7.4) |
| > 50 patients | 8 | 1159 | 276/599 | 139/560 | 2.0 (1.7 to 2.3) | 4.7 (3.8 to 6.3) |
| Outcome type | ||||||
| preferred outcomes | 8 | 920 | 198/462 | 85/458 | 2.3 (1.9 to 2.9) | 4.1 (3.3 to 5.4) |
| lower preference outcomes | 6 | 582 | 173/309 | 108/273 | 1.6 (1.4 to 1.9) | 6.1 (4.1 to 12) |
| Condition | ||||||
| knee osteoarthritis | 5 | 567 | 127/307 | 58/260 | 2.0 (1.6 to 2.6) | 5.3 (3.8 to 8.6) |
| other musculoskeletal | 9 | 935 | 244/464 | 135/471 | 1.9 (1.6 to 2.2) | 4.2 (3.3 to 5.6) |
Figure 1.
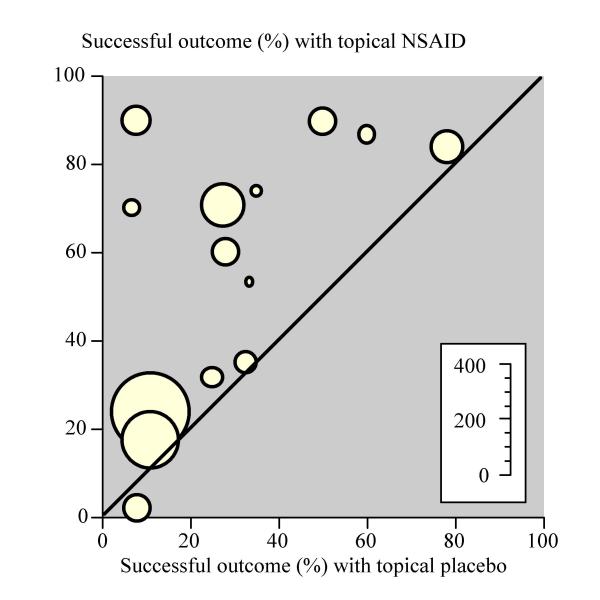
Topical NSAIDs in chronic musculoskeletal pain Randomised double-blind studies of topical NSAID compared to topical placebo for two-week outcome of successful treatment. Inset scale shows size of individual trials.
Sensitivity analyses (Table 1) showed no significantly greater effect for low quality trials (quality score 2/5) compared with higher quality trials (quality score 3–5/5) (z = 1.69, p = 0.091). There was no significant difference for smaller versus larger trials using 50 patients per group (median group size for topical NSAID was 49) as a cut off (z = 0.40, p = 0.69), for preferred outcomes versus lower preference outcomes (physician determined or general improvement) (z = 1.56, p = 0.12), or for patients with knee osteoarthritis compared with other musculoskeletal conditions (z = 0.99, p = 0.32) (Figure 2). The 10 trials with both a quality score of 3/5 or greater and a validity score of 9/16 or greater had an NNT of 4.4 (95% CI 3.6 to 5.6). There were insufficient data to allow comparisons of efficacy between different NSAIDs.
Figure 2.
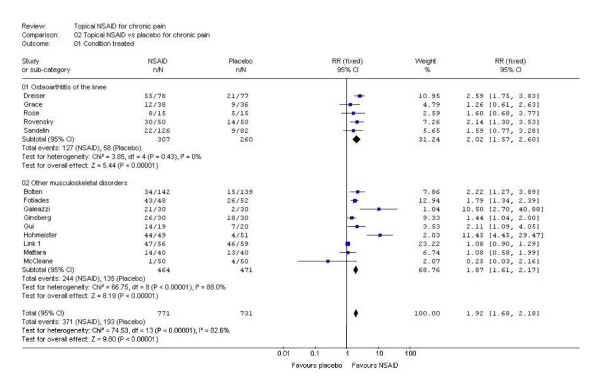
Analysis of trials of topical NSAIDs in chronic musculoskeletal pain by condition. This Forrest plot was created using RevMan 4.2. Details of the statistical tests used can be found in the Cochrane Handbook.
Harm
All 18 placebo controlled trials (2,032 patients) provided some information on adverse events (Table 2). There was no statistically significant difference between topical NSAID and topical placebo for the number of patients experiencing local adverse events (6%), systemic adverse events (3%), or the number withdrawing due to an adverse event (1%). With topical NSAID or topical placebo, local adverse events were usually described as rash, itching or stinging, and were predominantly mild.
Table 2.
Placebo contolled trials
| Number of | Events/total | ||||
| Type of adverse event | Trials | Patients | Treatment | Placebo | RR (95% CI) |
| Local adverse events | 15 | 1734 | 53/949 | 48/785 | 1.0 (0.7 to 1.4) |
| Systemic adverse events | 16 | 1838 | 33/1002 | 14/836 | 1.7 (0.96 to 2.85) |
| Withdrawals beacuse of adverse events | 10 | 1225 | 10/697 | 7/528 | 0.9 (0.4 to 2.1) |
| Active controlled trials: topical vs oral | |||||
| Number of | Events/total | ||||
| Type of adverse event | Trials | Patients | Treatment | Placebo | RR (95% CI) |
| Local adverse events | 2 | 443 | 19/243 | 4/118 | 3.0 (1.1 to 8.5) |
| Systemic adverse events | 3 | 764 | 82/408 | 87/356 | 0.83 (0.6 to 1.1) |
| Withdrawals because of adverse events | 3 | 764 | 19/408 | 24/356 | 0.7 (0.4 to 1.3) |
Active controlled trials
Efficacy
There was sufficient information to pool results only from the three trials comparing a topical NSAID with an oral NSAID in patients with osteoarthritis of the knee or finger joints. One trial [34] compared piroxicam 0.5% gel with oral ibuprofen 1200 mg daily, another [38] compared diclofenac 1% gel with oral ibuprofen 1200 mg daily, and the third [41] compared eltenac 1% gel with oral diclofenac 100 mg daily. In these trials, with 764 patients, 37% had a successful outcome both with topical NSAID and oral NSAID. There was no statistically significant difference (relative risk 1.1; 95% CI 0.9 to 1.3). The other seven studies used different topical preparations and different comparators in small trials (additional file 5: Outcome details of active-controlled trials).
Harm
Eight of the active controlled trials (1,461 patients) provided some information on adverse events (Table 2). In two active controlled trials comparing topical with oral NSAID, local adverse events occurred more frequently (8%) with topical than with oral NSAID (3%). Systemic adverse events and adverse event withdrawals did not differ between topical and oral NSAID. No study documented specific instances of upper gastrointestinal bleeding or symptomatic ulcers.
Discussion
Patients in these trials all had moderate to severe baseline pain, and for those with osteoarthritis, disease severity was generally mild to moderate. Patients with most severe disease were specifically excluded in several trials because authors regarded topical NSAID to be inappropriate for their treatment.
Both the original and this updated review concluded that topical NSAIDs were effective in chronic conditions. However, removing trials of lower quality, and topical agents that are not now regarded as topical NSAIDs, increased (worsened) the NNT from 3.1 (95% CI 2.7 to 3.8) to 4.7 (95% CI 3.8 to 5.9) for the outcome of at least half pain relief at two weeks for all topical NSAIDs compared to placebo. For every four or five patients with chronic pain treated with topical NSAID, one would benefit who would not have done with placebo. Three trials comparing topical with oral NSAID found no difference in efficacy.
There are a number of aspects of this review that might question this demonstration of efficacy. The trials spanned several decades and retrospective examination finds fault with them in several respects. Many trials were small, and small size can allow chance effects to influence treatment and placebo event rates [4]. Different preparations were used, with different formulations, concentrations of active ingredient, and application schedules. Reported outcomes were not consistent, and a hierarchy of outcomes had to be constructed. It was inevitable that there would be some clinical heterogeneity, even when similar patients were treated, and when trials were both randomised and double blind, and of appropriate duration.
We addressed these limitations with pre-planned sensitivity analyses. Using studies with higher quality and validity scores, larger size, or higher rather than lower preference outcomes made no difference. Patients treated for knee osteoarthritis derived the same degree of pain relief as those treated for general musculoskeletal conditions. The evidence was that topical NSAIDs were effective whatever strategy was used for sensitivity analysis, improving the robustness of the overall result.
A possible criticism might be that there has been selective publication of trials showing topical NSAIDs to be effective, and suppression of trials where there was no difference between topical NSAID and placebo. Funnel plots do not reliably detect publication bias [13,14], so we did not use them or make any adjustment for possible publication bias [42]. We did approach every company in the world that we could identify as being involved with topical NSAID manufacture or sale for any additional unpublished trials, but no more unpublished material was identified. When unpublished material is found, it often does not change the relevance of a result [43-45].
It is important to emphasise that both active and placebo treatments were rubbed on, making any effect of rubbing equal in both groups. The mean placebo response in the included trials was 26%, compared with the mean response of 48% with topical NSAID. The response with placebo is consistent with that found in acute and chronic pain with a variety of conditions and endpoints [46].
Local adverse events were reported with equal frequency for topical NSAID and topical placebo in placebo-controlled trials, but more often for topical NSAID than oral NSAID in active controlled trials. There were no differences between topical NSAID and topical placebo, or topical NSAID and oral NSAID, for systemic adverse events, or withdrawals due to adverse events. Studies of short duration will not capture important long-term safety information, and this may be important for ongoing applications of gels, creams or sprays in chronic conditions. There is, however, information that indicates that topical NSAIDs do not cause the gastrointestinal harm found with oral NSAIDs [47], nor are they associated with increased renal failure [48].
Clearly there is a body of evidence to support the efficacy of topical NSAIDs in chronic painful musculoskeletal conditions. Despite removing smaller studies that were not double blind, and substituting newer, larger trials of higher quality, topical NSAIDs remained effective, though the NNT was higher (worse) than originally estimated [1]. More information of high quality is required, to compare the relative efficacy of topical and oral NSAIDs, and between different topical NSAIDs.
We are able to compare the evidence for different topical analgesics in chronic musculoskeletal pain (Table 3). Systematic reviews of topical salicylate [49] and capsaicin [50], tell us what is known about those treatments. As Table 3 shows, topical NSAIDs have been tested in many more studies, and in four times as many patients as these other topical analgesics, and have the lowest (best) NNT. The limitation of this comparison is essentially the same limitation as with all these reviews, that the included trials were too short and too small to be sure of the result. Topical NSAIDs have the best evidence for chronic musculoskeletal pain, supporting the excellent evidence available in acute painful conditions [51].
Table 3.
Comparison of topical analgesics in chronic musculoskeletal pain
| Number of | Percent success with | ||||
| Topical analgesic | Trials | Patients | Treatment | Placebo | NNT (95% CI) |
| NSAID | 14 | 1502 | 48 | 26 | 4.6 (3.8 to 5.9) |
| Salicylates | 6 | 429 | 54 | 36 | 5.3 (3.6 to 10) |
| Capsaicin | 3 | 368 | 38 | 25 | 8.1 (4.6 to 34) |
Authors' contributions
LM was involved with planning the study, searching, data extraction, analysis, and preparing a manuscript; RAM with planning, data extraction, analysis and writing the manuscript; JE with searching, data extraction, and writing; SD with data extraction, analysis, and writing; HJM with planning, analysis and writing. All authors read and approved the final manuscript.
Competing interests
RAM & HJM have consulted for various pharmaceutical companies. RAM, HJM & JE have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. All authors have received research support from charities, government and industry sources at various times, but no such support was received for this work. No author has any direct stock holding in any pharmaceutical company.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Search strategy for RCTs of topical NSAIDs in chronic pain
Excluded studies
QUOROM flow diagram
Outcome details of placebo-controlled trials
Outcome details of active-controlled trials
Acknowledgments
Acknowledgements
The study was supported with Pain Research funds and by the Oxford Pain Relief Trust.
Contributor Information
Lorna Mason, Email: lornamason79@yahoo.co.uk.
R Andrew Moore, Email: andrew.moore@pru.ox.ac.uk.
Jayne E Edwards, Email: jayne.edwards@pru.ox.ac.uk.
Sheena Derry, Email: sheena.derry@pru.ox.ac.uk.
Henry J McQuay, Email: henry.mcquay@pru.ox.ac.uk.
References
- Moore RA, Tramer MR, Carroll D, Wiffen PJ, McQuay HJ. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ. 1998;316:333–338. doi: 10.1136/bmj.316.7128.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything – large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain. 1998;78:209–216. doi: 10.1016/S0304-3959(98)00140-7. [DOI] [PubMed] [Google Scholar]
- Li Wan Po A. PJ Practice Checklist: Topical Analgesics. The Pharmaceutical Journal. 1996. http://www.pharmj.com/Index.html
- British National Formulary No 45. London: British Medical Association, Royal Pharmaceutical Society; 2003. [Google Scholar]
- Reynolds JEF, editor. Martindale: the extra pharmacopoeia. 32. London: Royal Pharmaceutical Society; 1999. [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Smith LA, Oldman AD, McQuay HJ, Moore RA. Teasing apart quality and validity in systematic reviews: an example from acupuncture trials in chronic neck and back pain. Pain. 2000;86:119–132. doi: 10.1016/S0304-3959(00)00234-7. [DOI] [PubMed] [Google Scholar]
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG, editor. In Statistics with confidence – confidence intervals and statistical guidelines. London: British Medical Journal; 1995. pp. 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Abbe KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107:224–233. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- Gavaghan DJ, Moore RA, McQuay HJ. An evaluation of homogeneity tests in meta-analyses in pain using simulations of individual patient data. Pain. 2000;85:415–424. doi: 10.1016/S0304-3959(99)00302-4. [DOI] [PubMed] [Google Scholar]
- Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002;7:51–61. doi: 10.1258/1355819021927674. [DOI] [PubMed] [Google Scholar]
- Tramer MR, Reynolds DJ, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta-analysis: a case study. BMJ. 1997;315:635–640. doi: 10.1136/bmj.315.7109.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/S0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- Bolten W. Felbinac-Gel zur Behandlung lokalisierter extraartikulärer rheumatischer Beschwerden – eine multizentrische, placebokontrollierte, randomisierte Studie. [Felbinac gel for treatment of localized extra-articular rheumatic diseases – a multicenter, placebo controlled, randomized study] Z Rheumatol. 1991;50:109–113. [PubMed] [Google Scholar]
- Brühlmann P, Michel BA. Topical diclofenac patch in patients with knee osteoarthritis: a randomized, double-blind, controlled clinical trial. Clin Exp Rheumatol. 2003;21:193–198. [PubMed] [Google Scholar]
- Dreiser RL, Tisne-Camus M. DHEP plasters as a topical treatment of knee osteoarthritis – a double-blind placebo-controlled study. Drugs Exp Clin Res. 1993;19:117–123. [PubMed] [Google Scholar]
- Fotiades P, Bach GL. Wirkung einer flufenaminsaurehaltigen Salbe bei verschiedenen rheumatischen Erkrankungen. [The effect of a flufenamic acid containing ointment in various rheumatic diseases. A double blind study] Fortschr Med. 1976;94:1036–1038. [PubMed] [Google Scholar]
- Galeazzi M, Marcolongo R. A placebo-controlled study of the efficacy and tolerability of a nonsteroidal anti-inflammatory drug, DHEP plaster, in inflammatory peri- and extra-articular rheumatological diseases. Drugs Exp Clin Res. 1993;19:107–115. [PubMed] [Google Scholar]
- Ginsberg F, Famaey JP. Double-blind, randomized crossover study of the percutaneous efficacy and tolerability of a topical indomethacin spray versus placebo in the treatment of tendinitis. J Int Med Res. 1991;19:131–136. doi: 10.1177/030006059101900206. [DOI] [PubMed] [Google Scholar]
- Grace D, Rogers J, Skeith K, Anderson K. Topical diclofenac versus placebo: a double blind, randomized clinical trial in patients with osteoarthritis of the knee. J Rheumatol. 1999;26:2659–2663. [PubMed] [Google Scholar]
- Gui L, Pellacci F, Ghirardini G. Impiego dell'ibuprofen crema in pazienti ambulatoriali di interesse ortopedico. Confronto in doppia cecità con placebo. [Use of ibuprofen cream in ambulatory orthopedic patients. Double blind comparison with placebo] Clin Ter. 1982;101:363–369. [PubMed] [Google Scholar]
- Hohmeister R. Die Behandlung von weichteilrheumatischen Erkrankungen mit Mobilisin Gel. Resultate einer Doppelblind-Studie. [Treatment of rheumatic diseases of soft tissues with Mobilisin Gel. Results of a double blind study] Fortschr Med. 1983;101:1586–1588. [PubMed] [Google Scholar]
- Link R, Balint G, Pavlik G, Otto J, Krause W. Topische Behandlung von Weichteil-rheumatismus und Sportverletzungen. Wirksamkeit und Verträglichkeit eines neuen Ketoprofengels. [Topical treatment of soft tissue rheumatism and athletic injuries. Effectiveness and tolerance of a new ketoprofen gel] Fortschr Med. 1996;114:311–314. [PubMed] [Google Scholar]
- Mattara L, Trotta F, Biasi D, Cervetti R. Evaluation of the efficacy and tolerability of a new locally acting preparation of flurbiprofen in scapulohumeral periarthritis. Eur J Rheumatol Inflamm. 1994;4:15–20. [PubMed] [Google Scholar]
- Poul J, West J, Buchanan N, Grahame R. Local action transcutaneous flurbipofen in the treatment of soft tissue rheumatism. Br J Rheumatol. 1993;32:1000–1003. doi: 10.1093/rheumatology/32.11.1000. [DOI] [PubMed] [Google Scholar]
- Rose W, Manz G, Lemmel EM. Behandlung der aktivierten gonarthrose mit topisch appliziertem piroxicam-gel. [Topical application of Piroxicam-gel in the treatment of activated gonarthrosis] Münch Med Wschr. 1991;133:562–566. [Google Scholar]
- Roth SH. A controlled clinical investigation of 3% diclofenac/2.5% sodium hyaluronate topical gel in the treatment of uncontrolled pain in chronic oral NSAID users with osteoarthritis. Int J Tissue React. 1995;4:129–132. [PubMed] [Google Scholar]
- Rovensky J, Micekova D, Gubzova Z, Fimmers R, Lenhard G, Vogtle-Junkert U, Schreyger F. Treatment of knee osteoarthritis with a topical non-steroidal antiinflammatory drug. Results of a randomized, double-blind, placebo-controlled study on the efficacy and safety of a 5% ibuprofen cream. Drugs Exp Clin Res. 2001;27:209–221. [PubMed] [Google Scholar]
- Balthazar-Letawe D. Voltaren Emulgel en pratique rhumatologique. Essai comparatif avec Indocid gel. [Voltaren Emugel in clinical rheumatology. Comparative trial with Indocid gel] Acta Belg Med Phys. 1987;10:109–110. [PubMed] [Google Scholar]
- Burgos A, Busquier MP, Reino JG, Ferreiro JL, Navarro F, Valverde J, Moreno E. Double-blind, double-dummy comparative study of local action transcutaneous flurbiprofen (flurbiprofen LAT) versus piketoprofen cream in the treatment of extra-articular rheumatism. Clin Drug Invest. 2001;21:95–102. [Google Scholar]
- Dickson DJ. A double-blind evaluation of topical piroxicam gel with oral ibuprofen in osteoarthritis of the knee. Curr Ther Res. 1991;49:199–207. [Google Scholar]
- Geller O. Vergleich eines Salizylat/Heparin-Gels mit einem Mono-substanzpräparat. Ergebnisse einer Doppelblind-cross-over-Studie. [Comparison of a salicylate heparin gel with a monosubstance preparation. Results of a double blind cross over study] Munch Med Wochenschr. 1980;122:1231–1232. [PubMed] [Google Scholar]
- van Haselen RA, Fisher PA. A randomized controlled trial comparing topical piroxicam gel with a homeopathic gel in osteoarthritis of the knee. Rheumatology. 2000;39:714–719. doi: 10.1093/rheumatology/39.7.714. [DOI] [PubMed] [Google Scholar]
- Vitali G. Sperimentazione clinica controllata sull'impiego topico dell'acido 2-(3-benzoil-fenil) proprionico a tre diverse concentrazioni. [Controlled clinical experiment with the topical use of 2-(3-benzoyl-phenyl) propionic acid at 3 different concentrations] Clin Ter. 1980;94:257–273. [PubMed] [Google Scholar]
- Zacher J, Burger KJ, Farber L, Grave M, Abberger H, Bertsch K. Topical diclofenac versus oral ibuprofen: A double blind, randomized clinical trial to demonstrate efficacy and tolerability in patients with activated osteoarthritis of the finger joints (Heberden and/or Bouchard arthritis) Aktuelle Rheumatologie. 2001;26:7–14. doi: 10.1055/s-2001-11369. [DOI] [Google Scholar]
- McCleane G. The addition of piroxicam to topically applied glyceryl trinitrate enhances its analgesic effect in musculoskeletal pain: A randomised, double-blind, placebo-controlled study. Pain Clinic. 2000;12:113–116. doi: 10.1163/156856900750229861. [DOI] [Google Scholar]
- Ottillinger B, Gomor B, Michel BA, Pavelka K, Beck W, Elsasser U. Efficacy and safety of eltenac gel in the treatment of knee osteoarthritis. Osteoarthritis Cartilage. 2001;9:273–280. doi: 10.1053/joca.2000.0385. [DOI] [PubMed] [Google Scholar]
- Sandelin J, Harilainen A, Crone H, Hamberg P, Forsskahl B, Tamelander G. Local NSAID gel (eltenac) in the treatment of osteoarthritis of the knee. A double blind study comparing eltenac with oral diclofenac and placebo gel. Scand J Rheumatol. 1997;26:287–292. doi: 10.3109/03009749709105318. [DOI] [PubMed] [Google Scholar]
- Terrin N, Schmid CH, Lau J, Olkin I. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22:2113–2126. doi: 10.1002/sim.1461. [DOI] [PubMed] [Google Scholar]
- MacLean CH, Morton SC, Ofman JJ, Roth EA, Shekelle PG. How useful are unpublished data from the Food and Drug Administration in meta-analysis? J Clin Epidemiol. 2003;56:44–51. doi: 10.1016/S0895-4356(02)00520-6. [DOI] [PubMed] [Google Scholar]
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7:1–76. [PubMed] [Google Scholar]
- Burdett S, Stewart LA, Tierney JF. Publication bias and meta-analyses: a practical example. Int J Technol Assess Health Care. 2003;19:129–134. doi: 10.1017/S0266462303000126. [DOI] [PubMed] [Google Scholar]
- Kalso E, Moore RA. Five easy pieces on evidence-based medicine (2) Eur J Pain. 2000;4:321–324. doi: 10.1053/eujp.2000.0191. [DOI] [PubMed] [Google Scholar]
- Evans JM, McMahon AD, McGilchrist MM, White G, Murray FE, McDevitt DG, MacDonald TM. Topical non-steroidal anti-inflammatory drugs and admission to hospital for upper gastrointestinal bleeding and perforation: a record linkage case-control study. BMJ. 1995;311:22–26. doi: 10.1136/bmj.311.6996.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JM, McGregor E, McMahon AD, McGilchrist MM, Jones MC, White G, McDevitt DG, MacDonald TM. Non-steroidal anti-inflammatory drugs and hospitalization for acute renal failure. QJM. 1995;88:551–557. [PubMed] [Google Scholar]
- Mason L, Moore RA, Edwards JE, McQuay HJ, Derry S, Wiffen PJ. Systematic review of topical rubefacients containing salicylates for the treatment of acute and chronic pain. BMJ. 2004;238:995–998. doi: 10.1136/bmj.38040.607141.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;238:991–994. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for acute pain: a meta-analysis. BMC Fam Pract. 2004;5:10. doi: 10.1186/1471-2296-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy for RCTs of topical NSAIDs in chronic pain
Excluded studies
QUOROM flow diagram
Outcome details of placebo-controlled trials
Outcome details of active-controlled trials


