ABSTRACT
Microevolutionary mechanisms of resistance to a bacterial pathogen were explored in a population of the Greater wax moth, Galleria mellonella, selected for an 8.8-fold increased resistance against the entomopathogenic bacterium Bacillus thuringiensis (Bt) compared with a non-selected (suspectible) line. Defense strategies of the resistant and susceptible insect lines were compared to uncover mechanisms underpinning resistance, and the possible cost of those survival strategies. In the uninfected state, resistant insects exhibited enhanced basal expression of genes related to regeneration and amelioration of Bt toxin activity in the midgut. In addition, these insects also exhibited elevated activity of genes linked to inflammation/stress management and immune defense in the fat body. Following oral infection with Bt, the expression of these genes was further elevated in the fat body and midgut of both lines and to a greater extent some of them in resistant line than the susceptible line. This gene expression analysis reveals a pattern of resistance mechanisms targeted to sites damaged by Bt with the insect placing greater emphasis on tissue repair as revealed by elevated expression of these genes in both the fat body and midgut epithelium. Unlike the susceptible insects, Bt infection significantly reduced the diversity and richness (abundance) of the gut microbiota in the resistant insects. These observations suggest that the resistant line not only has a more intact midgut but is secreting antimicrobial factors into the gut lumen which not only mitigate Bt activity but also affects the viability of other gut bacteria. Remarkably the resistant line employs multifactorial adaptations for resistance to Bt without any detected negative trade off since the insects exhibited higher fecundity.
KeyWords: Bt, experimental evolution, immune response, insect, microevolution, resistance
Introduction
Both the pathogen and insect host are participants in a highly dynamic co-evolutionary arms race where the insect's defenses are continuously evolving to keep pace with the corresponding infection adaptations of the pathogen. The selective pressures driving these processes are strong and often require some form of trade off. For example, resistant and susceptible insects may differ in their color, development time and fecundity.1,2
Bacillus thuringiensis (Bt) is a widespread Gram positive bacterium that has been developed as a biopesticide to control insect pests attacking crops as well as disease vectors such as mosquitoes.3 Bt must be ingested in order to infect and kill its host. Bt virulence factors include enterotoxins, hemolysins, phospholipases and metalloproteases, which are transcribed in the vegetative cells and play an important role in the infection process.4 These factors are activated by the quorum-sensing system PlcR-PapR.5 The insecticidal activity of Bt is primarily due to proteinaceous crystal endotoxins (Cry), which are produced during sporulation and activated by the host's gut fluids.6 Cry toxins can act alone (as seen in genetically modified plants) but spores can also contribute to virulence.7 The binding of toxins to receptors in the midgut epithelial cell membrane either creates pores that subsequently lead to cell lysis, or they activate intracellular signaling pathways that result in cell death by oncosis.8,9
There are increasing reports of resistance in insect populations to Bt; this is particularly evident with crops genetically modified with the Cry toxin genes.10,11 The mechanisms of resistance to Bt endotoxins has been studied extensively and appears to be multifaceted.6 Even in those cases that seem to fit a monogenic model, resistance is rarely completely recessive, suggesting that resistant phenotypes contain major and minor genes contributing to overall resistance.12 This fact is particularly relevant where virulence factors such as the bacterial spore play a vital role in the overall toxicity of Bt -based insecticides in which case development of resistance is likely to be multigenic. Indeed, disparate mechanisms for resistance to Bt have been reported. The most commonly reported mechanism involves reduced binding of the toxins through the alteration or loss of midgut toxin-binding proteins.13-15 Other insect resistance mechanisms include sequestration of the toxin by lipophorin,16,17 esterases18 or alkaline phosphatase,19 absence of enzymes or environment to activate pro-toxin,20 and increased stem cell production in the gut to replace damaged epithelial cells.21 The insect gut biota can also influence Bt efficacy either by degrading the toxin or initiating septicaemia.22,23 Resistance to Bt is also linked to the host's immune response, but the role of the different defense components is often inconclusive, contradictory or variable. For example, some researchers report a correlation between phenoloxidase (PO) activity and Bt efficacy,24 whereas others noted no differences between Bt-resistant and Bt-susceptible insects.25 Futhermore, no differences were noted for haemocyte populations and nitric oxide levels.26 Bt mediated suppression of key immune components will increase the host's susceptibility to Bt infections and exacerbate secondary infections by opportunistic pathogens.27-30
This paper focuses on an artificial selection experiment designed to explore the evolution of resistance of Greater wax moth Galleria mellonella to natural peroral infections by Bt. The goal was to identify traits in the selected insects that could account for their increased resistance when challenged with a Bt spore-crystal mixture, and to assess any corresponding “trade-offs.” Since Bt resistance is multifaceted, the current study examined specific parameters: humoral immunity, stress management, resource re-allocation and changes to the gut microbiome in selected and non-selected lines.
Results
Selection with B. thuringiensis leads to enhanced resistance of wax moth
Wax moth, G. mellonella, were selected for resistance to B. thuringiensis over 20 generations, but the first indication of increased resistance to Bt was observed after 5 generations when larval survival was significantly higher (p < 0.05) for the selected resistant (R) line than for the non-selected susceptible (S) control line (Fig. 1). By the 20th generation resistance was observed at 3 different Bt concentrations tested, and was most striking at the highest dose where mortality was <40% for the R line fourth instar larvae compared with 100% for the S line fourth instar larvae (Fig. 2). At the 20th generation, the resistance ratio (RR) to Bt of R line larvae relative to the S line was 8.8. In a separate study using a cohort of 18th generation R line insects, no reversal of resistance was observed in 3 successive generations reared on a Bt-free diet (Fig. 3).
Figure 1.
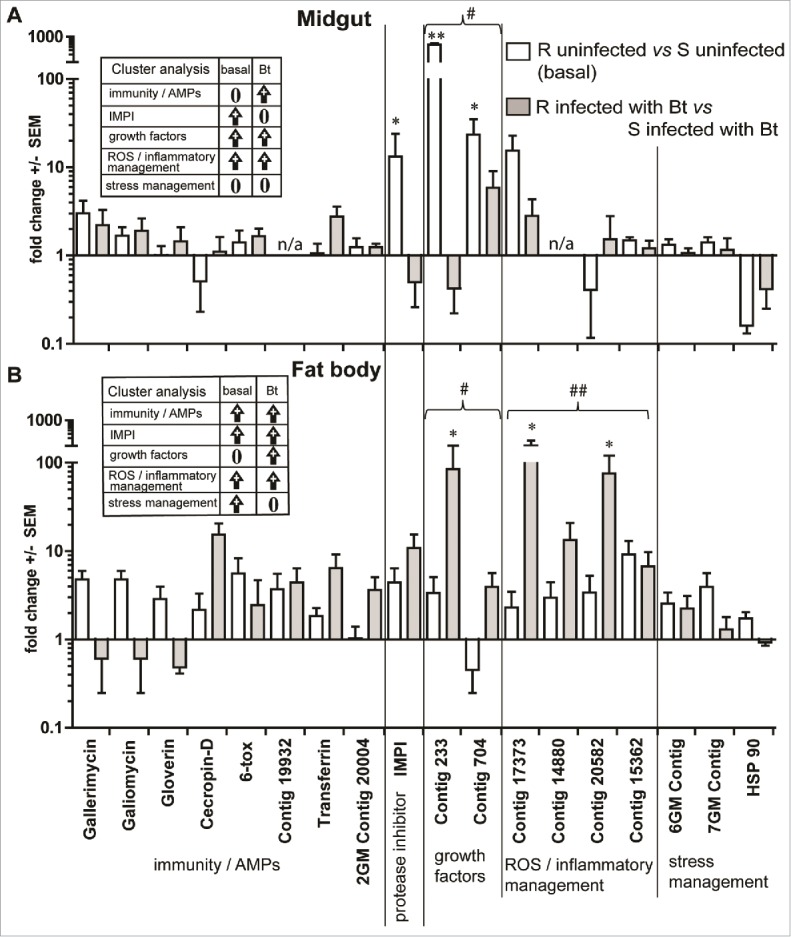
Basal (uninfected) and Bt induced (48 pi) expression of defense genes in the midgut (A) and fat body (B) of G. mellonella larvae. Expression of antimicrobial peptide genes and other immunity /stress-management genes in the fat body and midgut of resistant (R) and susceptible (S) fourth instar larvae. Expression was assessed under basal (uninfected) conditions and Bt-treated (infected) conditions 48 h post-infection. The y-axis represents basal expression in uninfected/infected R larvae as a fold change relative to S uninfected/infected larvae. Na = not assayed in midgut tissue; = *p < 0.05; = **p < 0.01; = ***p < 0.001 significant change in fold expression compared with S larvae; #-p < 0.05, ## = p < 0.01 show significant changes in expression of genes grouped in functional clusters in R vs S insects under Bt infection compared with uninfected R vs S. Data presented as mean ±SE and analyzed by one-way ANOVA (Kruskall-Wallis with Dunn's post test). Tables (cluster analysis) present trends in expression of defense genes grouped in clusters (arrow indicates significant upregulation, fold change cutoff ≥2 .0). Additional information is presented in Table S1.
Figure 2.
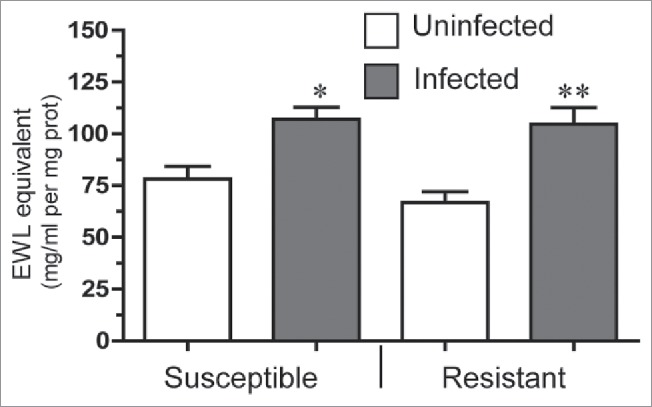
Lysozyme activity in infected and uninfected R and S line larvae. Lysozyme-like activity in midgut of fourth instar larvae from both susceptible and resistant wax moth lines 48 h following ingestion with Bt (data presented as mean +/−SEM; *P < 0.05, **P < 0.01, compared with uninfected larvae from the same line).
Figure 3.
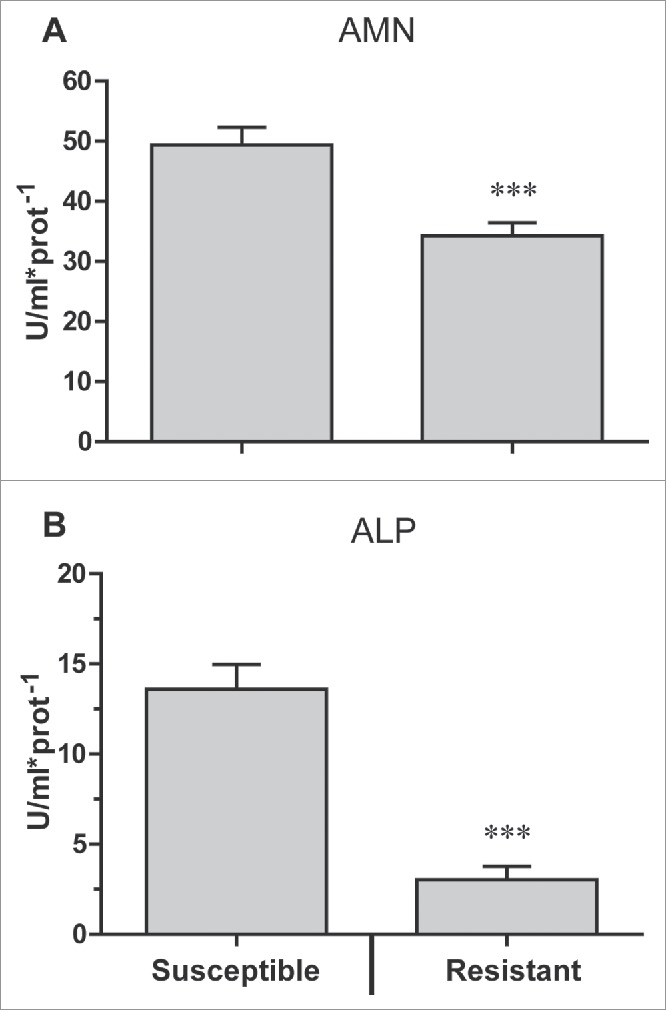
Midgut receptors of uninfected R and S line larvae Aminopeptidase-N (AMN) (A) and alkaline phosphatase (ALP) (B) activity in the midgut of fourth instar uninfected larvae from both the susceptible and resistant lines (*** p < 0.001 compared with susceptible).
High basal (uninfected) expression of immunity/stress-related genes in resistant insects
The expression of 15 immunity, stress and inflammatory management genes, inducible metalloproteases inhibitor (IMPI) and 2 growth factor genes was measured in the midgut and fat body of uninfected control insects of the 20th generation R and S lines. Several important trends were observed. The most notable differences in gene expression between the R and S lines arose in the midgut. Expression of IMPI, and the growth factors Contig 703 and Contig 233 was significantly higher in the midgut of R larvae compared with S larvae (13 (p < 0.05), 23 (p < 0.05) and 489 (p < 0.001) fold higher, respectively) (Fig. 1A). Also notable was the comparatively lower expression of HSP90 in the midgut of the R line compared with the S line larvae (Fig. 2A). Relative to the S line, the basal expression of most of the other immune, inflammatory and stress management genes in R larvae was slightly higher in the midgut of R line larvae (2-fold change) (Fig. 1A, Table S1). When the genes in fat body of R line insects are examined as functional clusters there is a trend toward increased expression of AMPs, IMPI, stress and inflammation management genes compared with the S larvae (3-6-fold change) (Fig. 1B, Table S1). Furthermore, in comparing the midgut with the fat body, the R line expression of growth factors was significantly higher (p < 0.01) but AMPs / immunity and stress management significantly lower (p < 0.05) (SI Fig. 4).
Enhanced expression of immunity/stress-related genes in infected resistant insects
Tissue-specific differences in gene expression were noted for both the R and S lines following infection (Fig. 1). Whereas expression of most genes increased relative to basal expression, particularly in the fat body, others appeared unchanged and a few were downregulated (Fig. 1). Genes coding for growth factors, ROS and inflammation management, which were already highly expressed in the fat body of uninfected R insects, were further elevated following infection with Bt (10–80 fold p < 0.05 and 5–100 fold p < 0.01, respectively; Fig. 1B; Fig. 5). Although Bt infection stimulates upregulation of immune genes in both lines (Fig. 5), the critical difference separating these lines is that immune gene expression is of a higher magnitude in the R line before infection and for the majority after infection (Fig. 1); this mirrors the pattern of expression observed for all other genes examined (Fig. 1, Fig. 5). Susceptible insects do show an increase in expression of growth factor genes (particularly Contig 233; 364 fold following infection; Fig. 5), but this is overshadowed by the significantly higher expression in the R insects, which even under basal conditions was 489 fold higher than the S insects (Fig. 1A). Similarly, infection triggered increased expression of IMPI in the midgut of both R and S lines (10 and 70 fold, respectively) but basal (uninfected) expression was higher in R larvae (Fig. 1A, Fig. 5).
Figure 5.
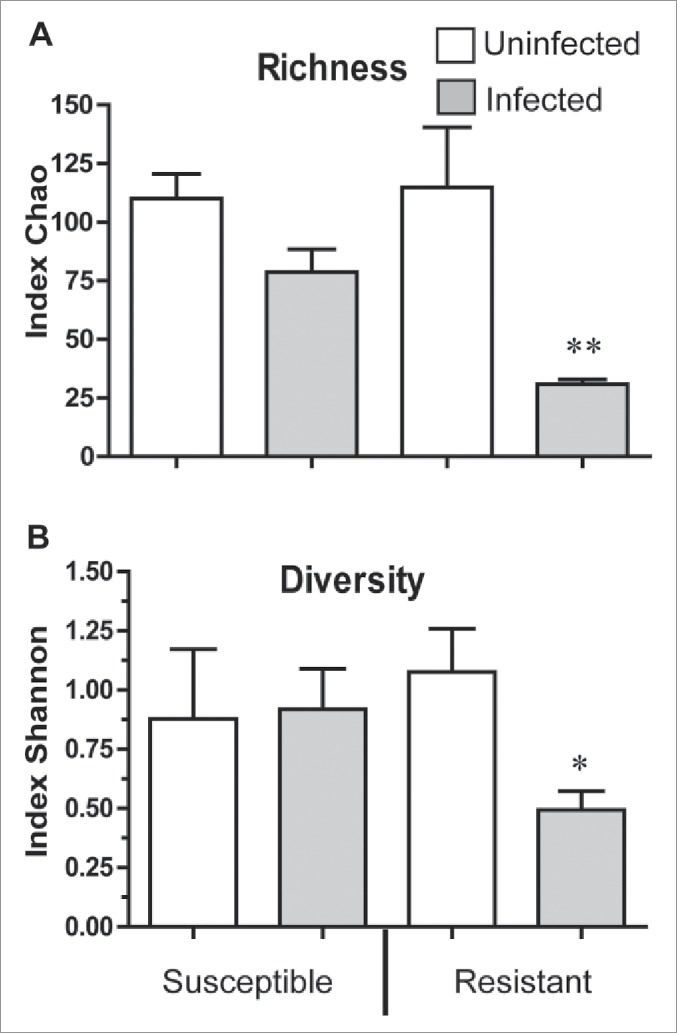
Richness and diversity of bacterial communities in infected and uninfected R and S lines (A) Chao community quantitative index reflecting richness (i.e. different bacterial phylotypes) in a dataset. (B) Shannon index reflecting diversity of bacterial communities for resistant and susceptible lines following infection with Bt (**p < 0.01, compared with other variants; *p < 0.05 compared with same non-infected line). This index quantifies how evenly the basic entities (such as phylotypes) are distributed. To prevent bias due to sampling depth, all samples were first rarefied (randomly standardized) to 3 700 sequences per sample.
Lysozyme activity in midgut elevated under Bt treatment in R and S lines
Lysozyme activity was elevated 1.5 times in the midgut of infected R (p < 0.05) and S (p < 0.01) lines compared with uninfected larvae from the same lines 48 hrs post infection (Fig. 2), however, there was no statistical difference in the level of activity of R and S line insects in either the basal or infected state (Fig. 2).
Alkaline phosphatase (ALP) and aminopeptidase N (AMN) activity is lower in Bt resistant lines
ALP and AMN activity in the brush border membrane of uninfected R line insects were ca. 82% and 31% lower than those of the S larvae, respectively (p < 0.001, Fig. 3).
Midgut bacterial community changes following Bt infection
Taxonomic classification based on 16 S rRNA gene sequencing of bacteria in the midgut of S and R line larvae revealed that bacterial communities were dominated by only a few phyla, with over 99.5% of the community being represented by 4 phyla (average relative abundance values averaged across all uninfected larvae): Firmicutes (80.7 ± 6.3%), Proteobacteria (11.8 ± 4.5%), Actinobacteria (3.9 ± 1.6) and Bacteroidetes (3.1 ± 1.1) (Fig. 4A).
Figure 4.
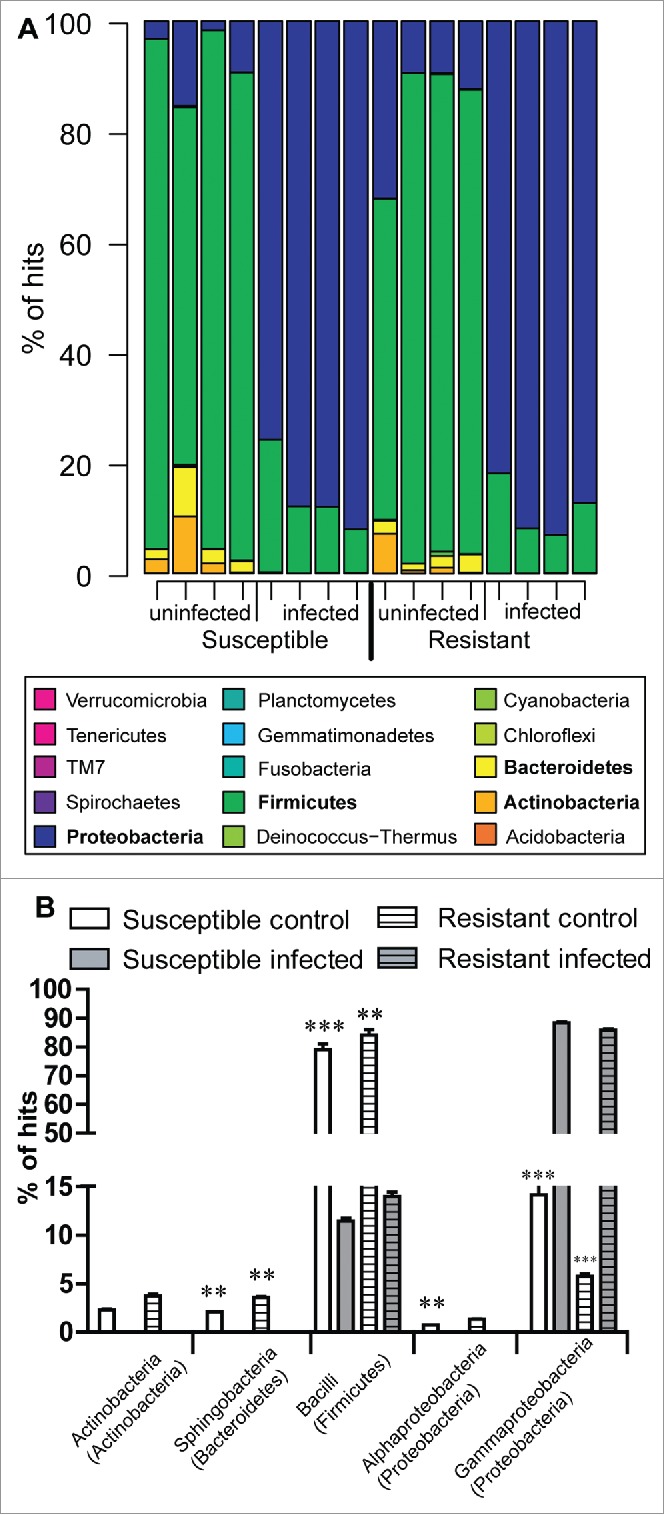
Gut biota profiles in Bt infected and uninfected R and S line larvae. Profile of the bacterial community in midguts from fourth instar larvae from both resistant and susceptible lines on the second day post Bt infection. Values are averaged across 4 independent control (uninfected) and 4 infected samples of each line. (A) Bacteria classified by phylum and (B) Comparison of community, classified by class, from infected and uninfected R and S line larvae (p < 0.01, p < 0.001 compared with infected insects from the corresponding line).
Infection of both lines with Bt led to a shift in dominance from the Firmicutes (80.7 ± 6.3%) to the Proteobacteria (86.3 ± 2.6%) (p < 0.001) (Fig. 4A, 4B). Uninfected R line had significantly more Enterobacter than the S larvae, however, upon infection with Bt the levels were both much elevated but to the same degree (SI, Fig. 6). Pseudomonas was present at similar but low levels in uninfected R and S larvae, but Bt infection resulted in opposite effects on the 2 lines. In the case of the R line no Pseudomonas was detected, while there was an increase in the S line relative to the uninfected insects (p < 0.05, Fig. 6). Phenomena common to both lines were the disappearance of several genera (e.g. Micrococcineae) post-infection and a huge shift in dominance from Enterococcus (Gram +ve) in uninfected to Enterobacter (Gram −ve) in infected insects (Fig. 4; Fig. 6). No Bacillaceae were detected in uninfected R and S lines but small amounts (2–3%) were detected post-infection (Fig. 6). Most striking was the significant reduction in richness and diversity of bacterial communities in the midgut of the infected R line, because such changes were not observed in the S line (Fig. 5). In the infected R line, there was a significant (p < 0.01) depletion of the community quantitative index (richness) i.e. there was a decrease in the number of detectable bacterial phylotypes (Fig. 5A). Similarly, the Shannon (diversity) index revealed a significant decline in abundance and species evenness of each phylotype in the infected R line (p < 0.05, Fig. 5B).
Figure 6.
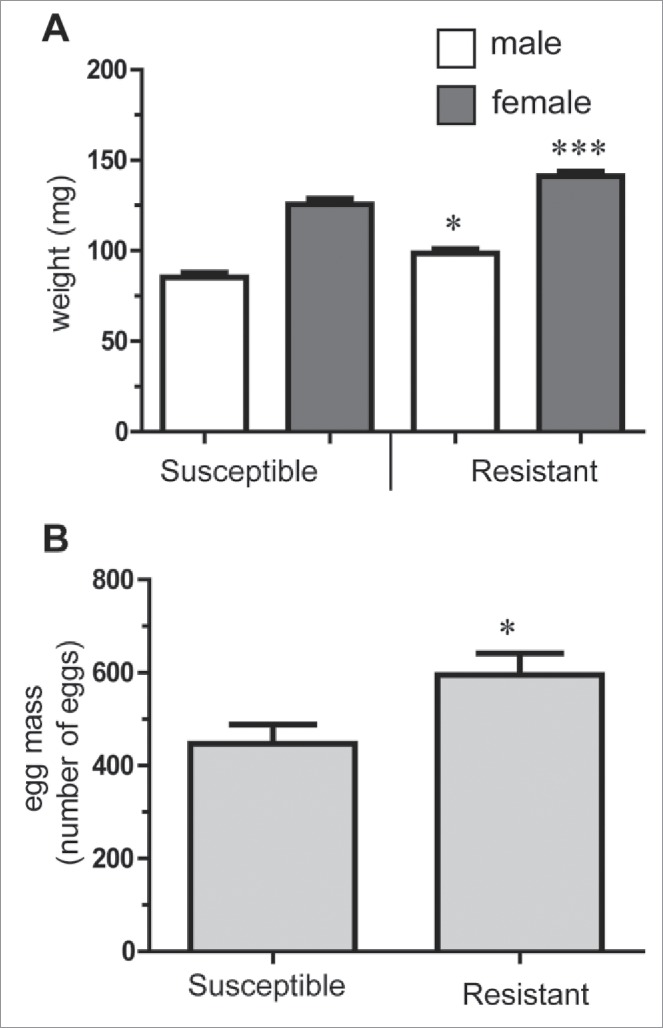
Increased fecundity: a positive trade-off in wax moth resistant to Bt Life-history traits in uninfected susceptible and resistant lines of 20th generation wax moth. (A) Pupal weights and (B) adult fecundity as measured by mean egg production over 5 d per female (*p < 0.05, *** p < 0.001 compared with susceptible line).
Life history traits of R and S line insects
There was no difference in survival rate between uninfected R and S insects. Interestingly, uninfected R line insects had significantly greater pupal biomass for both males (15%) and females (18%) compared with S line insects (both p < 0.05) (Fig. 6A). Adult fecundity was also significantly enhanced (up to 25%) with the average R moth laying more eggs than the S counterpart (p < 0.001) (Fig. 6B).
Discussion
This study shows that laboratory populations of wax moth larvae developed resistance to Bt in a relatively short time, and that this was retained even after removal of the selective pressure. The resistant (R) line implemented several complementary strategies, maintained even in the uninfected state, but which could be further activated upon infection. These included cellular repair, antimicrobial activity, limiting Cry toxin and toxin receptor sites, mitigating inflammation and stress. Besides midgut repair and reduced receptor sites, which are well known mechanisms, this study is the first to implicate the possible role of antimicrobial peptides (AMPs) and inflammation/stress management in evolution of resistance to Bt, and to demonstrate the importance of their elevated, constitutive activity. In addition, it reports an unusual positive trade-off resulting in increased fecundity of R line insects.
It can be hypothesized that elevated basal expression of defense and repair genes enables the R line to pre-empt infection or rapidly mitigate the damage caused by Bt. This rarely reported phenomenon was also described as a strategy for resistance to entomopathogenic fungi in melanic wax moth larvae.31,32 It appears that insects resistant to pathogens also adapt their response according to the pathogen's route of entry. Thus, the focal point of fungus-resistant melanic wax moth larvae is the integument31 whereas in the current study, the foci are the gut and fat body. It is likely that resistant insects balance energy allocation between the midgut and fat body defenses. Activity in the midgut appears to be directed toward repair and limiting toxin damage, while additional support is provided by the fat body in secreting AMPs that could combat microbial breaches of the midgut barrier, thereby preventing septicaemia. Elevated expression of AMPs was also observed in Spodoptera exigua larvae in response to Bt Cry and Vip toxins, however, the study was limited to local midgut responses in a susceptible line.33 In the current study, it is unclear if the fat body is responding to signals generated by and transmitted by the injured midgut and/or direct exposure to bacteria that subsequently breach the gut barrier. Systemic immune responses are well documented in other insects following exposure to ingested bacteria or topical infections by fungal pathogens.34 The present study shows that not only is the R line much more responsive than the S line but its expression profile, especially that of AMPs, is different and deserves further investigation. Moreover, it also highlights the importance of the contribution of midgut immunity to larval resistance to Bt. Lysozyme was induced by both R and S lines and appears to be a generic response in most insects to injury, infection or stress.35 Lysozyme is, therefore, not a reliable indicator of insect resistance to Bt.
Central to Bt pathogenicity is activation of Cry proteins, of which the earliest stages are mediated by the host proteases and bacterial metalloproteases.36 Here the R larvae had enhanced basal expression of an inducible metalloprotease inhibitor (IMPI), with its expression increasing during Bt infection in both R and S larvae. Thus, R line insects would be in the position to limit proteolysis of the protoxins to active Cry toxins and subsequent damage to the midgut, whereas the S line would first have to synthesize IMPI and this delay could profoundly influence their survival. Moreover, IMPI could inactivate the Bt zinc immune inhibitor metalloproteases (e.g., InhA), which are known to digest the hosts AMPs.37 Elevated IMPI is complemented in the R line by reduced Cry toxin binding receptors (alkaline phosphatase and aminopeptidase N) and strong inflammation and repair responses. Together these activities could contribute to damage limitation by Bt toxins.
Bt toxins can disrupt the redox-regeneration balance in insects.38 In the current study, the patterns of gene expression suggest that R line insects have the capacity to ameliorate oxidative/inflammation damage caused by later stages of Bt infection i.e., invasion of the gut epithelium. Consequently, the greatest upregulation of oxidative/inflammation genes is in post infection R line insects. In contrast, S line insects are incapable of mounting a similar response. Although differences were observed in the expression of stress management genes in R and S lines, suggesting a role for these genes in resistance, it was unclear exactly how they mitigated Bt damage. Interestingly, the constitutive expression of growth factor genes was higher in R than S lines but elevated upon infection with Bt, which corroborates the findings of others that repair of the midgut epithelium was one of the mechanisms insects resisted Bt.21,39
The gut of the infected R line appears to offer a hostile environment to microbes as reflected in the Shannon index, which is an indicator of richness and diversity. This would have significant benefit by reducing the risk of secondary infections and septicaemia. The latter is one mechanism by which Bt successfully kill and colonise their hosts.4,40,41 The exact mechanisms altering the gut environment have not been identified but may include changes in pH, secretion of AMPs into the gut lumen, and removal of antagonistic microbes. There are minor fluctuations in the representation of certain bacterial groups which are specific for the R line e.g. complete loss of Pseudomonas in Bt infected R insects. The pathological significance of these changes is hard to determine without further investigation of the role of the specific bacteria involved.
A striking feature of the R line was their larger pupal mass and higher fecundity than the S line. This positive trade-off is a rare and unusual phenomenon since most micro-evolutionary trade-offs are negative, such as small size and reduced fecundity, which compensate for beneficial traits such as increased resistance to pathogens or insecticides.25,31,42 The success of the R line may partly be linked to contig 233, a growth-blocking peptide, that not only controls cell proliferation and blocks juvenile hormone (JH) esterase activity,18 but may also elevate immune responses.43 Thus, contig 233 would not only prevent the onset of metamorphosis from larva to pupa but also influence body size. Contig 233 has high constitutive expression in the R line relative to the S line but after infection expression is highly elevated in the fat body, which presumably allows the insect to retain juvenility until it has attained sufficient body mass or reserves to progress to the next development stage.
Materials and methods
Insects
For artificial selection we used insects from a laboratory population of the Greater wax moth, Galleria mellonella, from the Institute of Systematics and Ecology of Animals (ISEA), Siberian Branch Russian Academy of Sciences. The starting population was separated into 2 lines the first was exposed to B. thuringiensis (Bt), and selected for increased resistance to the pathogen (R line) while the second consisted of the untreated susceptible control (S line). The 20th generation R and S insects were compared to elucidate the resistance mechanism(s) to Bt. Also a group of 400 larvae from the 18th generation R line was reared over 3 generations without Bt (non-selected, NS line) to determine if resistance was reversible. The resistance ratio was calculated based on the LC50 of R and S lines. Fourth instar larvae have been used in all experiments. Full details of insect rearing and selection are provided in the Supplementary Information Experimental Procedures.
Bacterial infection
The insect pathogen, Bacillus thuringiensis ssp. galleria, H-serotype V, strain 69–6 was provided by the ISEA bacterial collection. Insects from the 20th generation were Bt naïve until initiation of the experiments whereupon the susceptibility of R and S lines to Bt was determined by natural peroral application of a spore-crystal mixture. To quantify the differential susceptibility of the R and S lines, a cohort of fourth instar larvae were starved for 2 h before being exposed to different doses of Bt. The R and S larvae received predetermined sub-lethal, half-lethal, and lethal doses corresponding to 5 × 108, 1 × 109 and 5×109 per ml which result in 15%, 50% and 100% mortality of S larvae within 5 days, respectively. To determine the resistance ratio (RR) of 20th generation S and R line larvae, the LC50 of R line was divided by the LC50 of the S line. In a parallel study, infected fourth instar insects from both lines were collected 48 h post-exposure to Bt to: (1) determine the bacterial content of the midgut (n = 20 larvae per treatment), (2) quantify genes expression in the midgut and fat body (n = 9 larvae per treatment) and (3) determine haemolymph lysozyme activity (n = 40 larvae per treatment) in control and half-lethal treatments. Experiments were carried out in triplicate. Full details of bacterial culture and inoculation methods are provided in the supplemental material online.
QRT-PCR analysis of insect immunity-related gene expression
To identify resistance factors, a comparison was made in the R and S larvae of the expression of genes operational under basal conditions (uninfected) and during Bt infection in both fat body and midgut samples. Eighteen genes previously detected as part of immune response, repair, regeneration and stress regulation in wax moth were investigated.31,32 These were the genes coding for the antimicrobial peptides gallerimycin, galiomicin, gloverin, cecropin D and 6-tox, the siderophore transferrin, the insect metalloproteinase inhibitor (IMPI), 3 coding for heat-shock proteins (HSP-90, contig 21310 and 1489) whose activities ameliorate stress,44,45 4 coding for enzymes dealing with oxidative stress (Contigs 17373, 14880, 20582 and 15362), and 2 involved with cell proliferation (Contigs 704 and 233). Gene expression was measured by real-time quantitative RT-PCR using normalized cDNA samples with the Rotor-Gene 6000 (Corbett Research), with Rotor-Gene SYBR Green PCR mix (Qiagen), relative to 2 reference genes, 18 S rRNA (AF286298) and Elongation Factor 1-α (EF1; AF423811). Full details are provided in Table S2.
Midgut lysozyme-like activity
Antibacterial activity in midgut was determined by a zone-of-clearance assay using freeze-dried Micrococcus lysodeikticus as a substrate suspended in agarose. The radius of the digested zone was compared with a standard curve made with egg white lysozyme (EWL) and expressed as an EWL equivalent per mg of protein in the samples.46 The experiment was repeated independently 3 times. Full details are provided in the Supplementary Information Experimental Procedures.
Quantification of alkaline phosphatase (ALP) and aminopeptidase-N (APN) activities
Brush border membrane vesicles (BBMV) were prepared by Mg2+ precipitation. Specific alkaline phosphatase (ALP) and N-aminopeptidase (APN) enzymatic activities of BBMV proteins were measured using p-nitrophenyl phosphate disodium (pNPP) and leucine-p-nitroanilide (Sigma, St. Louis, MO, USA) respectively. One enzymatic unit was defined as the amount of enzyme that would hydrolyze 1.0 µmole of substrate to chromogenic product per min per mg of protein at the specific reaction pH and temperature. Sixty larvae were examined for each enzyme per insect line. Midguts from 3 insects were pooled in one sample. Data are presented as the mean specific activities from 20 independent BBMV samples. The experiment was repeated independently 3 times. Full details are provided in the Supplementary Information Experimental Procedures.
16 S rRNA bacterial diversity analysis
The bacterial community in the midgut of Bt infected (48 hrs post exposure) and uninfected R and S larvae was analyzed by 16 S pyrosequencing with a MiSeq Illumina sequencer. Midguts with intact contents were frozen in liquid nitrogen before being homogenized using a pestle and mortar. DNA was extracted from midguts using the MoBIO PowerSoil-htp 96 Well DNA Isolation kit (Carlsbad, California). Each sample was amplified with bacterial 16 S rRNA gene primers that amplify the V3-V4 region. The experiment was repeated independently 4 times. The mean number of analyzed sequences for each variant was 10701 sequences (min 3730, max 24303) for the non-infected S line, 21027 sequences (min 9047, max 48050) for the S line infected with Bt, 12670 sequences (min 4267, max 33005) for the non-infected R line, and 16902 sequences (min 8508, max 29322) for the R line infected with Bt. Profile of the bacterial community and comparison were made with CloVR-16 S version 1.0 package.47
Additional processing of sequence data was performed using the “Rarefied” datasets (with equivalent sampling depths) generated in QIIME by randomly subsampling 3700 (high quality, chimera-free) sequences from each sample. The Shannon diversity index and Chao1 richness estimates were calculated for “Rarefied” data sets with CLoVR. Diversity metrics computed for OTUs for each sample. Full details are provided in the supplemental material online.
Life history traits
The following life history traits were monitored in the 20th generation uninfected R and S insects: survival rate of insects over a period of whole ontogenesis (300 individuals per line), pupal weight (200 individuals per line) and adult fecundity (mean fertile egg production over 5 d per female) with 30 pairs per line. Full details are provided in the supplemental material online.
Data analyses
Data was analyzed using GraphPad Prism v4.0 (GraphPad Software Inc., USA) and Statistic v6.0 (StatSoft Inc., USA). Data were checked for normal (Gaussian) distribution using the Agostino-Pearson omnibus test, and if abnormally distributed a more conservative non-parametric analysis was applied. In Q-RT-PCR data with a Gaussian distribution, Grubbs' extreme studentized deviate (ESD) test was used to exclude extreme outliers. In order to assess overall trends associated with selection for Bt resistance in basal and induced gene expression, the data from 3 independently repeated experiments were pooled for different gene clusters: immunity / AMPs (Gallerimycin, Galiomycin, Gloverin, Cecropin-D, 6-tox, Contig 19932, Transferrin, 2 GM Contig 20004), IMPI, growth factors (Contig 233, Contig 704), ROS / inflammatory management (Contig 17373, Contig 14880, Contig 20582, Contig 15362), and stress management (6 GM Contig, 7 GM Contig, HSP 90). Individual, clustered gene and bacterial diversity (Chao and Shannon) comparisons were made with t-test and non-parametric one-way ANOVA (Kruskall-Wallis with Dunn's post test) respectively. Cox's proportional hazards survival regression was used to quantify differences in mortality rates after bacterial infections between R and S larvae. No mortality was recorded for uninfected control larvae in dose mortality studies, therefore, it was unnecessary to compare R and S controls. One-way ANOVA (with Tukey's post test) was used to assess differences between lysozyme responses, and life history traits in R and S insects. Differences between R and S larvae, or between treated and control samples, were considered significant when P < 0.05. DNA sequence data from gut bacterial communities (profiles of the microbiota) were analyzed using CLoVR (metastats).47
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The authors gratefully acknowledge funding from the RFBR (Grant Number 15-34-20488 mol_a_ved (IMD), 14-04-31507 mol_a (EVG), and D.F.G Priority Program 1399 ‘Host–parasite-coevolution—rapid reciprocal adaptation and its genetic basis’, and the Hessian Ministry for Science and Art via the LOEWE research focus Insect Biotechnology VI 219/3-1 (AV). VVG gratefully acknowledge funding from the Russian Science Foundation (project №15-14-10014) for support in study of bacterial community. TMB was supported by a grant funded jointly by the Biotechnology and Biological Sciences Research Council, the Department for Environment, Food and Rural affairs, the Economic and Social Research Council, the Forestry Commission, the Natural Environment Research Council and the Scottish Government, under the Tree Health and Plant Biosecurity Initiative.
References
- [1].Janmaat AF, Bergmann L, Ericsson J. Effect of low levels of Bacillus thuringiensis exposure on the growth, food consumption and digestion efficiencies of Trichoplusia ni resistant and susceptible to Bt. J Invertebrate Pathol 2014; 119:32-9; PMID:24727193; http://dx.doi.org/ 10.1016/j.jip.2014.04.001 [DOI] [PubMed] [Google Scholar]
- [2].Paris M, David JP, Despres L. Fitness costs of resistance to Bti toxins in the dengue vector Aedes aegypti. Ecotoxicology 2011; 20:1184-94; PMID:21461926; http://dx.doi.org/ 10.1007/s10646-011-0663-8 [DOI] [PubMed] [Google Scholar]
- [3].Raymond B, Johnston PR, Nielsen-LeRoux C, Lereclus D, Crickmore N. Bacillus thuringiensis: an impotent pathogen? Trends Microbiol 2010; 18:189-94; PMID:20338765; http://dx.doi.org/ 10.1016/j.tim.2010.02.006 [DOI] [PubMed] [Google Scholar]
- [4].Nielsen-LeRoux C, Gaudriault S, Ramarao N, Lereclus D, Givaudan A. How the insect pathogen bacteria Bacillus thuringiensis and Xenorhabdus/Photorhabdus occupy their hosts. Curr Opin Microbiol 2012; 15:220-31; PMID:22633889; http://dx.doi.org/ 10.1016/j.mib.2012.04.006 [DOI] [PubMed] [Google Scholar]
- [5].Slamti L, Perchat S, Huillet E, Lereclus D. Quorum Sensing in Bacillus thuringiensis Is Required for Completion of a Full Infectious Cycle in the Insect. Toxins 2014; 6:2239-55; PMID:25089349; http://dx.doi.org/ 10.3390/toxins6082239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bravo A, Gill SS, Soberon M. Bacillus thuringiensis: mechanisms and use In: Gilbert LI, Kostas I, Gill SS, eds Comprehensive Molecular Insect Science. Amsterdam: Elsevier, 2005:175-205. [Google Scholar]
- [7].Li RS, Jarrett P, Burges HD. Importance of spores, crystals, and ∂-endotoxins in the pathogenicity of different varieties of Bacillus thuringiensis in Galleria mellonella and Pieris brassicae. J Invertebrate Pathol 1987; 50:277-84; http://dx.doi.org/ 10.1016/0022-2011(87)90093-0 [DOI] [Google Scholar]
- [8].Zhang X, Candas M, Griko NB, Taussig R, Bulla LA Jr. A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc Natl Acad Sci U S A 2006; 103:9897-902; PMID:16788061; http://dx.doi.org/ 10.1073/pnas.0604017103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Soberon M, Gill SS, Bravo A. Signaling versus punching hole: How do Bacillus thuringiensis toxins kill insect midgut cells? Cell Mol Life Sci 2009; 66:1337-49; PMID:19132293; http://dx.doi.org/ 10.1007/s00018-008-8330-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tabashnik BE, Brevault T, Carriere Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 2013; 31:510-21; PMID:23752438; http://dx.doi.org/ 10.1038/nbt.2597 [DOI] [PubMed] [Google Scholar]
- [11].Griffitts JS, Aroian RV. Many roads to resistance: how invertebrates adapt to Bt toxins. BioEssays 2005; 27:614-24; PMID:15892110; http://dx.doi.org/ 10.1002/bies.20239 [DOI] [PubMed] [Google Scholar]
- [12].Ferre J, Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu Rev Entomol 2002; 47:501-33; PMID:11729083; http://dx.doi.org/ 10.1146/annurev.ento.47.091201.145234 [DOI] [PubMed] [Google Scholar]
- [13].Gahan LJ, Gould F, Heckel DG. Identification of a gene associated with Bt resistance in Heliothis virescens. Science 2001; 293:857-60; PMID:11486086; http://dx.doi.org/ 10.1126/science.1060949 [DOI] [PubMed] [Google Scholar]
- [14].Darboux I, Pauchet Y, Castella C, Silva-Filha MH, Nielsen-LeRoux C, Charles JF, Pauron D. Loss of the membrane anchor of the target receptor is a mechanism of bioinsecticide resistance. Proc Natl Acad Sci U S A 2002; 99:5830-5; PMID:11983886; http://dx.doi.org/ 10.1073/pnas.092615399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bravo A, Soberon M. How to cope with insect resistance to Bt toxins? Trends Biotechnol 2008; 26:573-9; PMID:18706722; http://dx.doi.org/ 10.1016/j.tibtech.2008.06.005 [DOI] [PubMed] [Google Scholar]
- [16].Ma G, Sarjan M, Preston C, Asgari S, Schmidt O. Mechanisms of inducible resistance against Bacillus thuringiensis endotoxins in invertebrates. Insect Science 2005; 12:319-30; http://dx.doi.org/ 10.1111/j.1005-295X.2005.00039.x [DOI] [Google Scholar]
- [17].Ma G, Rahman MM, Grant W, Schmidt O, Asgari S. Insect tolerance to the crystal toxins Cry1Ac and Cry2Ab is mediated by the binding of monomeric toxin to lipophorin glycolipids causing oligomerization and sequestration reactions. Dev Comp Immunol 2012; 37:184-92; PMID:21925538; http://dx.doi.org/ 10.1016/j.dci.2011.08.017 [DOI] [PubMed] [Google Scholar]
- [18].Gunning RV, Dang HT, Kemp FC, Nicholson IC, Moores GD. New resistance mechanism in Helicoverpa armigera threatens transgenic crops expressing Bacillus thuringiensis Cry1Ac toxin. Appl Environ Microbiol 2005; 71:2558-63; PMID:15870346; http://dx.doi.org/ 10.1128/AEM.71.5.2558-2563.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Caccia S, Moar WJ, Chandrashekhar J, Oppert C, Anilkumar KJ, Jurat-Fuentes JL, Ferré J. Association of Cry1Ac toxin resistance in Helicoverpa zea (Boddie) with increased alkaline phosphatase levels in the midgut lumen. Appl Environ Microbiol 2012; 78:5690-8; PMID:22685140; http://dx.doi.org/ 10.1128/AEM.00523-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Oppert B, Kramer KJ, Johnson DE, MacIntosh SC, McGaughey WH. Altered protoxin activation by midgut enzymes from a Bacillus thuringiensis resistant strain of Plodia interpunctella. Biochem Biophys Res Commun 1994; 198:940-7; PMID:8117300; http://dx.doi.org/ 10.1006/bbrc.1994.1134 [DOI] [PubMed] [Google Scholar]
- [21].Martinez-Ramirez AC, Gould F, Ferre J. Histopathological effects and growth reduction in a susceptible and a resistant strain of Heliothis virescens (Lepidoptera : Noctuidae) caused by sublethal doses of pure Cry1A crystal proteins from Bacillus thuringiensis. Biocontrol Sci Techn 1999; 9:239-46; http://dx.doi.org/ 10.1080/095-83159929811 [DOI] [Google Scholar]
- [22].Broderick NA, Robinson CJ, McMahon MD, Holt J, Handelsman J, Raffa KF. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC biology 2009; 7:11; PMID:19261175; http://dx.doi.org/ 10.1186/1741-7007-7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Patil CD, Borase HP, Salunke BK, Patil SV. Alteration in Bacillus thuringiensis toxicity by curing gut flora: novel approach for mosquito resistance management. Parasitology research 2013; 112:3283-8; PMID:23820604; http://dx.doi.org/ 10.1007/s00436-013-3507-z [DOI] [PubMed] [Google Scholar]
- [24].Valadez-Lira JA, Alcocer-Gonzalez JM, Damas G, Nunez-Mejia G, Oppert B, Rodriguez-Padilla C, Tamez-Guerra P. Comparative evaluation of phenoloxidase activity in different larval stages of four lepidopteran pests after exposure to Bacillus thuringiensis. Journal of insect science 2012; 12:80; PMID:23414117; http://dx.doi.org/ 10.1673/031.012.8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gassmann AJ, Carriere Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis. Annu Rev Entomol 2009; 54:147-63; PMID:19067630; http://dx.doi.org/ 10.1146/annurev.ento.54.110807.090518 [DOI] [PubMed] [Google Scholar]
- [26].Ribeiro LMD, Wanderley-Teixeira V, da Cunha FM, Teixeira AAC, de Siqueira HAA. Immunological response of resistant and susceptible Plutella xylostella (Lepidoptera: Plutellidae) to Bacillus thuringiensis. Rev Colomb Entomol 2012; 38:208-14 [Google Scholar]
- [27].Richards EH, Dani MP. A recombinant immunosuppressive protein from Pimpla hypochondriaca (rVPr1) increases the susceptibility of Lacanobia oleracea and Mamestra brassicae larvae to Bacillus thuringiensis. J Invertebrate Pathol 2010; 104:51-7; PMID:20123105; http://dx.doi.org/ 10.1016/j.jip.2010.01.010 [DOI] [PubMed] [Google Scholar]
- [28].Shrestha S, Hong YP, Kim Y. Two chemical derivatives of bacterial metabolites suppress cellular immune responses and enhance pathogenicity of Bacillus thuringiensis against the diamondback moth, Plutella xylostella. J Asia-Pac Entomol 2010; 13:55-60; http://dx.doi.org/ 10.1016/j.aspen.2009.11.005 [DOI] [Google Scholar]
- [29].Broderick NA, Raffa KF, Handelsman J. Chemical modulators of the innate immune response alter gypsy moth larval susceptibility to Bacillus thuringiensis. BMC Microbiol 2010; 10:129; PMID:20423490; http://dx.doi.org/ 10.1186/1471-2180-10-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hwang J, Kim Y. RNA interference of an antimicrobial peptide, gloverin, of the beet armyworm, Spodoptera exigua, enhances susceptibility to Bacillus thuringiensis. J Invertebrate Pathol 2011; 108:194-200; PMID:21925182; http://dx.doi.org/ 10.1016/j.jip.2011.09.003 [DOI] [PubMed] [Google Scholar]
- [31].Dubovskiy IM, Whitten MM, Kryukov VY, Yaroslavtseva ON, Grizanova EV, Greig C, Mukherjee K, Vilcinskas A, Mitkovets PV, Glupov VV, et al.. More than a colour change: insect melanism, disease resistance and fecundity. P Roy Soc B-Biol Sci 2013; 280:20130584; http://dx.doi.org/ 10.1098/rspb.2013.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dubovskiy IM, Whitten MMA, Yaroslavtseva ON, Greig C, Kryukov VY, Grizanova EV, Mukherjee K, Vilcinskas A, Glupov VV, Butt TM. Can insects develop resistance to insect pathogenic fungi? Plos One 2013; 8:e60248; http://dx.doi.org/ 10.1371/journal.pone.0060248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Crava CM, Jakubowska AK, Escriche B, Herrero S, Bel Y. Dissimilar Regulation of Antimicrobial Proteins in the Midgut of Spodoptera exigua Larvae Challenged with Bacillus thuringiensis Toxins or Baculovirus. Plos One 2015; 10:e0125991; PMID:25993013; http://dx.doi.org/ 10.1371/journal.pone.0125991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol 2007; 7:862-74; PMID:17948019; http://dx.doi.org/ 10.1038/nri2194 [DOI] [PubMed] [Google Scholar]
- [35].Wojda I, Kowalski P, Jakubowicz T. Humoral immune response of Galleria mellonella larvae after infection by Beauveria bassiana under optimal and heat-shock conditions. J Insect Physiol 2009; 55:525-31; PMID:19232408; http://dx.doi.org/ 10.1016/j.jinsphys.2009.01.014 [DOI] [PubMed] [Google Scholar]
- [36].Oppert B. Protease interactions with Bacillus thuringiensis insecticidal toxins. Arch Insect Biochem Physiol 1999; 42:1-12; PMID:10467052; http://dx.doi.org/ 10.1002/(SICI)1520-6327(199909)42:1%3c1::AID-ARCH2%3e3.0.CO;2- [DOI] [PubMed] [Google Scholar]
- [37].Lovgren A, Zhang M, Engstrom A, Dalhammar G, Landen R. Molecular characterization of immune inhibitor A, a secreted virulence protease from Bacillus thuringiensis. Mol Microbiol 1990; 4:2137-46; PMID:2089225; http://dx.doi.org/ 10.1111/j.1365-2958.1990.tb00575.x [DOI] [PubMed] [Google Scholar]
- [38].Dubovskiy IM, Martemyanov VV, Vorontsova YL, Rantala MJ, Gryzanova EV, Glupov VV. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comparative biochemistry and physiology Toxicology & pharmacology : CBP 2008; 148:1-5; http://dx.doi.org/ 10.1016/j.cbpc.2008.02.003 [DOI] [PubMed] [Google Scholar]
- [39].Loeb MJ, Martin PA, Hakim RS, Goto S, Takeda M. Regeneration of cultured midgut cells after exposure to sublethal doses of toxin from two strains of Bacillus thuringiensis. J Insect Physiol 2001; 47:599-606; PMID:11249948; http://dx.doi.org/ 10.1016/S0022-1910(00)001-50-5 [DOI] [PubMed] [Google Scholar]
- [40].Broderick NA, Raffa KF, Handelsman J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc Natl Acad Sci U S A 2006; 103:15196-9; PMID:17005725; http://dx.doi.org/ 10.1073/pnas.06048-65103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Raymond B, Johnston PR, Wright DJ, Ellis RJ, Crickmore N, Bonsall MB. A mid-gut microbiota is not required for the pathogenicity of Bacillus thuringiensis to diamondback moth larvae. Environ Microbiol 2009; 11:2556-63; PMID:19555371; http://dx.doi.org/ 10.1111/j.1462-2920.2009.01980.x [DOI] [PubMed] [Google Scholar]
- [42].Rivero A, Magaud A, Nicot A, Vezilier J. Energetic cost of insecticide resistance in Culex pipiens mosquitoes. J Med Entomol 2011; 48:694-700; PMID:21661333; http://dx.doi.org/ 10.1603/ME10121 [DOI] [PubMed] [Google Scholar]
- [43].Tsuzuki S, Ochiai M, Matsumoto H, Kurata S, Ohnishi A, Hayakawa Y. Drosophila growth-blocking peptide-like factor mediates acute immune reactions during infectious and non-infectious stress. Scientific reports 2012; 2:210; PMID:22355724; http://dx.doi.org/ 10.1038/srep00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Semighini CP, Heitman J. Dynamic duo takes down fungal villains. Proc Natl Acad Sci U S A 2009; 106:2971-2; PMID:19251661; http://dx.doi.org/ 10.1073/pnas.0900801106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Freitak D, Schmidtberg H, Dickel F, Lochnit G, Vogel H, Vilcinskas A. The maternal transfer of bacteria can mediate trans-generational immune priming in insects. Virulence 2014; 5:547-54; PMID:24603099; http://dx.doi.org/ 10.4161/viru.28367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Grizanova EV, Dubovskiy IM, Whitten MM, Glupov VV Contributions of cellular and humoral immunity of Galleria mellonella larvae in defence against oral infection by Bacillus thuringiensis. Journal of invertebrate pathology 2014; 119:40-6 [DOI] [PubMed] [Google Scholar]
- [47].Angiuoli SV, Matalka M, Gussman A, Galens K, Vangala M, Riley DR, Arze C, White JR, White O, Fricke WF. CloVR: A virtual machine for automated and portable sequence analysis from the desktop using cloud computing. Bmc Bioinformatics 2011; 12:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


