ABSTRACT
Short-chain fatty acids (SCFAs), e.g. acetic acid, propionic acid and butyric acid, generated through colonic fermentation of dietary fibers, have been shown to reach the systemic circulation at micromolar concentrations. Moreover, SCFAs have been conferred anti-obesity properties in both animal models and human subjects. Branched SCFAs (BSCFAs), e.g., isobutyric and isovaleric acid, are generated by fermentation of branched amino acids, generated from undigested protein reaching colon. However, BSCFAs have been sparsely investigated when referring to effects on energy metabolism. Here we primarily investigate the effects of isobutyric acid and isovaleric acid on glucose and lipid metabolism in primary rat and human adipocytes. BSCFAs inhibited both cAMP-mediated lipolysis and insulin-stimulated de novo lipogenesis at 10 mM, whereas isobutyric acid potentiated insulin-stimulated glucose uptake by all concentrations (1, 3 and 10 mM) in rat adipocytes. For human adipocytes, only SCFAs inhibited lipolysis at 10 mM. In both in vitro models, BSCFAs and SCFAs reduced phosphorylation of hormone sensitive lipase, a rate limiting enzyme in lipolysis. In addition, BSCFAs and SCFAs, in contrast to insulin, inhibited lipolysis in the presence of wortmannin, a phosphatidylinositide 3-kinase inhibitor and OPC3911, a phosphodiesterase 3 inhibitor in rat adipocytes. Furthermore, BSCFAs and SCFAs reduced insulin-mediated phosphorylation of protein kinase B. To conclude, BSCFAs have effects on adipocyte lipid and glucose metabolism that can contribute to improved insulin sensitivity in individuals with disturbed metabolism.
KEYWORDS: adipocyte, branched short-chain fatty acids, metabolism, obesity, short-chain fatty acids, type 2 diabetes
Introduction
A diet rich in dietary fibers influences the regulation of weight and appetite control as well as energy homeostasis.1-3 These effects are most likely mediated by the short-chain fatty acids (SCFAs), generated through colonic fermentation of dietary fibers.4-11 The most abundant SCFAs, namely acetic acid, propionic acid and butyric acid, appear to have anti-obesity properties in both animal models and human subjects when orally distributed.12-21 For example, a high fat diet supplemented with acetic acid, propionic acid or butyric acid improved both insulin sensitivity and protected against weight gain in animal models.18,19 Furthermore, inulin-propionate, a dietary fiber combined with the ester of propionic acid, reduced energy intake, accumulation of intra-abdominal adiposity and lipid content in the liver in obese human individuals.20
Branched SCFAs (BSCFAs), e.g. isobutyric and isovaleric acid, are generated by fermentation of branched amino acids, valine, leucine and isoleucine,22 generated from undigestible protein reaching colon.23,24 However, an increase of isobutyric acid has also been demonstrated after ingestion of certain dietary fibers, such as polydextrose.25 To the best of our knowledge, less is known regarding the role of gut-derived BSCFAs in the regulation of metabolism. Nevertheless, a recent study showed that a diet composed of brown beans increased colonic production of isobutyric acid and propionic acid, which was associated with lowered glucose and insulin concentrations in the blood as well as increased production of the satiety hormone pancreatic peptide YY (PYY).26 Furthermore, by supplementing the drinking water with leucine, a branched amino acid, to mice fed a high-fat diet has been shown to improve glucose tolerance and insulin signaling as well as decrease inflammation in adipose tissue.27 In obesity, adipose tissue shows a number of structural, morphological and functional alterations associated with a deteriorated fat storage capacity, an imbalance in the circulating levels of fatty acids and adipose tissue-derived hormones as well as pro-inflammatory cytokines that promote insulin resistance.28-31 Thus, adipose tissue is a key target tissue for prevention of type 2 diabetes. We and others have previously demonstrated health beneficial effects of SCFAs on adipocyte function, involving effects on lipolysis, lipogenesis and glucose uptake.32-38 BSCFAs have not been associated with outcomes on host health as is the case for SCFAs and specifically, effects of BSCFAs have not been reported when referring to effects on adipocyte metabolism. In the current study, we investigate whether the 2 BSCFAs isobutyric acid and isovaleric acid have the ability to modulate adipocyte function.
Results
Branched short-chain fatty acids inhibit isoproterenol-stimulated lipolysis in a PI3-kinase and PDE3 independent manner
Adipocyte lipolysis is mediated by hormones that increase cAMP, leading to protein kinase A (PKA)-mediated phosphorylation and activation of hormone sensitive lipase (HSL), a rate limiting enzyme in hormone-stimulated lipolysis.30 The effects of the BSCFAs isobutyric acid and isovaleric acid, and the SCFA acetic acid on basal and isoproterenol-stimulated lipolysis were investigated in primary rat adipocytes. As shown in Table 1, BSCFAs and acetic acid inhibited isoproterenol-stimulated lipolysis, which was associated with reduced phosphorylation of HSL (Fig. 1, Table 2).
Table 1.
BSCFAs and acetic acid inhibit lipolysis in rat adipocytes. Lipolysis was measured after 30 minutes of stimulation without (BASAL) or with 30 nM isoproterenol (ISO) in the absence (CTRL) or presence of isobutyric acid (I-BA), isovaleric acid (I-VA) and acetic acid (AA). The values for I-BA, I-VA or AA are related to respective CTRL (set to 1) in BASAL and ISO condition. The mean ± SD (n = 3-5) were used and significance levels were accepted when *p < 0.05, **p < 0.01 and ***p < 0.001. a8.4-fold, b8.3-fold, c6.4-fold increase in ISO-stimulated lipolysis compared to BASAL.
| 1 I-BA |
3 I-BA |
10 I-BA |
|||||
|---|---|---|---|---|---|---|---|
| CTRLMean | Mean | SD | Mean | SD | Mean | SD | |
| BASAL | 1 | 0.74 | 0.57 | 0.90 | 0.52 | 0.60* | 0.31 |
| ISO | 1a | 1.07 | 0.07 | 0.98 | 0.12 | 0.39** | 0.26 |
| 1 I-VA |
3 I-VA |
10 I-VA |
|||||
| |
CTRLMean |
Mean |
SD |
Mean |
SD |
Mean |
SD |
| BASAL | 1 | 1.73* | 0.18 | 0.95 | 0.02 | 0.75 | 0.27 |
| ISO | 1b | 1.10* | 0.03 | 1.05 | 0.03 | 0.50** | 0.05 |
| 1 AA |
3 AA |
10 AA |
|||||
| |
CTRLMean |
Mean |
SD |
Mean |
SD |
Mean |
SD |
| BASAL | 1 | 1.54* | 0.29 | 0.81 | 0.21 | 1.16 | 0.42 |
| ISO | 1c | 1.05 | 0.03 | 0.93 | 0.09 | 0.33*** | 0.08 |
Figure 1.
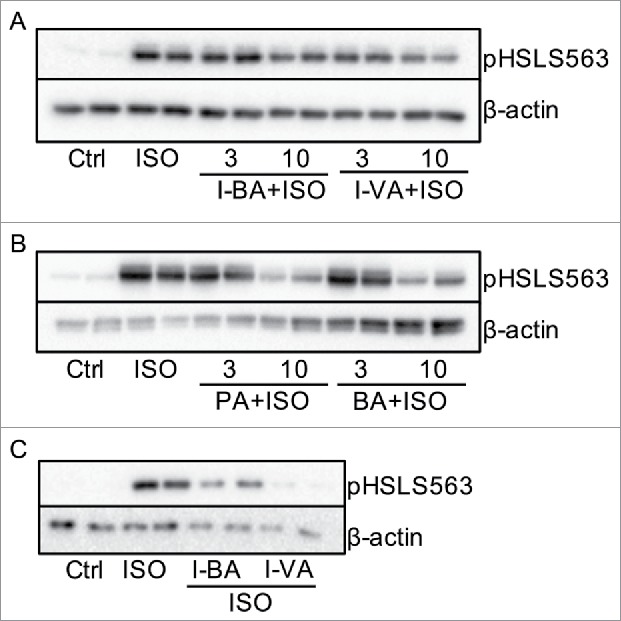
BSCFAs and SCFAs inhibit ISO-potentiated phosphorylation of HSL in primary rat and human adipocytes. In A-B, rat adipocytes were stimulated without (Ctrl) and with isoproterenol (ISO) in the presence or absence of 3 and 10 mM isobutyric acid (I- BA), isovaleric acid (I-VA), propionic acid (PA) and butyric acid (BA) for 10 minutes (n = 3). In C, human adipocytes were stimulated with ISO in the presence or absence of 10 mM I-BA or I-VA for 10 minutes (n = 3). Homogenates were subjected to immunoblot analysis and membranes were probed with antibodies against pHSLS563 and β-actin. Representative blots are shown.
Table 2.
BSFAs and SCFAs reduce phosphorylation of HSLS563 in primary human and rat adipocytes. Rat adipocytes were stimulated without (CTRL) or with ISO in the absence or presence of 3 and 10 mM isobutyric acid (I-BA), isovaleric acid (I-VA), propionic acid (PA) and butyric acid (BA) for 10 minutes. Human adipocytes were stimulated without (CTRL) or with ISO in the absence or presence of 10 mM I-BA or I-VA for 10 minutes (n = 2-3).Homogenates were subjected to immunoblot analysis, membranes were probed with antibodies against pHSLS563 and quantification was made using Image Lab Software (Bio-Rad Laboratories). Data are presented as fold of isoproterenol (ISO). Mean ± SD were used and significance levels were accepted when *p< 0.05, **p < 0.01 and ***p < 0.001. N/A; not applicable.
| HSLS563 in primary rat adipocytes | ||||||
|---|---|---|---|---|---|---|
| +ISO |
||||||
| n = 2#-3 | CTRL | ISO | 3 I-BA# | 10 I-BA | 3 I-VA# | 10 I-VA |
| Mean | 0.02*** | 1 | 0.77 | 0.5* | 0.66 | 0.51*** |
| SD | 0.12 | N/A | 0.35 | 0.25 | 0.52 | 0.03 |
| + ISO |
||||||
| n = 3 |
CTRL |
ISO |
3 PA |
10 PA |
3 BA |
10 BA |
| Mean | 0.09*** | 1 | 0.62** | 0.26** | 0.58 | 0.24** |
| SD | 0.04 | N/A | 0.15 | 0.19 | 0.42 | 0.16 |
| HSLS563 in human adipocytes | ||||||
| +ISO |
||||||
| n = 3 |
CTRL |
ISO |
10 I-BA |
10 I-VA |
||
| Mean | 0.20** | 1 | 0.61** | 0.31** | ||
| SD | 0.20 | N/A | 0.10 | 0.24 | ||
Insulin mediates its antilipolytic effect to a large extent via a phosphatidylinositide 3-kinase (PI3 kinase)- and protein kinase B (PKB)-dependent activation of the cAMP-degrading enzyme phosphodiesterase (PDE) 3B.39,40 However, as shown in Figure 2, the PI3 kinase- selective inhibitor wortmannin and the PDE3-selective inhibitor OPC3911 did not prevent the antilipolytic effect of either branched or non-branched SCFAs on isoproterenol-mediated lipolysis, whereas the antilipolytic effect of insulin was blocked by the inhibitors. To get the human perspective, we also studied lipolysis in adipocytes isolated from subcutaneous adipose tissue of human donors. BSCFAs and SCFAs reduce phosphorylation of HSL whereas significant inhibition of lipolysis was obtained with SCFAs (Fig. 1, Fig. 3, Table 2).
Figure 2.
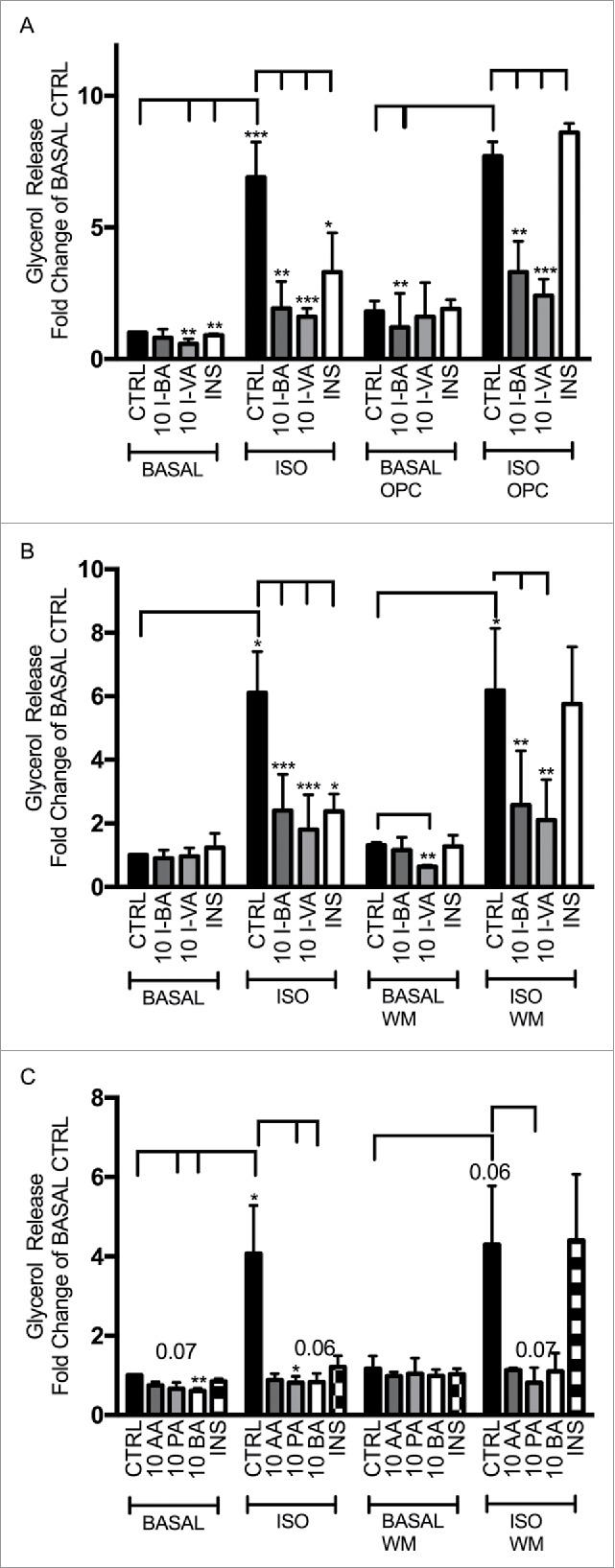
BSCFAs inhibit lipolysis independent of PDE3B and PI3K in primary rat adipocytes. Lipolysis was measured after 1 hour of stimulation without (BASAL) or with 30 nM isoproterenol (ISO), in the absence (CTRL) or presence of 10 mM of isobutyric acid (I-BA), isovaleric acid (I-VA), acetic acid (AA) propionic acid (PA), butyric acid (BA) or insulin (INS) (1nM). The inhibitor for PDE3 (OPC3911) and the inhibitor for PI3K (Wortmannin;WM) were used in BASAL or ISO-stimulated lipolysis in the presence of the branched or non-branced SCFAs. A) I-BA, I-VA and INS in combination with OPC3911; B) I-BA, I-VA and INS in combination with WM; C) AA, PA, BA and INS in combination with WM. The values for I-BA, I-VA, AA, PA, BA and INS are related to CTRL (control without branched or non-branched SCFAs) in BASAL condition. For A-C, mean ± SD (n = 3) were used and significance levels were accepted when *p < 0.05, **p < 0.01 and ***p < 0.001.
Figure 3.
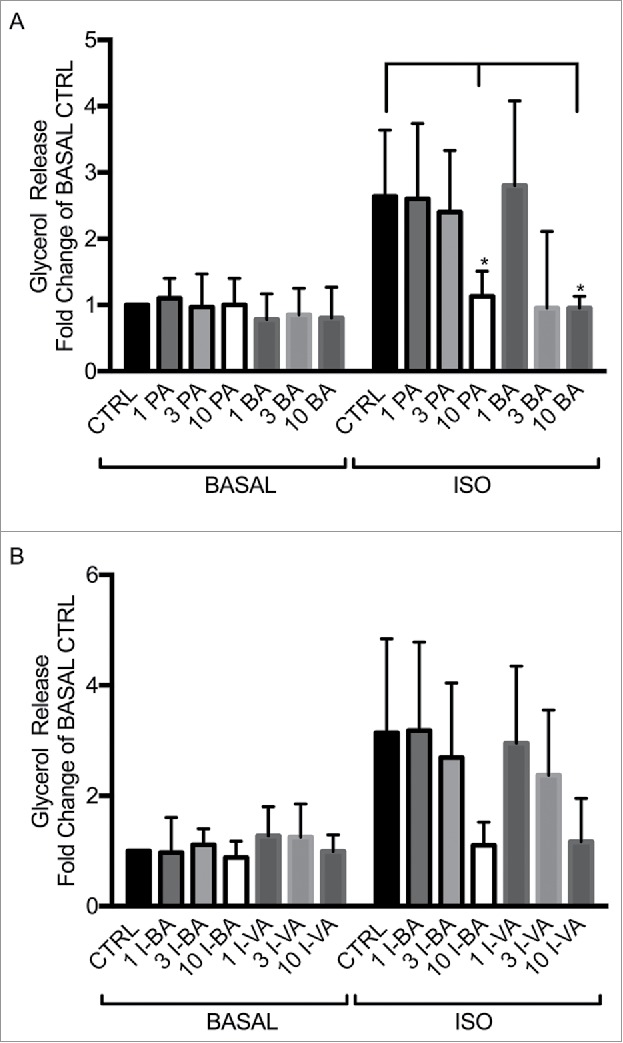
SCFAs and BSCFAs inhibit ISO-potentiated lipolysis in human adipocytes. Lipolysis was measured after 1 hour of stimulation without (BASAL) and with 30 nM isoproterenol (ISO) in the absence (CTRL) or presence of 3 and 10 mM of propionic acid (PA), butyric acid (BA), isobutyric acid (I-BA) or isovaleric acid (I-VA), as shown in A) for PA and BA and B) for I-BA and I-VA. The values are related to BASAL CTRL (condition without lipolytic agent and SCFA or BSCFAs). Mean ± SD (n = 3-4) were used and significance levels were accepted when *p < 0.05, **p < 0.01 and ***p < 0.001.
Branched short-chain fatty acids inhibit basal and stimulated lipogenesis
The effect of BSCFAs and acetic acid on lipogenesis was studied in primary rat adipocytes. Both basal and insulin-stimulated lipogenesis, measured as the incorporation of [3H]-labeled glucose into cellular lipids, were inhibited by isobutyric and isovaleric acid, although to a lesser extent for isovaleric acid in the basal condition (Table 3). Also, acetic acid resulted in inhibition of lipogenesis (Table 3).
Table 3.
BSCFAs and acetic acid inhibit lipogenesis in primary rat adipocytes. Lipogenesis was measured after 30 minutes of stimulation without (BASAL) or with 1 nM insulin (INS) in the absence (CTRL) or presence of 1, 3 and 10 mM isobutyric acid (I-BA), isovaleric acid (I-VA) or acetic acid (AA). The values for I-BA, I-VA and acetic acid are related to respective CTRL (set to 1), either in a basal or an insulin-stimulated state. Mean ± SD (n = 3-4) were used and significance levels were accepted when *p < 0.05,**p < 0.01 and ***p < 0.001. a11.2-fold, b6.4-fold, c5.7-fold increase in insulin-stimulated lipogenesis compared to BASAL.
| 1 I-BA |
3 I-BA |
10 I-BA |
|||||
|---|---|---|---|---|---|---|---|
| CTRLMean | Mean | SD | Mean | SD | Mean | SD | |
| BASAL | 1 | 0.90 | 0.23 | 0.90** | 0.23 | 0.58*** | 0.12 |
| ISO | 1a | 0.98 | 0.09 | 0.88 | 0.14 | 0.61* | 0.3 |
| 1 I-VA |
3 I-VA |
10 I-VA |
|||||
| |
CTRLMean |
Mean |
SD |
Mean |
SD |
Mean |
SD |
| BASAL | 1 | 0.89 | 0.08 | 0.95 | 0.03 | 0.88 | 0.14 |
| ISO | 1b | 1.01 | 0.02 | 1.00 | 0.02 | 0.61* | 0.11 |
| 1 AA |
3 AA |
10 AA |
|||||
| |
CTRLMean |
Mean |
SD |
Mean |
SD |
Mean |
SD |
| BASAL | 1 | 0.69 | 0.26 | 0.64** | 0.07 | 0.22** | 0.12 |
| ISO | 1c | 0.84 | 0.11 | 0.62 | 0.21 | 0.05*** | 4E-4 |
Isobutyric acid potentiates insulin-stimulated glucose uptake
The effect BSCFAs as well as acetic acid on glucose uptake was studied in primary rat adipocytes. As shown in Table 4, isobutyric acid induced a small, but significant potentiation of both basal and insulin-stimulated glucose uptake. The highest concentration of isovaleric acid inhibited basal glucose uptake, while no effect on insulin-stimulated glucose uptake was observed, which was also the case for acetic acid.
Table 4.
Isobutyric acid potentiates insulin-stimulated glucose uptake in primary rat adipocytes. Glucose uptake was measured after 30 minutes of stimulation without (BASAL) or with 1 nM insulin (INS) in the absence (CTRL) or presence of 1, 3 and 10 mM isobutyric acid (I-BA), isovaleric acid (I-VA) and acetic acid (AA). The values for I-BA, I-VA or AA are related to respective CTRL (set to 1), either in a basal or an insulin-stimulated state. Mean ± SD (n = 6-7) were used and significance levels were accepted when *p < 0.05, **p < 0.01 and ***p < 0.001. a2.9-fold, b2.8-fold, c2.1-fold increase in insulin-stimulated glucose uptake compared to BASAL.
| 1 I-BA |
3 I-BA |
10 I-BA |
|||||
|---|---|---|---|---|---|---|---|
| CTRLMean | Mean | SD | Mean | SD | Mean | SD | |
| BASAL | 1 | 1.29* | 0.19 | 1.3** | 0.18 | 1.24* | 0.21 |
| INS | 1a | 1.24*** | 0.05 | 1.27*** | 0.1 | 1.25** | 0.11 |
| 1 I-VA |
3 I-VA |
10 I-VA |
|||||
| |
CTRLMean |
Mean |
SD |
Mean |
SD |
Mean |
SD |
| BASAL | 1 | 0.94 | 0.12 | 0.95 | 0.05 | 0.69** | 0.1 |
| INS | 1b | 1.02 | 0.03 | 0.74 | 0.2 | 1.01 | 0.12 |
| 1 AA |
3 AA |
10 AA |
|||||
| |
CTRLMean |
Mean |
SD |
Mean |
SD |
Mean |
SD |
| BASAL | 1 | 0.98 | 0.09 | 0.87 | 0.14 | 0.82** | 0.1 |
| INS | 1c | 0.98 | 0.06 | 1.06 | 0.11 | 1.14 | 0.21 |
BSCFAs and SCFAs inhibit insulin-induced phosphorylation of protein kinase B
To elucidate whether BSCFAs and SCFAs mediate their effect via PKB, a kinase involved in most acute insulin-mediated metabolic responses, we investigated their effect on PKB phosphorylation. The two regulatory sites, Thr308 and Ser473 in PKBα, are phosphorylated by PDK1 and the TORC2 complex, respectively, and are necessary for full activation of the kinase.41 Figure 4 and Table 5 depict that insulin-mediated phosphorylation of PKB at Thr308 and Ser473 was decreased in response to BSCFAs and SCFAs in rat and human adipocytes.
Figure 4.
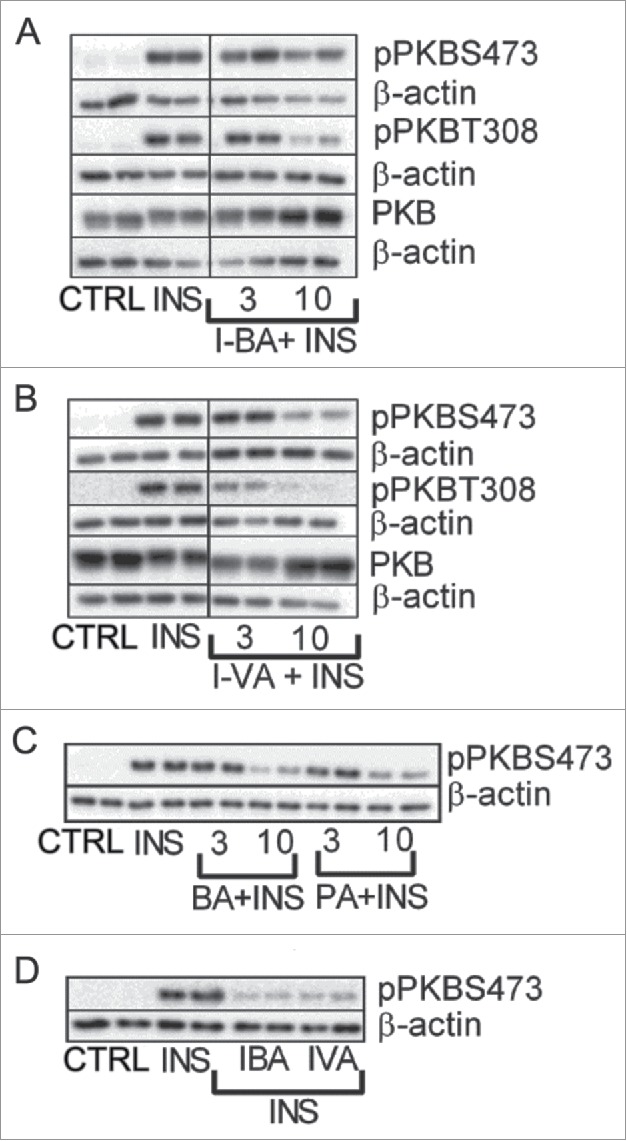
BSCFAs and SCFAs decrease phosphorylation of PKB in primary rat and human adipocytes. Primary rat or human adipocytes were stimulated with insulin (INS) in the absence (CTRL) or presence of 3 and 10 mM of SCFA or BCFA for 10 minutes. Homogenates were subjected to immunoblot analysis and membranes were probed with antibodies against PKB, PKB at serine 473 (S473) and PKB at threonine 308 (T308) as well as β-actin. For primary rat adipocytes, results (n = 3-6) are shown for isobutyric acid (I-BA) and isovaleric (I-VA) (A, B). For human adipocytes, results (n = 3–4) are shown for BA, PA, I- BA and I-VA (C, D). Representative blots are shown.
Table 5.
BSCFAs and SCFAs decrease phosphorylation of PKB in primary rat and human adipocytes. Rat adipocytes were stimulated without (CTRL) or with INS in the absence or presence of 3 and 10 mM isobutyric acid (I-BA) and isovaleric acid (I-VA) for 10 minutes (n = 3). Human adipocytes were stimulated without (CTRL) or with INS in the absence or presence of 10 mM I-BA or I-VA for 10 minutes (n = 3) or 3 mM and 10 mM propionic acid (PA) or butyric acid (BA) for 10 minutes (n = 2-3). Homogenates were subjected to immunoblot analysis and membranes were probed with antibodies against PKB and PKB at serine 473 (S473) as well as β-actin and quantification was made using Image Lab Software (Bio-Rad Laboratories). Data are presented as fold of isoproterenol (ISO).Mean ± SD were used and significance levels were accepted when *p < 0.05, **p < 0.01 and ***p < 0.001.
| PKBS473 in primary rat adipocytes1 | ||||||
|---|---|---|---|---|---|---|
| + INS |
||||||
| n = 3 | CTRL | INS | 10 I-BA | 10 I-VA | ||
| Mean | 0.09*** | 1 | 0.45** | 0.46** | ||
| SD | 0.07 | 0 | 0.14 | 0.14 | ||
| PKBS473 in human adipocytes2 | ||||||
| + INS |
||||||
| n = 3 |
CTRL |
INS |
10 I-BA |
10 I-VA |
||
| Mean | 0.04*** | 1 | 0.60** | 0.65* | ||
| SD | 0.04 | 0 | 0.10 | 0.22 | ||
| PKBS473 in human adipocytes2 | ||||||
| + INS |
||||||
| n = 2#-3 |
CTRL |
INS |
3 BA# |
10 BA |
3 PA# |
10 PA |
| Mean | 0.02*** | 1 | 0.84 | 0.30*** | 0.98 | 0.35*** |
| SD | 0.12 | 0 | 0.13 | 0.14 | 0.27 | 0.05 |
Materials and methods
Animal model
Sprague Dawley male rats, 36-41 d of age, purchased from Charles River Laboratories (Germany), were kept under standardized conditions in the animal house facilities at Lund University. The Committee of ethical animal research in Malmö and Lund has approved all experimental procedures (permission number: M245-12).
Human adipose tissue
Patients with scheduled reconstructive breast surgery, recruited by the surgeons at Skåne University Hospital, Sweden, were included in the study. Adipose tissue, removed from the abdominal subcutaneous region of the patients, reached the laboratory within one hour after surgery. The preparation of adipocytes was immediately initiated and usually 3 g of subcutaneous adipose tissue was used for an experiment. All experiments were approved by the Regional Ethics Committee in Lund, Sweden (Dnr 2013/298).
Adipocyte isolation
Epididymal white adipose tissue was isolated and digested in collagenase (Sigma Aldrich/C6885) for 30 minutes at 37°C with agitation and then washed as previously described.42 The total volume of packed cells (lipocrit) in the adipocyte suspension was determined as described by Fine et al.43 The adipocytes were resuspended and diluted to the final cell concentration (1-10%), depending on experimental setup.
Lipolysis
Lipolysis was measured as described by Dole et al.44 Aliquots (400 μl) of 5% (rat) or 10% (human) suspensions of adipocytes were prepared in Krebs Ringers HEPES (KRH) containing 25 mM HEPES (pH 7.4), 120 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, 1% BSA, 2 mM glucose, and 200 nM adenosine and incubated with or without 30 nM DL-Isoproterenol hydrochloride (ISO) (Sigma-Aldrich, St. Louis, MO, USA) in the presence or absence of 1, 3 and 10 mM isobutyric acid (Sigma-Aldrich), isovaleric acid (Merck, Kenilworth, NJ, USA) or acetic acid (Merck) at 37°C for 30 minutes in a shaking device (150 rpm). The cell-free medium was transferred to wells and buffer containing 50 mM glycine, 0.05% hydrazinehydrate, 1 mM MgCl2, 0.75 mg/ml ATP (Sigma-Aldrich), 0.375 mg/ml NAD (Roche, Pleasanton, CA, USA), 25 μg/ml glycerol-3-phosphate dehydrogenase (Roche) and 0.5 μg/ml glycerol kinase (Roche) was added. Lipolysis was measured at optical density of 340 as the release of glycerol into the cell-free medium.
De novo lipogenesis from radio labeled glucose
Lipogenesis was measured as described by Moody et al.45 Aliquots (700μl) of a 2% suspension of adipocytes were prepared in KRH with low glucose (containing 0.55 mM glucose and 3.5% BSA without adenosine) and 14 μl D-[6−3H (N)] glucose (22 μCi/ml) 0.81 Bq/ml (Perkin Elmer, Waltham, MA, USA) was added as substrate for lipogenesis. Cells were stimulated with 1 nM insulin (Novonordisk, Bagsvaerd, Denmark) in the presence or absence of 1, 3 and 10 mM isobutyric acid, isovaleric acid or acetic acid in a shaking device (100 rpm) at 37°C for 30 minutes. Incubations were stopped by addition of toluene-based scintillation liquid (VWR Chemicals, Radnor, PA, USA) containing 0.3 mg/ml 2.5-diphenyloxazole (PPO) (Sigma-Aldrich) and 5 mg/ml 1.4-bis-[4-methyl-5-phenyl-2-oxazolyl]benzene (POPOP) (Sigma-Aldrich) and the incorporation of 3H into cellular lipids in the non-water soluble phase was determined by scintillation counting.
Glucose uptake
Glucose uptake was measured as described by Foley et al.46 Aliquots (200 μl) of a 5% suspension of adipocytes were prepared in a glucose-free KRBH buffer containing 120 mM NaCl, 4 mM KH2OP4, 1 mM MgSO4, 0.75 mM CaCl2, 10 mM NaHCO3, 1% BSA and 30 mM HEPES (pH 7.4). Radiolabeled glucose [100 μl D-[14C (U)] (57.8 mCi/mmol) (2.1 GBq/mmol)] was added as substrate for glucose uptake. The adipocyte suspensions were incubated with or without 1 nM insulin in the presence or absence of 1, 3 and 10 mM isobutyric acid, isovaleric acid or acetic acid in a shaking device (100 rpm) at 37°C for 30 minutes. Adipocytes were separated from the medium by addition of 60 μl dinonylphthalate and then briefly centrifuged. Ultima Gold scintillation liquid (PerkinElmer, Waltham, MA, USA) was added to the cell suspensions and uptake of [14C] glucose was determined by scintillation counting.
SDS-PAGE and Immunoblot Analysis
Aliquots of a 10% suspension of adipocytes were used for 10 minute incubations with 1 nM insulin in the presence or absence of different concentrations of isobutyric acid, isovaleric acid, acetic acid, propionic acid and butyric acid. Cells were homogenized with a lysis buffer containing 50 mM Tris-HCl (pH 7.5), 1mM EGTA, 1 mM EDTA, 1% NP40, 1 mM Na- orthovanadate, 50 mM NaF, 5 mM Na-pyrophosphate, 0.27 M sucrose, Nonidet P 40 (Sigma- Aldrich) and Complete Protease Inhibitor (containing inhibitors for serine-, cysteine-, and metalloproteases as well as calpains) (Roche) and centrifuged at 13 600 × g for 15 minutes. The total protein amount was determined according to Bradford. Samples (15-30 μg) were supplemented with NuPAGE sample reducing agent (Novex, Grand Island, NY, USA) and subjected to SDS-PAGE. Proteins were electrotransferred to Amersham Hybond-C Extra nitrocellulose membranes (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) and the membranes were stained with Ponceau S (0.1% in 5% acetic acid) and then blocked with 10% milk in a buffer, consisting of 20 mM Tris-HCl, pH 7.6, 137 mM NaCl and 0.1% (v/w) Tween-20 for 60 min. Membranes with proteins were probed with antibodies for protein kinase B (PKB) (Cell Signaling Technology Inc., Danvers, MA, USA) , phospho-PKB (S473) (Cell Signaling) and phospho-PKB (T308) (Cell Signaling), phospho-HSL (S563) (Cell Signaling) and β-actin (Sigma Aldrich) and incubated overnight at 4°C. Proteins were detected using the chemiluminescent Super Signal West Pico Luminol/Enhancer solution (Thermo Fisher Scientific, Waltham, MA, USA) and the ChemiDoc XRS+ Imager (Bio-Rad Laboratories, Hercules, CA, USA.). Image Lab Software (Bio-Rad Laboratories) was used for quantification.
Statistics
Data are presented as mean ± SD from the indicated number of experiments. Statistically significant differences were analyzed using Student´s t-test and significance levels were accepted when *p < 0.05, **p < 0.01 and ***p < 0.001.
Discussion
The ability of colon-produced SCFAs and BSCFAs to act upon peripheral tissues like adipose tissue requires that a fraction of these molecules reaches the systemic circulation. Beyond the liver, the concentration of all 3 SCFAs (acetic acid > propionic acid > butyric acid) as well as isobutyric acid have been estimated to be in the micromolar range in the systemic circulation.4,21,47-50 Furthermore, using stable isotope technology, systemic availability of colonic-administered acetate, propionate and butyrate was shown to be 36%, 9% and 2%, respectively.51 In another study, a net uptake of the SCFAs by adipose tissue, measured as the arteriovenous difference across the tissue times the tissue could be demonstrated.50 In summary, SCFAs and BSCFAs are available in the systemic circulation albeit at lower concentration than used in the current study having isolated primary adipocytes as in vitro model.
The mechanisms whereby BSCFAs and SCFAs mediate their effects observed on biological responses and cellular signaling that have been reported herein, are still unclear. However, it has become increasingly evident that all macronutrients, carbohydrates, proteins and lipids, also play an important role in the regulation of energy metabolism as signaling molecules.52 Moreover, free fatty acid receptors (FFARs) are likely to play important roles in fatty acid- mediated regulation of energy metabolism.10 Specifically, FFAR2 and 3 have been linked to a number of effects exerted by SCFAs in adipocytes, for example, FFAR2 was shown to suppress lipolysis and stimulate adipogenesis as well as adipocyte differentiation whereas FFAR3 was shown to induce leptin expression.34-36,53 With regards to BSCFAs and their binding to FFARs, to our knowledge, less is known. In addition to acting as signaling molecules, BSCFAs and SCFAs could mediate metabolic effects in adipocytes by entering various metabolic routes as discussed below.
The finding that isobutyric and isovaleric acid inhibited isoproterenol-mediated lipolysis and decreased isoproterenol-induced phosphorylation of HSL, indicate a role for BSCFAs in the regulation of energy homeostasis by protecting against lipotoxicity, as has previously been reported for SCFAs.33-35 Importantly, the mechanism behind the anti-lipolytic effect of BSCFAs and SCFAs appears not to involve components utilized by insulin to mediate inhibition of lipolysis. Namely, BSCFAs and SCFAs induced inhibition of lipolysis in the presence of inhibitors for PI3 kinase and PDE3B, whereas the anti-lipolytic effect of insulin was inhibited as expected under those conditions. Furthermore, phosphorylation of PKB, a key kinase in mediating acute metabolic effects of insulin such as inhibition of lipolysis, was downregulated both at Ser473 and Thr308, 2 activity controlling phosphorylation sites.41 Having that in mind, it will be interesting to test the ability of BSCFAs and SCFAs to inhibit lipolysis in insulin-resistant adipocytes isolated from obese individuals. Obese adipose tissue often has dysregulated lipolysis, leading to excessive release of fatty acids and decreased insulin sensitivity.29-31 As shown in this paper, both BSCFAs and SCFAs reduce phosphorylation of HSL whereas significant inhibition of lipolysis was only obtained with SCFAs.
The finding that BSCFAs decrease the rate of de novo lipogenesis,54 measured as the incorporation of [3H]-labeled glucose into cellular lipids, is in agreement with previous results with non-branched SCFAs.33 Altogether, these findings indicate that BSCFAs and SCFAs facilitate storage of diet-derived lipids in adipocytes rather than performing de novo synthesis, which at least under conditions of fat over-consumption appears metabolically beneficial. Also, the effect to diminish de novo lipogenesis might lead to an increased β-oxidation due to reduced level of malonyl CoA, a metabolite known to inhibit the transport of fatty acids into the mitochondria.55 The inhibitory effect on PKB phosphorylation observed in response to the branched and non-branched SCFAs and the phosphorylation and inactivation of ACC33 might be molecular mechanisms for diminished de novo lipogenesis. Isovaleric acid-containing porpoise oil, given in the diet, has ameliorating effects on fatty liver in OLETF (Otsuka Long- Evans Tokushima Fatty) rat, a model for type 2 diabetes, by increasing serum levels of adiponectin and enhancing lipoprotein synthesis and secretion.56 However, isobutyric and isovaleric acid have been scarcely investigated with regard to effects on de novo lipogenesis.
The finding that isobutyric acid significantly increased both basal and insulin-stimulated glucose uptake is in agreement with recently obtained results for propionic acid and to some extent also for butyric acid in rodent primary and differentiated adipocytes.33 Phosphorylation of PKB is one key event in insulin-induced glucose uptake,41 however, phosphorylation of PKB was shown to be downregulated by both branched and non-branched SCFAs. In agreement with our findings on PKB, Kimura et al have observed a decreased insulin- mediated phosphorylation of PKB at Ser473 in response to acetic acid in adipocytes, an effect that was mediated by GPR43.36
In addition to acting as signaling molecules, BSCFAs and SCFAs could act as energy substrates in adipocytes by entering routes in lipid and carbohydrate metabolism as has been described in colonocytes and hepatocytes.6,9,21,57 In hepatocytes, acetate and butyrate have been described to be mostly introduced into lipid biosynthesis, whereas propionate will mainly be incorporated into gluconeogenesis.6 With regards to adipocytes, BSCFAs and SCFAs might enter the lipogenic route after uptake and activation by short chain CoA synthases.58-60 Thus, the inhibitory effect we observe on de novo lipogenesis from glucose could, at least to some extent, be explained by the ability of BSCFAs/SCFAs to generate “competing” non-labeled acetyl-CoAs for the synthesis of fatty acids. Likewise, it is possible that SCFAs and BSCFAs, by the generation of long chain acyl-CoAs, inhibit lipolysis by the inhibition of ATGL (triglyceride lipase) and/or HSL.61-63
To summarize, in the current investigation we have observed a number of effects in response to BSCFAs and SCFAs on lipid and glucose metabolism and signaling in adipocytes, possible mediated by FFAR 2 and 3. However, the possibility that the effects observed are mediated by BSCFAs and SCFAs acting as substrates by entering various routes in lipid and carbohydrate metabolism in adipocytes should not be excluded.
Abbreviations
- SCFA
short-chain fatty acid
- BSCFA
branched short-chain fatty acids
- BA
butyric acid
- PA
propionic acid
- I-BA
isobutyric acid
- I-VA
isovaleric acid
- PDE3B
phosphodiesterase 3B
- HSL
hormone sensitive lipase
- PKB
protein kinase B
- PI3 kinase
phosphatidyl inositol 3-kinase
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Eva Ohlson is acknowledged for excellent technical assistance. Dr. Jens Martin Larsson, Britt-Louise Boman, Maria Borg Jönsson, Dr. Henny Svensson and Dr. Carolin Freccero and co-workers at the Department of Plastic and Reconstructive Surgery in Malmö in Sweden are all gratefully acknowledged for the delivery of human adipose tissue.
Funding
The study was financially supported by the Antidiabetic Food Center (AFC), a VINNOVA VINN Excellence Center at Lund University, Royal Physiographic Society in Lund, the Swedish Society of Medicine and the Albert Påhlsson Foundation.
Author contributions
All authors contributed to the design of the study and manuscript writing. EH contributed to the conduct of the study and data collection, whereas all authors contributed with analysis as well as data interpretation.
References
- [1].Brownawell AM, Caers W, Gibson GR, Kendall CW, Lewis KD, Ringel Y, Slavin JL. Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. J Nutr 2012; 142(5):962-74. [DOI] [PubMed] [Google Scholar]
- [2].Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med 2013; 34(1):39-58; PMID:23159341; http://dx.doi.org/ 10.1016/j.mam.2012.11.001 [DOI] [PubMed] [Google Scholar]
- [3].Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem 2008; 19(2):71-84; PMID:17618108; http://dx.doi.org/ 10.1016/j.jnutbio.2007.02.009 [DOI] [PubMed] [Google Scholar]
- [4].Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016; 7(3):189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Woting A, Blaut M. The intestinal microbiotain metabolic disease. Nutrients 2016; 8(4):202; http://dx.doi.org/ 10.3390/nu8040202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N1. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Utzschneider KM, Kratz M, Damman CJ, Hullarg M. Mechanisms linking the Gut microbiome and glucose metabolism. J Clin Endocrinol Metab 2016; 101(4):1445-54; PMID:26938201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015; 11(10):577-91; PMID:26260141; http://dx.doi.org/ 10.1038/nrendo.2015.128 [DOI] [PubMed] [Google Scholar]
- [9].den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The roleof short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. JLipid Res 2013; 54(9):2325-40; PMID:23821742; http://dx.doi.org/ 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL Jr. Short chain fatty acids and their receptors: new metabolic targets. Transl Res 2013; 161(3):131-40; PMID:23146568; http://dx.doi.org/ 10.1016/j.trsl.2012.10.007 [DOI] [PubMed] [Google Scholar]
- [11].Nyangale EP, Mottram DS, Gibson GR. Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J Proteome Res 2012; 11(12):5573-85; http://dx.doi.org/ 10.1021/pr300637d [DOI] [PubMed] [Google Scholar]
- [12].Boillot J, Alamowitch C, Berger AM, Luo J, Bruzzo F, Bornet FR, Slama G. Effects of dietary propionate on hepatic glucose production, whole-body glucose utilization, carbohydrate and lipid metabolism in normal rats. Br J Nutr 1995; 73(2):241-51. [DOI] [PubMed] [Google Scholar]
- [13].Berggren AM, Nyman EM, Lundquist I, Bjorck IM. Influence of orally and rectally administered propionate on cholesterol and glucose metabolism in obese rats. Br J Nutr 1996; 76(2):287-94. [DOI] [PubMed] [Google Scholar]
- [14].Todesco T, Rao AV, Bosello O, Jenkins DJ. Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am J Clin Nutr 1991; 54(5):860-5. [DOI] [PubMed] [Google Scholar]
- [15].Wolever TM, Spadafora P, Eshuis H. Interaction between colonic acetate and propionate in humans. Am J Clin Nutr 1991; 53(3):681-7. [DOI] [PubMed] [Google Scholar]
- [16].Chen WJ, Anderson JW, Jennings D. Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats. Proc Soc Exp Biol Med 1984; 175(2):215-8; http://dx.doi.org/ 10.3181/00379727-175-41791 [DOI] [PubMed] [Google Scholar]
- [17].Laurent C, Simoneau C, Marks L, Braschi S, Champ M, Charbonnel B, Krempf M. Effect of acetate and propionate on fasting hepatic glucose production in humans. Eur J Clin Nutr 1995; 49(7):484-91. [PubMed] [Google Scholar]
- [18].Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009; 58(7):1509-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lin HV, Frassetto A, Kowalik EJ Jr., Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, et al.. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 2012; 7(4):e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, et al.. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015; 64(11):1744-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Al-Lahham S, Roelofsen H, Rezaee F, Weening D, Hoek A, Vonk R, Venema K. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur J Clin Invest 2012; 42(4):357-64. [DOI] [PubMed] [Google Scholar]
- [22].Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014; 10(12):723-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yao CK, Muir JG, Gibson PR. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther 2016; 43(2):181-96. [DOI] [PubMed] [Google Scholar]
- [24].Zarling EJ, Ruchim MA. Protein origin of the volatile fatty acids isobutyrate and isovalerate in human stool. J Lab Clin Med 1987; 109(5):566-70. [PubMed] [Google Scholar]
- [25].Jie Z, Bang-Yao L, Ming-Jie X, Hai-Wei L, Zu-Kang Z, Ting-Song W, Craig SA. Studies on the effects of polydextrose intake on physiologic functions in Chinese people. Am J Clin Nutr 2000; 72(6):1503-9. [DOI] [PubMed] [Google Scholar]
- [26].Nilsson A, Johansson E, Ekstrom L, Bjorck I. Effects of a brown beans evening meal on metabolic risk markers and appetite regulating hormones at a subsequent standardized breakfast: a randomized cross-over study. PLoS One 2013; 8(4):e59985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Gall W and Kahn CR. Dietary leucine–an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One 2011; 6(6):e21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011; 29:415-45; PMID:21219177 [DOI] [PubMed] [Google Scholar]
- [29].Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab 2014; 25(5):255-62; PMID:24731595 [DOI] [PubMed] [Google Scholar]
- [30].Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie 2016; 125:259-66; PMID:26542285 [DOI] [PubMed] [Google Scholar]
- [31].Lafontan M. Advances inadipose tissue metabolism. Int JObes(Lond) 2008; 32(Suppl 07):S39-51. [DOI] [PubMed] [Google Scholar]
- [32].Rumberger JM, Arch JR, Green A. Butyrate and other short-chain fatty acids increase the rate of lipolysis in 3T3-L1 adipocytes. PeerJ 2014; 2:e61; http://dx.doi.org/ 10.7717/peerj.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Heimann E, Nyman M, Degerman E. Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocyte 2015; 4(2):81-8; PMID:26167409; http://dx.doi.org/ 10.4161/21623945.2014.960694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, et al.. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005; 146(12):5092-9; PMID:16123168; http://dx.doi.org/ 10.1210/en.2005-0545 [DOI] [PubMed] [Google Scholar]
- [35].Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen JL, et al.. Activation of G protein- coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008; 149(9):4519-26; PMID:18499755; http://dx.doi.org/ 10.1210/en.2008-0059 [DOI] [PubMed] [Google Scholar]
- [36].Zaibi MS, Stocker CJ, O'Dowd J, Davies A, Bellahcene M, Cawthorne MA, Brown AJ, Smith DM, Arch JR. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett 2010; 584(11):2381-6; PMID:20399779; http://dx.doi.org/ 10.1016/j.febslet.2010.04.027 [DOI] [PubMed] [Google Scholar]
- [37].Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et al.. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 2013; 4:1829; PMID:23652017; http://dx.doi.org/ 10.1038/ncomms2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Aberdein N, Schweizer M, Ball D. Sodium acetate decreases phosphorylation of hormone sensitive lipase in isoproterenol-stimulated 3T3-L1 mature adipocytes. Adipocyte 2014; 3(2):121-5; PMID:24719785; http://dx.doi.org/ 10.4161/adip.27936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Degerman E, Ahmad F, Chung YW, Guirguis E, Omar B, Stenson L, Manganiello V. From PDE3B to the regulation of energy homeostasis. Curr Opin Pharmacol 2011; 11(6):676-82; PMID:22001403; http://dx.doi.org/ 10.1016/j.coph.2011.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Berggreen C, Gormand A, Omar B, Degerman E, Goransson O. Protein kinase B activity is required for the effects of insulin on lipid metabolism in adipocytes. Am J Physiol Endocrinol Metab 2009; 296(4):E635-46; PMID:19158325; http://dx.doi.org/ 10.1152/ajpendo.90596.2008 [DOI] [PubMed] [Google Scholar]
- [41].Toker A, Marmiroli S. Signaling specificity in the Akt pathway in biologyanddisease. AdvBiolRegul 2014;55:28-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Honnor RC, Dhillon GS, Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes.I. Cell preparation, manipulation, and predictabilityin behavior. J Biol Chem 1985; 260(28):15122-9; PMID:2415513 [PubMed] [Google Scholar]
- [43].Fine JB, DiGirolamo M. A simple method to predict cellular density in adipocyte metabolic incubations. Int J Obes Relat Metab Disord 1997; 21(9):764-8; PMID:9376888; http://dx.doi.org/ 10.1038/sj.ijo.0800469 [DOI] [PubMed] [Google Scholar]
- [44].Dole VP, Meinertz H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem 1960; 235:2595-9; PMID:13817286 [PubMed] [Google Scholar]
- [45].Moody AJ, Stan MA, Stan M, Gliemann J. A simple free fatcell bioassayfor insulin. HormMetabRes 1974; 6(1):12-6. [DOI] [PubMed] [Google Scholar]
- [46].Foley JE, Kashiwagi A, Verso MA, Reaven G, Andrews J. Improvement in in vitro insulin action after one month of insulin therapy in obese noninsulin-dependent diabetics. Measurements of glucose transport and metabolism, insulin binding, and lipolysis in isolated adipocytes. J Clin Invest 1983; 72(6):1901-9; PMID:6358258; http://dx.doi.org/ 10.1172/JCI111153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cummings JH1, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987; 28(10):1221-7; PMID:3678950; http://dx.doi.org/ 10.1136/gut.28.10.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Peters SG, Pomare EW, Fisher CA. Portal and peripheral blood short chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut 1992; 33(9):1249-52; http://dx.doi.org/ 10.1136/gut.33.9.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pomare EW, Branch WJ, Cummings JH. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J Clin Invest 1985; 75(5):1448-54; PMID:3998144; http://dx.doi.org/ 10.1172/JCI111847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 2005; 82(3):559-67; PMID:16155268 [DOI] [PubMed] [Google Scholar]
- [51].Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM, Annaert P, et al.. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol 2016. August 11. http://dx.doi.org/ 10.1113/JP272613[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism bylong-chain fattyacids. Prog LipidRes 2014; 53:124-44. [DOI] [PubMed] [Google Scholar]
- [53].Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein- coupled receptor GPR41. Proc Natl Acad Sci U S A 2004; 101(4):1045-50; PMID:14722361; http://dx.doi.org/ 10.1073/pnas.2637002100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Czech MP, Tencerova M, Pedersen DJ, Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 2013; 56(5):949-64; PMID:23443243; http://dx.doi.org/ 10.1007/s00125-013-2869-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta- oxidation as targets for treatment of the metabolic syndrome. Obes Rev 2010; 11:380-8; PMID:19694967; http://dx.doi.org/ 10.1111/j.1467-789X.2009.00642.x [DOI] [PubMed] [Google Scholar]
- [56].Shirouchi B, Nagao K, Furuya K, Nagai T, Ichioka K, Tokairin S, et al.. Physiological functions of iso-type short-chain fatty acid and omega 3 polyunsaturated fatty acids containing oil in obese OLETF rats. J Oleo Sci 2010;59(6):299-305; PMID:20484835 [DOI] [PubMed] [Google Scholar]
- [57].Astbury SM1, Corfe BM. Uptake and metabolism of the short-chain fatty acid butyrate, a critical review of the literature. Curr Drug Metab 2012; 13(6):815-21; PMID:22571479; http://dx.doi.org/ 10.2174/138920012800840428 [DOI] [PubMed] [Google Scholar]
- [58].Grevengoed TJ, Klett EL, Coleman RA. Acyl-CoA metabolism and partitioning. Annu Rev Nutr 2014; 34:1-30; PMID:24819326; http://dx.doi.org/ 10.1146/annurev-nutr-071813-105541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Garrido-Sánchez L, García-Fuentes E, Fernández-García D, Escoté X, Alcaide J, Perez-Martinez P, Vendrell J, Tinahones FJ. Zinc- Alpha 2-glycoprotein gene expression in adipose tissue is related with insulin resistance and lipolytic genes in morbidly obese patients. PLoS One 2012; 7(3):e33264; PMID:22442679; http://dx.doi.org/ 10.1371/journal.pone.0033264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nakamura Y, Sato T, Shiimura Y, Miura Y, Kojima M. FABP3 and brown adipocyte- characteristic mitochondrial fatty acid oxidation enzymes are induced in beige cells in a different pathway from UCP1. Biochem Biophys Res Commun 2013; 441(1):42-6; PMID:24129192; http://dx.doi.org/ 10.1016/j.bbrc.2013.10.014 [DOI] [PubMed] [Google Scholar]
- [61].Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytesby high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol 2010; 298(4):C961-71; PMID:20107043; http://dx.doi.org/ 10.1152/ajpcell.00547.2009 [DOI] [PubMed] [Google Scholar]
- [62].Smith AJ, Thompson BR, Sanders MA, Bernlohr DA. Interaction of the adipocyte fatty acid-binding protein with the hormone-sensitive lipase: regulationby fatty acids and phosphorylation. J Biol Chem 2007; 282(44):32424-32; PMID:17785468; http://dx.doi.org/ 10.1074/jbc.M703730200 [DOI] [PubMed] [Google Scholar]
- [63].Hu L, Deeney JT, Nolan CJ, Peyot ML, Ao A, Richard AM, Luc E, Faergeman NJ, Knudsen J, Guo W, Sorhede-Winzell M et al.. Regulation of lipolytic activityby long-chain acyl-coenzyme A in islets and adipocytes. Am J Physiol Endocrinol Metab 2005; 289(6):E1085-92; PMID:16091387; http://dx.doi.org/ 10.1152/ajpendo.00210.2005 [DOI] [PubMed] [Google Scholar]
- [64].Nagy HM, Paar M, Heier C, Moustafa T, Hofer P, Haemmerle G, Lass A, Zechner R, Oberer M, Zimmermann R. Adipose triglyceride lipase activity is inhibitedby long-chain acyl-coenzyme A. Biochim Biophys Acta 2014; 1841(4):588-94; PMID:24440819; http://dx.doi.org/ 10.1016/j.bbalip.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


