ABSTRACT
Invasive aspergillosis is a life-threatening infection caused by the opportunistic filamentous fungus Aspergillus fumigatus. Patients undergoing haematopoietic stem cell transplant (HSCT) for the treatment of hematological malignancy are at particularly high risk of developing this fatal infection. The susceptibility of HSCT patients to infection with A. fumigatus is a consequence of a complex interplay of both fungal and host factors. Here we review our understanding of the host-pathogen interactions underlying the susceptibility of the immunocompromised host to infection with A. fumigatus with a focus on the experimental validation of fungal and host factors relevant to HSCT patients. These include fungal factors such as secondary metabolites, cell wall constituents, and metabolic adaptations that facilitate immune evasion and survival within the host microenvironment, as well as the innate and adaptive immune responses involved in host defense against A. fumigatus.
KEYWORDS: aspergillosis, aspergillus, immunity, pathogenesis, stem cell transplant, virulence
Introduction
Aspergillus fumigatus is ubiquitous within the environment and produces abundant spores (conidia) that are disseminated by air currents. Healthy humans inhale hundreds of conidia daily without developing disease. There are multiple levels of host defense that mediate protection against this constant fungal exposure. The majority of inhaled conidia undergo mechanical elimination by mucociliary action within the airways, or are rapidly phagocytosed and killed by innate immune cells including epithelial cells and alveolar macrophages.1 Conidia that escape these first lines of defense, and which undergo germination, induce epithelial cells, alveolar macrophages and dendritic cells to initiate a pro-inflammatory response through the release of cytokines and chemokines. This response leads to the recruitment of neutrophils, which exhibit potent activity against hyphae. In addition to these innate immune responses, adaptive Th1 and Th17 type responses can also mediate protection against infection.2,3 Impairment in these innate or adaptive immune responses to A. fumigatus permits the growth of hyphae, which rapidly invade pulmonary tissues, and if unchecked, can disseminate to other deep organs. Although A. fumigatus conidia comprise only a small fraction of the spores which humans inhale daily, this species accounts for the majority of invasive mold infections in immunocompromised patients.1 The ability of A. fumigatus to grow at 37°C and to produce conidia that are small enough to penetrate deep within the airways undoubtedly contributes to the success of this pathogen,4 however these properties are not unique to A. fumigatus and are shared by many other Aspergillus species and non-Aspergillus fungi. These observations suggest that A. fumigatus has developed unique virulence traits that contribute to its success as a human pathogen.
Given the array of human host defenses, invasive Aspergillosis (IA) is largely a disease of severely immunocompromised patients including those with primary immunodeficiencies such as chronic granulomatous disease; patients undergoing haematopoietic stem cell and solid organ transplantation; patients with hematological malignancies and prolonged neutropenia; and those receiving corticosteroid or other immunosuppressive therapies.5 The burden of disease due to IA has increased significantly in the last 30 y in large part due to the increased number of patients undergoing immunosuppressive treatments for hematological malignancies and haematopoietic stem cell transplants (HSCT).6 IA has become a leading cause of death in HSCT patients, accounting for 10% of all deaths in this population.6
In the allogeneic HSCT population, IA can occur early, during the neutropenic pre-engraftment phase, and late, during the post-engraftment period.7,8 During the post-engraftment period patients are usually non-neutropenic but are immunosuppressed largely as a consequence of graft-versus-host-disease (GVHD) and the therapies given for this condition. Early IA is more common in patients undergoing myeloablative transplants due to the prolonged neutropenia resulting from the extensive chemotherapy and radiation used to destroy the native bone marrow. The rise of non-myeloablative transplantation, with a shorter neutropenic period and higher risk of GVHD, has led to a decrease in the incidence of early IA with a shift to increasing rates of late IA.9,10 The late risk period associated with GVHD can last for months to years, making prophylactic and monitoring strategies challenging to implement.11 Further, the sensitivity of galactomannan screening, which is commonly used for the detection of IA in neutropenic patients, is low in non-neutropenic hosts.12 Thus, IA is often diagnosed at a relatively advanced stage of infection in this population, compromising the efficacy of antifungal therapy.
The pathology and immunobiology of IA is different in pre-engraftment neutropenic patients and post-engraftment non-neutropenic patients. In neutropenic patients, A. fumigatus infection likely begins as a bronchopneumonia, but progresses rapidly, with abundant fungal growth, early angioinvasion, thrombosis and hemorrhage often leading to fungal dissemination and eventually death.1,13-15 The natural history of IA during neutropenia is therefore characterized by rapid fungal growth with low levels of inflammation. Pulmonary computed tomography (CT) imaging early in infection may reveal a bronchopneumonia, however nodular infiltrates, often surrounded by a ring of ground glass appearance (the halo sign) are more commonly detected.5,16 In contrast, non-neutropenic patients receiving corticosteroids for prophylaxis or treatment of GVHD have an overabundant inflammatory response to A. fumigatus characterized by pyogranulomatous infiltrates and tissue necrosis, with more limited fungal growth and angioinvasion.1,14 Exaggerated and uncontrolled inflammation is thought to contribute to death in this population. CT findings also include nodules but may also extend to other airway invasive type of presentations including tree-in-bud lesions and non-specific signs of bronchopneumonia.17
Given these important clinical, pathologic and radiologic differences between early and late IA, understanding the pathogenesis of aspergillosis in HSCT patients requires studies in both neutropenic and non-neutropenic experimental models. Multiple neutropenic models of IA have been reported, including treatment of mice with chemotherapeutic agents such as cyclophosphamide or using antibody depletion of neutrophils.18,19 Non-neutropenic models of IA commonly rely on the treatment of mice with corticosteroids such as cortisone acetate to induce susceptibility to infection.1 These models result in experimental IA that mirrors the differences seen in human pathology between neutropenic and non-neutropenic patients.20 Both of these models frequently use intranasal and intratracheal administration of high doses of conidia, however inhalational chamber methods have been described which induce reproducible infection in immunocompromised mice with an inoculum of 103 to 104 conidia per animal.19,21 Although these studies have largely relied on the use of BALB/C or C57BL6 mice, a comparison of 10 inbred mouse strains using the neutropenic model of IA revealed marked differences in their intrinsic susceptibility to A. fumigatus.22 A haplotype-based computational genetic analysis wide-association study identified a correlation between survival of these mouse strains and polymorphisms in several genes including the gene encoding for plasminogen.22 Further studies testing the role of these polymorphisms in the pathogenesis of IA have not yet been reported. The use of transgenic mice in models of IA has also provided a powerful tool to study the role of host immune factors in governing defense against A. fumigatus, although many of these studies have been performed in immunocompetent mice infected with a very high inoculum (up to 107 conidia per animal)23 and thus extrapolation to IA in HSCT requires caution. Relatively few studies have used a murine model of myeloablative HSCT-related aspergillosis in which mice are transplanted with purified haematopoietic stem cells following myeloablative irradiation before A. fumigatus infection.24,25 Mouse models of acute leukemia and GVHD have been developed but have not been used for the study of IA.26-28 Despite these limitations, studies with these experimental models have begun to shed light on the pathogenesis of IA in HSCT patients. In this review, we will examine a selection of experimental studies that highlight key factors governing the host-pathogen interaction in IA, with a focus on fungal factors that play a distinct role in neutropenic vs. non-neutropenic hosts and host factors that have been directly linked to IA susceptibility in patients undergoing HSCT.
Fungal virulence factors
Secondary metabolites as virulence factors and implications for HSCT
Like many molds, A. fumigatus can produce a variety of secreted bioactive secondary metabolites. A list of characterized A. fumigatus virulence factors is shown in Table 1. The epipolythiodioxopiperazine gliotoxin has been the best studied of these mycotoxins. Gliotoxin is produced by mature hyphae of A. fumigatus both in vitro, and during human infection.29 Studies in vitro have found that gliotoxin is a potent immunosuppressive molecule that mediates a variety of functions including inhibition of NF-κB activation and NADPH oxidase function, thereby preventing macrophage and neutrophil phagocytosis and oxidative killing29-32; alteration in neutrophil and macrophage cytoskeletal structure to facilitate immune evasion33; inhibition of macrophage phosphoinositide signaling protein phosphatidylinositol-3,4,5-trisphosphate, impairing phagosome formation34; and induction of leukocyte apoptosis.35,36 Studies in mouse models have been helpful in understanding the relative importance of these various functions in the pathogenesis of IA. Initial studies in neutropenic models of IA found that A. fumigatus gliotoxin-deficient mutants are fully virulent.37-39 However, in a non-neutropenic mouse model, gliotoxin-deficient mutants display attenuated virulence and induce lower levels of apoptosis in neutrophils surrounding fungal lesions.40,41 Collectively, these studies suggest that gliotoxin contributes to virulence largely through the induction of neutrophil apoptosis and thus this toxin plays little role in the pathogenesis of IA in neutropenic hosts such as HSCT patients pre-engraftment.
Table 1.
Fungal virulence factors reviewed in this paper and their role in virulence in immunocompetent, neutropenic and non-neutropenic mouse models. – indicates that the contribution of the indicated factor to virulence in this experimental model is not known.
| Mouse Model and Contribution to Virulence |
|||
|---|---|---|---|
| Virulence Factor | Immunocompetent | Neutropenic | Non-neutropenic |
| Gliotoxin | — | NO | YES |
| LaeA | — | YES | YES |
| RodA | YES | — | — |
| Melanin/Alb1 | YES | — | — |
| Ags1, 2, 3 | YES | YES | — |
| GAG/Uge3 | — | YES | YES |
| RbhA | — | YES | YES |
| SidA, C, D, F, G | — | YES | YES |
| HapX | — | YES | YES |
| AcuM | — | — | YES |
| AcuK | — | — | YES |
| SrbA | — | YES | YES |
| SrbB | — | — | YES |
| RbdA | YES | — | YES |
| HorA | — | YES | YES |
It is likely that secondary metabolites other than gliotoxin also contribute to virulence. Support for this hypothesis comes from studies of the global regulator of secondary metabolite production LaeA. Deletion of laeA leads to a near complete repression of all secondary metabolite biosynthetic gene clusters in A. fumigatus, including the genes required for the production of gliotoxin.42 In contrast to gliotoxin-deficient mutants, the ΔlaeA mutant is attenuated in virulence in both neutropenic and non-neutropenic murine models of IA.42-44 While the attenuated virulence of the LaeA-deficient mutant in non-neutropenic mice may be due in part to decreased gliotoxin expression, this mutant is much more attenuated in virulence than gliotoxin-deficient strains, suggesting that LaeA controls other secondary metabolites important for virulence. This hypothesis is supported by the observation that the LaeA-deficient mutant is also virulent in neutropenic mice in which gliotoxin is dispensible for virulence.42 LaeA has been observed to mediate A. fumigatus-dependent inhibition of angiogenesis in a neutropenic mouse model by both gliotoxin-dependent and -independent factors.43 It has been suggested that inhibiting angiogenesis may help sequester the fungus from host defenses and antifungals to promote infection. Importantly however, LaeA also governs other fungal processes in addition to the regulation of secondary metabolite production, including the expression of hydrophobins and of alb1, a gene required for synthesis of conidial pigment.44 As detailed below, both of these factors have been reported to play a role in the pathogenesis of IA in experimental mouse models. Studies of individual secondary metabolites are likely required to elucidate their role in the pathogenesis of IA and to determine their potential as therapeutic targets in the context of HSCT-associated IA.
Cell wall components that play an important role in the pathogenesis of IA
As the site of first contact between the host and fungus during infection, the fungal cell wall plays a key role in the pathogenesis of IA. Although a comprehensive discussion of the role of the fungal cell wall components in virulence is beyond the scope of this review, a number of more recent experimental findings will be highlighted.
Components of the cell wall of conidia
Resting conidia of A. fumigatus are extremely hydrophobic due to the presence of an organized layer of immunologically inert rodlet proteins (RodA) that cover and conceal the underlying cell wall polysaccharides.45 Studies in vitro have demonstrated that this rodlet layer confers a variety of phenotypes on resting conidia including rendering them adhesive to host macromolecules and masking β-glucans and other polysaccharides from recognition by host macrophages and dendritic cells.45-47 Studies in mouse models, however, have yielded conflicting results. In immunocompetent mice, loss of RodA results in a dramatic increase in pulmonary inflammatory responses, and impaired virulence in a corneal model of A. fumigatus infection.46 However, conidia deficient in RodA are fully virulent in a corticosteroid treated, non-neutropenic mouse model of pulmonary IA.45 Studies in neutropenic mice have not yet been reported. Thus, although RodA clearly plays a role in preventing potentially deleterious pulmonary inflammatory responses to daily conidial exposure in healthy individuals, its role in the pathogenesis of IA in HSCT patients remains unresolved.
A. fumigatus conidia are heavily pigmented due to the presence of cell wall melanin. Studies in vitro have identified a number of roles for melanin in the host-pathogen interaction including contributing to cell wall integrity, modulating macrophage cytokine responses, and inhibition of phagolysosome acidification of alveolar macrophages, monocytes and neutrophils.48-52 The mechanisms by which melanin interacts with phagolysosomes to contribute to disease are beginning to be elucidated. It has been suggested that melanin interferes with LC3-associated phagocytosis (LAP), an Atg5-dependent autophagy pathway that promotes fungal killing.53,54 When Atg5 was inactivated in macrophages and murine haematopoietic cells, the virulence of a melanin-deficient A. fumigatus strain was restored. Consistent with these in vitro observations, conidia melanin production is essential for normal virulence.55,56 Mutant A. fumigatus strains lacking the conidial pigmentation gene alb1 (also known as pksP) are significantly attenuated in virulence in immunocompetent mice.57 It is therefore likely that melanin contributes to the establishment of both early and late IA in HSCT patients.
Although α-1,3-glucans are an important component of both conidia and hyphae, recent studies have highlighted a role for these glycans in the cell wall of conidia during experimental IA. Synthesis of α-1,3-glucans is mediated by one of 3 glucan synthases: ags1, ags2 and ags3.58,59 Single deletions of each of these genes is associated with minimal changes in cell wall composition, however loss of all 3 genes results in a complete absence of α-1,3-glucan synthesis, and extensive structural modification of the cell wall.60 Absence of α-1,3-glucans is associated with the production of an amorphous layer of glycoproteins that covers the normal rodlet layer, leading to enhanced recognition of conidia by macrophages.61 The α-1,3-glucan deficient mutant is markedly impaired in virulence in both immunocompetent and neutropenic models of aspergillosis, possibly through enhanced elimination of conidia by pulmonary macrophages.61 Studies in corticosteroid-treated mice have not been reported. The dramatic effect on virulence in a neutropenic host suggests the possibility that inhibition of α-1,3-glucan synthesis could be an effective antifungal strategy in the future for the treatment of early IA in the HSCT population.
Components of the hyphal cell wall that play a role in virulence
Hyphae of A. fumigatus are covered in a layer of galactosaminogalactan (GAG), a partially deacetylated heteropolymer of α-1,4 linked galactose and N-acetyl galactosamine.61-63 GAG has been described to mediate a number of virulence-associated functions in vitro including mediating adhesion and biofilm formation64; masking β-1,3-glucan from host recognition by dectin-1 on dendritic cells; mediating resistance to neutrophil extracellular traps65; inducing natural killer cell-mediated neutrophil apoptosis66; and the induction of expression of the immunosuppressive cytokine IL-1RA.67 In contrast to gliotoxin, in vivo studies using GAG-deficient mutants found that GAG effects on virulence are more marked in neutropenic mice,64 suggesting that neutrophil-independent functions of GAG are more important in mediating virulence. Conversely, it was found that increasing GAG expression in the naturally GAG-deficient species Aspergillus nidulans enhances virulence in non-neutropenic but not neutropenic mice.65 In this system, the predominant role of GAG is to mediate resistance to killing by neutrophil extracellular traps. Further studies are required to reconcile these findings and better understand the role of GAG in virulence in neutropenic and non-neutropenic hosts.
Other fungal factors involved in the pathogenesis of IA
Adaptation to the host environment is a critical factor for the survival of A. fumigatus during infection. For example, A. fumigatus mutants defective in nitrogen metabolism, such as the ΔrbhA mutant, exhibit significantly reduced growth and virulence in both neutropenic and non-neutropenic mouse models of infection.68,69 The ability to acquire micronutrients such as iron is similarly critical for fungal growth in vivo. Not surprisingly, siderophore biosynthesis has also been implicated in virulence in both neutropenic and non-neutropenic mouse models.70-73 The A. fumigatus ΔsidA mutant, which is completely deficient in iron siderophore synthesis, grows poorly in low iron conditions and within alveolar macrophages and is completely avirulent.73 Attenuated virulence has also been reported in other strains of A. fumigatus with mutations in the siderophore synthesis pathway and with other mutations affecting iron metabolism, including HapX, AcuM, and AcuK.72,74-76 Taken together, these studies strongly suggest that the ability of A. fumigatus to acquire and utilize iron from the host is essential for pathogenicity. This hypothesis is supported by the observation that iron overload within HSCT patients is a risk factor for the development of IA.77,78
Recent studies have also implicated the ability of A. fumigatus to adapt to hypoxic conditions as a critical factor in the development of IA. During A. fumigatus infection, the organism grows within a hypoxic microenvironment resulting from a number of factors including impaired blood supply from thrombosis and tissue necrosis, inhibition of angiogenesis, and increased oxygen consumption by host inflammatory cells.79 Fungal adaptation to hypoxia therefore is critical for pathogenicity.80 A. fumigatus SrbA and SrbB, members of the highly conserved sterol regulatory element binding protein (SREBP) family of transcription factors, have been reported to play critical roles in fungal adaptation to hypoxia.81,82 In vitro, SrbA is required for normal hyphal growth and cell wall morphology under hypoxic conditions, and increases ergosterol biosynthesis, thereby contributing to azole antifungal resistance.81 Interestingly, SrbA also regulates iron acquisition in response to hypoxia and low iron conditions by increasing siderophore biosynthesis.83 SrbB mediates similar functions to SrbA but also regulates heme biosynthesis, as well as carbohydrate and sterol metabolism under hypoxic conditions.82 Mutants deficient in SrbA display significantly reduced growth and are avirulent in both neutropenic and non-neutropenic mouse models, while SrbB-deficient mutants are markedly reduced in virulence in a non-neutropenic mouse model.81-83 The virulence of SrbB-deficient mutants in neutropenic mouse models has not been reported. Notably, mutants deficient in both SrbA and SrbB are more impaired in growth and virulence than mutants deficient in either protein.82 This observation suggests that SrbA and SrbB regulate both shared and distinct genes that are crucial to fungal metabolism and virulence. Another protein in the SREBP family, SrbC, remains to be characterized. Roles for other proteins in the regulation of the hypoxic response are now emerging. The iron metabolism regulators HapX and SreA have been found to play a role in hypoxia adaptation.83,84 Most recently, the signal peptide peptidase SppA, the protease RbdA, and the hypoxia-induced dehydrogenase HorA have also been linked to hypoxia adaptation and virulence, possibly through interactions with the Srb pathways.80,85,86 Together these data suggest a model whereby the ability of A. fumigatus to thrive in hypoxic microenvironments is a critical factor in the pathogenesis of IA and suggest a potential role for hyperbaric oxygen therapy for IA in HSCT patients.
Recent advances in microfluidics have provided new tools for the in vitro study of the effect of micro-environmental conditions on fungal growth and secondary metabolite production. A micrometabolomics platform has been developed which couples a microfluidic solid or liquid culture system to liquid chromatography-mass spectrometry for the profiling and discovery of secondary metabolite production in response to environmental variations.87 Use of this system revealed a clear relationship between A. fumigatus colony diameter and the secondary metabolite profile produced by the active culture that was independent of biomass.87 Similarly, marked differences were observed in the metabolite profile of A. fumigatus grown in blood as compared with grown in standard culture media.87 A similar system has been used to study the dynamics of germination in the pathogenic yeast Cryptococcus neoformans.88 The use of this and other similar microsystems may provide a useful tool to improve our understanding of the importance of the microenvironment on the expression and function of virulence factors of A. fumigatus.
Factors underlying the virulence of non-fumigatus Aspergillus species
Although A. fumigatus accounts for the majority of cases of IA, other species such as A. terreus and A. flavus can also cause disease in HSCT patients. There are relatively few studies probing the host-pathogen interactions of these Aspergillus species during infection. The virulence of A. flavus has been evaluated in intravenous models of disseminated IA in both immunocompetent and neutropenic mice. In intravenous infection models, A. flavus was reported to exhibit increased virulence as compared with A. fumigatus.89 However, it has been postulated that the larger spore size of this species limits its penetration of the lower airways, thus reducing the ability of this species to establish infection. The virulence and natural history of A. terreus infection in neutropenic and non-neutropenic mouse models have also been compared. In neutropenic mice, infection with A. terreus resulted in a degree of mortality that was similar to previous reports of A. fumigatus infection in the same model.90 In contrast, A. terreus exhibited attenuated virulence in corticosteroid-treated mice as compared with prior reports of A. fumigatus in this model.90 Interestingly, in both models, fatty degeneration of hepatocytes was noted, a finding not previously reported in A. fumigatus infection.90 Confirmation of these findings awaits a direct head-to-head comparison of A. terreus and A. fumigatus infection in these models. Unlike studies of pathogenic yeast, the heterologous expression of putative A. fumigatus virulence factors in less virulent Aspergillus species has only been reported in a single study.65 In this work, detailed above, heterologous expression of an A. fumigatus glucose epimerase was used to enhance the production of cell wall galactosaminogalactan in A. nidulans, and was associated with increased virulence in neutropenic mice.65 Heterologous expression of non-fumigatus Aspergillus virulence factors in A. fumigatus and the converse holds promise for the study of other species-specific virulence factors in mouse models.
Host response to aspergillosis
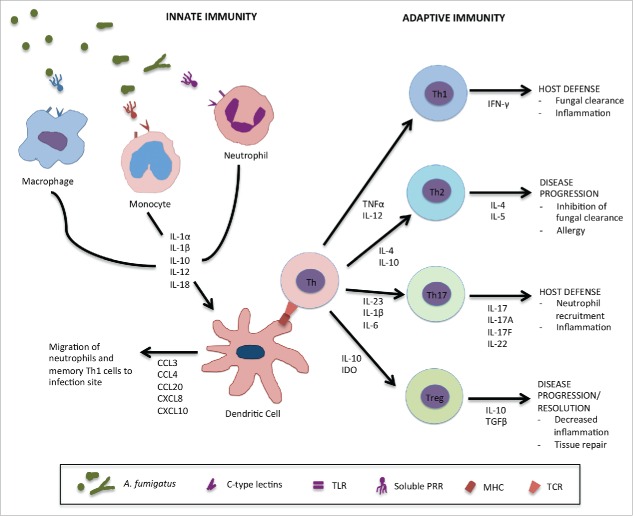
Our understanding of the host response to A. fumigatus has greatly increased in recent years. As an exhaustive review of fungal immunology studies is beyond the scope of this review, we will highlight advances in our understanding of host defense against IA, with an emphasis on immune factors that have been linked to susceptibility of HSCT populations to this infection. A general schematic of host immune factors involved in defense against A. fumigatus is shown in Figure 1.
Figure 1.
Host immune response against A. fumigatus. Conidia which enter the lungs and escape airway epithelial cells are recognized by innate immune effector cells via cell surface pattern recognition receptors (PRRs) such as dectin-1 or toll-like receptors (TLRs) and soluble factors such as pentraxin-3 (PTX-3), surfactant proteins A and D (SP-A,D) or mannose-binding lectin (MBL). Alveolar macrophages recognize and clear conidia leading to the activation of inflammatory pathways and the secretion of various proinflammatory cytokines by epithelial cells, other macrophages and dendritic cells. The cytokine environment contributes to the recruitment of other innate immune effectors such as inflammatory monocytes and neutrophils which mediate fungal killing of conidia that have escaped initial recognition and germinated into hyphae and further stimulate proinflammatory responses. Dendritic cells (DCs) are the link between the innate and adaptive immune responses to A. fumigatus. During infection and after interaction with other innate effectors, they secrete various chemokines such as CCL3, CCL4, CCL20, CXCL8 and CXCL10 to allow migration of more inflammatory cells to the site of infection. They also interact with immature helper T cells (Th) through the major histocompatibility complex (MHC) and T cell receptor (TCR), resulting in the secretion various cytokines driving protective Th1 and Th17 responses against A. fumigatus. They can also inhibit Th2 responses which favor disease progression and allergy. Regulatory T cells are producers of IL-10 which is linked to disease progression in IA.
Innate immune defenses against invasive aspergillosis in HSCT patients
Conidia that escape elimination by pulmonary mucociliary action or by alveolar macrophages shed their hydrophobin coat and undergo germination. Recognition of these germinated conidia and hyphae by the innate immune system is critical for the control of infection. Effective innate immune response to early IA begins with the recognition of fungal elements by cell surface pattern recognition receptors (PRRs) such as the C-type lectins dectin-1 and dectin-2; members of the toll-like receptor (TLR) family; and soluble factors such as pentraxin-3, surfactant proteins A and D (SP-A, SP-D) and mannose-binding lectin (MBL) (Figure 1).11 Effective fungal recognition leads to the activation of inflammatory programs including the NFκ-B pathway and the NLRP3 inflammasome resulting in secretion of a variety of pro-inflammatory cytokines and chemokines by epithelial cells, inflammatory monocytes, dendritic cells (DCs) and alveolar macrophages (Figure 1). This cytokine environment contributes to the recruitment and activation of effector cells such as neutrophils which mediate fungal killing.11 Polymorphisms in a number of key genes involved in mediating these innate responses to A. fumigatus have been identified in HSCT donors and recipients.91,92
Cellular pattern recognition receptors
Several cellular PRRs have been implicated in the recognition of A. fumigatus, of which dectin-1 and members of the TLR family have been best studied. Dectin-1 is a C-type lectin expressed by macrophages and DCs that recognizes the A. fumigatus cell wall constituent β-1,3-glucan.93 Interaction of dectin-1 with A. fumigatus conidia promotes their phagocytosis, enhances macrophage activation, and induces proinflammatory responses including the secretion of tumor necrosis factor (TNF).94 Dectin-1 mediates recruitment of the autophagy protein LC3 II to phagosomes in monocytes, a process that is inhibited by corticosteroids.95,96 Immunocompetent mice deficient in dectin-1 are more susceptible to A. fumigatus infection.97-99 In an experimental HSCT mouse model investigators observed that dectin-1 deficient mice are more susceptible to IA than wild-type mice following transplantation with wild-type stem cells. Dectin-1 deficient mice transplanted with stem cells from donors deficient in dectin-1 exhibit the highest susceptibility to IA, while wild-type mice transplanted with dectin-1 deficient stem cells do not exhibit increased susceptibility to infection.98 These findings suggest that expression of dectin-1 by non-haematopoietic cells may also play an important role in mediating protection against IA during HSCT.
Experimental studies also suggest that dectin-1 may hold promise for therapy of IA. Transient transfection experiments in neutropenic mice have demonstrated protective effects of augmenting expression of native dectin-1 or of a fusion protein consisting of the extracellular domain of dectin-1 linked to the Fc portion of murine immunoglobulin G1.100,101 Human studies of dectin-1-based therapeutics have not been reported.
Cell-surface expressed TLRs also contribute to the recognition of A. fumigatus. TLR2 and TLR4, which signal through the adaptor protein MyD88, enhance pro-inflammatory cytokine production in response to A. fumigatus.49,102 TLR2 forms a heterodimer with TLR1 and TLR6 in mouse cells, but only TLR1 in human cells has been hypothesized to play an accessory role to dectin-1 in the induction of TNF responses to A. fumigatus.102,103 Two studies have examined the susceptibility of TLR2-deficient mice to infection with A. fumigatus. In both studies, TLR2-/- mice developed higher pulmonary fungal burdens during infection; however increased mortality in neutropenic TLR2-/- mice was only observed in one of these 2 studies.104,105 In this neutropenic mouse model, TLR4-/- mice were more susceptible to A. fumigatus infection than TLR2-/- mice.105 The role of these TLRs in the pathogenesis of non-neutropenic IA has not yet been studied.
The intracellular PRR TLR9, which recognizes unmethylated CpG DNA, traffics to phagosomes containing A. fumigatus.106,107 It has been suggested that TLR9 recruitment to phagolysosomes acts to inhibit inflammatory responses and prevent over-exuberant inflammation. In support of this hypothesis, neutrophil-depleted TLR9-/- mice displayed reduced pulmonary fungal growth when infected with resting conidia, and prolonged survival when infected with swollen conidia. A more recent study suggests that in non-neutropenic hosts TLR9 may play a different role and induce TNF production and neutrophil recruitment via calcineurin-NFAT mediated signaling.108 This observation raises the possibility that loss of TLR9 signaling may play an important role in the susceptibility of non-neutropenic HSCT patients to IA in the post-engraftment phase.
Studies in human patients have confirmed the importance of many of these PRRs in mediating immunity to A. fumigatus. HSCT patients who received allogeneic stem cells with TLR1, TLR4, TLR6 and dectin-1 polymorphisms have significantly increased susceptibility to IA.98,109-111 One of the best studied of these mutations is the dectin-1 Y238X polymorphism, which results in the production of a truncated dectin-1 protein that is unable to localize to the cell membrane and mediate fungal recognition.98 The presence of this mutation in HSCT donors or recipients is associated with a significantly increased susceptibility to IA.98 Interestingly, normal patients who received donor stem cells with the Y238X polymorphism had an increased risk of IA in both the early (0–30 days) and late (30–120 days) stages after transplantation while recipients carrying the polymorphism receiving normal donor cells largely developed IA very late (after 120 days) following transplantation. As was reported in mouse models, the worst prognosis was observed in cases in which both donor and recipient carried this dectin-1 polymorphism.98 Furthermore, significantly lower levels of proinflammatory cytokines IFNγ, IL-10, IL-1β, IL-6 and IL-17A were detected in the serum of patients with the Y238X polymorphism during Aspergillus infection, consistent with a role for dectin-1 in mediating innate host defense against A. fumigatus.98 These observations suggest the possibility that mapping of these polymorphisms may allow identification of subpopulations of patients at higher risk for IA that may benefit from more aggressive screening or prophylactic strategies.
Soluble factors that are critical for defense against invasive aspergillosis
Experimental studies in transgenic mice have identified a role for the soluble PRR pentraxin-3 in host defense against A. fumigatus. Pentraxin-3 binds to fungal galactomannan and enhances the uptake and killing of conidia by macrophages as well as activation of DCs.97,112,113 Immunocompetent mice deficient in pentraxin-3 were more susceptible to A. fumigatus infection and failed to mount a protective Th1 adaptive immune response to this organism.113 Administration of recombinant pentraxin-3 protects against A. fumigatus infection in a murine model of allogeneic transplantation in which mice were challenged with A. fumigatus.114 In this model, treatment with pentraxin-3 results in accelerated recovery of pulmonary phagocytic cell populations and Th1 lymphocytes and was synergistic with amphotericin B therapy.115,116 Consistent with these experimental studies, it has been reported that HSCT recipients transplanted with donor cells containing a polymorphism in the pentraxin-3 gene PTX3, displayed a higher incidence of IA.117 The presence of this polymorphism was associated with reduced levels of pentraxin-3 production by neutrophils and a functional defect in neutrophil phagocytosis and killing of A. fumigatus in vitro.117 Collectively these data suggest a possible role for screening of HSCT patients with IA in order to identify pentraxin-3 deficient patients that may benefit from replacement therapy. Genome-wide association studies are underway to explore host susceptibility to IA based on genetic defects, which can be used as a basis for clinical trials involving medical intervention based on patient genetic screening and immune profiling.118
Two recent experimental studies have examined the role of IL-1α and inflammasome-dependent IL-1β in the pathogenesis of IA.119,120 In neutropenic mice, the processing of IL-1β by the NLRP3 inflammasome is critical for defense against pulmonary challenge by A. fumigatus.119 In contrast, a more modest role for inflammasome-dependent IL-1β was reported in immunocompetent mice challenged with a high dose of conidia.121 In this model, IL-1α played a dominant role in enhancing the recruitment and activity of neutrophils.121 The role of IL-1β and IL-1α in the pathogenesis of IA in non-neutropenic immunocompromised hosts remains unstudied. Interestingly, a polymorphism in NLRP3 (rs35829419, Q705K) was found to be associated with the development of IA in HSCT patients when present in either the donor or recipient.122 Surprisingly, however, this polymorphism was reported to be associated with increased activation of the inflammasome and IL-1β production. Further studies will be required in human populations to better understand the importance of the inflammasome in mediating antifungal defense during HSCT.
Epithelial cells in the innate defense against A. fumigatus infection
Successful infection of pulmonary epithelial cells by A. fumigatus conidia and hyphae has long been hypothesized to play an important role in the pathogenesis of invasive aspergillosis.123-126 In a mouse model of chemotherapy-induced neutropenia, treatment of mice with an inhaled synergic combination of TLR2, 6 and 9 agonists induced protection against A. fumigatus, by enhancing mucosal and epithelial cell responses.28 Similar protection was observed against other pathogens including Pseudomonas aeruginosa, suggesting that enhancing epithelial cell resistance to infection may be a useful strategy to reduce mortality due to opportunistic pneumonia in the HSCT population.
The adaptive immune response to aspergillosis in HSCT patients
The importance of cellular and acquired immunity in the protection against A. fumigatus infection is well established in mouse models of IA. Early studies in these models showed that production of Th1-type cytokines such as IFNγ, TNFα and IL-12 is associated with resistance to disease, while the production of Th2 cytokines such as IL-4 is associated with disease progression (Figure 1).127 The association of a Th2 cytokine environment with IA was shown through early experiments using transgenic mice deficient in IL-4. In both immunocompetent and neutropenic mouse models, IL-4-/- mice were protected against IA.128 Interventional studies with adoptive transfer of immune cells have highlighted the importance of Th1-mediated immunity in resistance to A. fumigatus infection. Vaccination of immunocompetent and neutropenic mice with T cells and DCs pulsed with Aspergillus antigens in order to induce Th1 mediated resistance was associated with enhanced survival following fungal challenge.129,130 Studies of human DCs in vitro have found that these cells produce chemokines and other ligands CCL3, CCL4, CXCL8, CXCL10 and CCL20 that stimulate migration of polymorphonuclear cells (PMNs) and memory Th1 cells to the site of infection aiding in host resistance (Figure 1).131,132 Interestingly in humans, polymorphisms in chemokine ligand CXCL10 resulting in decreased DC CXCL10 production have been associated with increased risk of IA in HSCT patients.22,133 Further studies, using DC vaccines to promote Th1 immunity, have shown promise in HSCT mouse models.134,135 In these studies, adoptive transfer of DCs pulsed with A. fumigatus conidia or transfected with A. fumigatus conidial RNA induced the production of IFNγ producing Th1 cells in mice following HSCT and increased resistance to A. fumigatus challenge.134 Interestingly, resistance to IA was not observed when DCs were pulsed with A. fumigatus hyphae. In other studies, HSCT mice treated with Aspergillus specific Th1 cells by adoptive transfer also exhibited increased resistance to A. fumigatus infection.134,136 More recently, the adoptive transfer of transgenic T-cells engineered to express a chimeric antigen receptor which incorporated the extracellular portion of the β-glucan receptor, dectin-1, was reported to reduce fungal burden in a neutropenic model of IA.137 Building on these experimental studies, the role of fungal specific T-cells in the treatment or prevention of IA in HSCT patients is an area of intense clinical interest, and holds great potential for the management of this infection, although significant challenges remain in translating these experimental techniques to clinical practice.
As with the Th2 cytokine IL-4, the immunosuppressive cytokine, IL-10, produced by regulatory T cells, enhances susceptibility to IA (Figure 1). In non-neutropenic mice pre-treated with corticosteroids, high levels of pulmonary IL-10 expression were associated with IA mortality.138,139 In both immunocompetent and non-neutropenic mouse models, IL-10-/- mice were better able to control fungal infections and survived longer when challenged with lethal doses of A. fumigatus.138,139 Polymorphisms in the IL-10 promoter gene have been identified in HSCT patients and have been linked to GVHD and susceptibility to IA.140-142 IL-10 promoter polymorphisms resulting in increased transcript levels are associated with increased susceptibility to both GVHD and IA,141 while patients with promoter polymorphisms resulting in low to undetectable transcript levels were much more tolerant to A. fumigatus infection and disease.142 Further study of IL-10 promoter polymorphisms in humans may be useful both to identify HSCT patients susceptible to IA and to understand the role this cytokine plays in host immunity.
The role of the IL-23 – IL-17 axis in the pathogenesis of IA is somewhat controversial. IL-23 produced by macrophages and DCs in response to A. fumigatus leads to Th17 polarization and IL-17 production during experimental infection.3,143 An initial study reported that neutralization of IL-17 and IL-23 in immunocompetent mice improved the outcome of IA and suggested that the IL-23/IL-17 axis inhibits protective Th1 mediated responses while inducing damaging host inflammatory responses.144 However, other studies using anti-IL-17 antibodies have reported that neutralization of IL-17A renders mice more susceptible to A. fumigatus infection.93 Similarly, in an experimental model of pulmonary A. fumigatus colonization in immunocompetent mice, clearance of the fungus from the airways was associated with the development of Th17 responses,145 and IL-17 production by neutrophils has been found to be protective in a model of A. fumigatus keratitis.144 Similar conflicting results have been reported from human studies. A. fumigatus was a weak inducer of IL-17 production in human T-cells in vitro, and low levels of IL-17 were detected in the bronchoalveolar lavage (BAL) of patients with IA.146,147 Active down regulation of IL-17 production through fungal tryptophan metabolism and kynurenine production was suggested as a mechanism whereby A. fumigatus evades Th17 responses.146 Polymorphisms in IL-23 but not IL-17A and IL-17F genes were associated with lower rates of IA in a cohort analysis of T-cell depleted allogeneic HSCT patients.148 Overall, the role of the Th17 response and IL-17 in IA remains poorly understood, and further studies are required to evaluate the utility of treatment strategies targeting this immune axis.
Finally, although Aspergillus antibody responses have largely been viewed as non-protective in IA, several studies using carbohydrate antigens and anti-carbohydrate monoclonal antibodies have challenged this dogma. Active vaccination of mice with β-glucan conjugated to diphtheria toxoid protected immunocompetent mice from intravenous challenge with A. fumigatus, although protection of immunosuppressed mice and efficacy in pulmonary disease were not evaluated.149 In a more recent study, administration of a monoclonal IgM antibody targeting a sialylated β-1,3-linked oligosaccharide was found to protect mice from intravenous challenge with A. fumigatus.150 Excitingly, treatment with this antibody also improved the survival of neutropenic mice infected intratracheally with A. fumigatus though not when these mice were infected intravenously. The mechanism by which this monoclonal antibody mediates protection in neutropenic mice is unclear, but may involve complement and/or enhancing phagocytic killing by non-neutrophil leukocytes. The findings of these studies suggest the exciting possibility that passive therapy with anti-Aspergillus antibodies could be an effective strategy for the prevention of IA during HSCT.
Conclusion
The pathogenesis of IA is governed by a complex interplay between environmental conditions, fungal virulence factors and the host immune response. Patients undergoing HSCT encompass a range of different physiological and immunological environments as a function of their underlying hematological disease; the chemotherapy and conditioning regimens administered pre-transplant; the engraftment status and degree of neutropenia; the presence of secondary infections that modulate host immunity such as CMV; and the degree of GVHD and the immunosuppressive therapies used for this condition. Many fungal virulence factors and host immune parameters are therefore likely to play an important role in pathogenesis in select groups of patients at specific times during their course of treatment. This level of complexity is a major challenge for the study of host-pathogen interactions in this population and will necessitate the validation of experimental results in multiple animal models as well as human populations. The development of robust and practical animal models for leukemia, HSCT and GVHD will be critical tools for the advancement of this field. Nonetheless, there is cause for optimism. Human genetic association studies have validated many of the findings in mouse studies of Aspergillus immunity. The identification of these key host determinants of susceptibility may lead to pre-transplant screening algorithms that can identify very high-risk patients who would be candidates for aggressive surveillance or antifungal prophylactic strategies and the development of novel immunotherapies for the prevention and treatment of IA. Similarly, the recent identification of novel fungal virulence factors may lead to the development of new antifungal strategies for this important disease. Collectively, these advances hold promise for an era of new therapies targeting the host-fungal interphase in order to reduce mortality due to IA in this vulnerable population.
Abbreviations
- BAL
bronchoalveolar lavage
- CT
computed tomography
- DC
dendritic cell
- GAG
galactosaminogalactan
- GVHD
graft-vs-host disease
- HSCT
haematopoietic stem cell transplantation
- IA
invasive aspergillosis
- MBL
mannose-binding lectin
- PMN
polymorphonuclear cell
- PRR
pattern recognition receptor
- SP
surfactant protein
- SREBP
sterol regulatory element binding protein
- TLR
toll-like receptor
- TNF
tumor necrosis factor
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin Microbiol Rev 2009; 22:447-65; PMID:19597008; http://dx.doi.org/ 10.1128/CMR.00055-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Latge JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol 2001; 9:382-9; PMID:11514221; http://dx.doi.org/ 10.1016/S0966-842X(01)02104-7 [DOI] [PubMed] [Google Scholar]
- [3].Zelante T, Bozza S, De Luca A, D'Angelo C, Bonifazi P, Moretti S, Giovannini G, Bistoni F, Romani L. Th17 cells in the setting of Aspergillus infection and pathology. Med Mycol 2009; 47 Suppl 1:S162-9; PMID:18608926; http://dx.doi.org/ 10.1080/13693780802140766 [DOI] [PubMed] [Google Scholar]
- [4].Bhabhra R, Askew DS. Thermotolerance and virulence of Aspergillus fumigatus: role of the fungal nucleolus. Med Mycol 2005; 43 Suppl 1:S87-93; http://dx.doi.org/ 10.1080/13693780400029486 [DOI] [PubMed] [Google Scholar]
- [5].Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med 2006; 173:707-17; PMID:16387806; http://dx.doi.org/ 10.1164/rccm.200505-727SO [DOI] [PubMed] [Google Scholar]
- [6].McNeil MM, Nash SL, Hajjeh RA, Phelan MA, Conn LA, Plikaytis BD, Warnock DW. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin Infect Dis 2001; 33:641-7; PMID:11486286; http://dx.doi.org/ 10.1086/322606 [DOI] [PubMed] [Google Scholar]
- [7].Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis 2007; 44:531-40; PMID:17243056; http://dx.doi.org/ 10.1086/510592 [DOI] [PubMed] [Google Scholar]
- [8].Paterson DL, Singh N. Invasive aspergillosis in transplant recipients. Medicine (Baltimore) 1999; 78:123-38; PMID:10195093; http://dx.doi.org/ 10.1097/00005792-199903000-00003 [DOI] [PubMed] [Google Scholar]
- [9].Marks DI, Pagliuca A, Kibbler CC, Glasmacher A, Heussel CP, Kantecki M, Miller PJ, Ribaud P, Schlamm HT, Solano C, et al.. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br J Haematol 2011; 155:318-27; PMID:21880032; http://dx.doi.org/ 10.1111/j.1365-2141.2011.08838.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, Gersten ID, Mendizabal AM, Leather HL, Confer DL, et al.. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010; 116:5111-8; PMID:20826719; http://dx.doi.org/ 10.1182/blood-2010-02-268151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Segal BH. Aspergillosis. N Engl J Med 2009; 360:1870-84; PMID:19403905; http://dx.doi.org/ 10.1056/NEJMra0808853 [DOI] [PubMed] [Google Scholar]
- [12].Meersseman W, Lagrou K, Maertens J, Wilmer A, Hermans G, Vanderschueren S, Spriet I, Verbeken E, Van Wijngaerden E. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med 2008; 177:27-34; PMID:17885264; http://dx.doi.org/ 10.1164/rccm.200704-606OC [DOI] [PubMed] [Google Scholar]
- [13].Chiang LY, Sheppard DC, Gravelat FN, Patterson TF, Filler SG. Aspergillus fumigatus stimulates leukocyte adhesion molecules and cytokine production by endothelial cells in vitro and during invasive pulmonary disease. Infect Immun 2008; 76:3429-38; PMID:18490455; http://dx.doi.org/ 10.1128/IAI.01510-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stergiopoulou T, Meletiadis J, Roilides E, Kleiner DE, Schaufele R, Roden M, Harrington S, Dad L, Segal B, Walsh TJ. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am J Clin Pathol 2007; 127:349-55; PMID:17276936; http://dx.doi.org/ 10.1309/UJRV9DLC11RM3G8R [DOI] [PubMed] [Google Scholar]
- [15].Nucci M, Nouer SA, Cappone D, Anaissie E. Early diagnosis of invasive pulmonary aspergillosis in hematologic patients: an opportunity to improve the outcome. Haematologica 2013; 98:1657-60; PMID:24186309; http://dx.doi.org/ 10.3324/haematol.2013.094359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bergeron A, Porcher R, Sulahian A, de Bazelaire C, Chagnon K, Raffoux E, Vekhoff A, Cornet M, Isnard F, Brethon B, et al.. The strategy for the diagnosis of invasive pulmonary aspergillosis should depend on both the underlying condition and the leukocyte count of patients with hematologic malignancies. Blood 2012; 119:1831-7; quiz 956; PMID:22010103; http://dx.doi.org/ 10.1182/blood-2011-04-351601 [DOI] [PubMed] [Google Scholar]
- [17].Xu XY, Sun HM, Zhao BL, Shi Y. Diagnosis of airway-invasive pulmonary aspergillosis by tree-in-bud sign in an immunocompetent patient: case report and literature review. J Mycol Med 2013; 23:64-9; PMID:23375859; http://dx.doi.org/ 10.1016/j.mycmed.2012.12.050 [DOI] [PubMed] [Google Scholar]
- [18].Cenci E, Mencacci A, Spreca A, Montagnoli C, Bacci A, Perruccio K, Velardi A, Magliani W, Conti S, Polonelli L, et al.. Protection of killer antiidiotypic antibodies against early invasive aspergillosis in a murine model of allogeneic T-cell-depleted bone marrow transplantation. Infect Immun 2002; 70:2375-82; PMID:11953373; http://dx.doi.org/ 10.1128/IAI.70.5.2375-2382.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sheppard DC, Rieg G, Chiang LY, Filler SG, Edwards JE Jr, Ibrahim AS. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2004; 48:1908-11; PMID:15105158; http://dx.doi.org/ 10.1128/AAC.48.5.1908-1911.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Balloy V, Huerre M, Latge JP, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun 2005; 73:494-503; PMID:15618189; http://dx.doi.org/ 10.1128/IAI.73.1.494-503.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Steinbach WJ, Benjamin DK Jr, Trasi SA, Miller JL, Schell WA, Zaas AK, Foster WM, Perfect JR. Value of an inhalational model of invasive aspergillosis. Med Mycol 2004; 42:417-25; PMID:15552643; http://dx.doi.org/ 10.1080/13693780410001712034 [DOI] [PubMed] [Google Scholar]
- [22].Zaas AK, Liao G, Chien JW, Weinberg C, Shore D, Giles SS, Marr KA, Usuka J, Burch LH, Perera L, et al.. Plasminogen alleles influence susceptibility to invasive aspergillosis. PLoS Genet 2008; 4:e1000101; PMID:18566672; http://dx.doi.org/ 10.1371/journal.pgen.1000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, Hohl TM. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis 2009; 200:647-56; PMID:19591573; http://dx.doi.org/ 10.1086/600380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mencacci A, Perruccio K, Bacci A, Cenci E, Benedetti R, Martelli MF, Bistoni F, Coffman R, Velardi A, Romani L. Defective antifungal T-helper 1 (TH1) immunity in a murine model of allogeneic T-cell-depleted bone marrow transplantation and its restoration by treatment with TH2 cytokine antagonists. Blood 2001; 97:1483-90; PMID:11222397; http://dx.doi.org/ 10.1182/blood.V97.5.1483 [DOI] [PubMed] [Google Scholar]
- [25].BitMansour A, Burns SM, Traver D, Akashi K, Contag CH, Weissman IL, Brown JM. Myeloid progenitors protect against invasive aspergillosis and Pseudomonas aeruginosa infection following hematopoietic stem cell transplantation. Blood 2002; 100:4660-7; PMID:12393415; http://dx.doi.org/ 10.1182/blood-2002-05-1552 [DOI] [PubMed] [Google Scholar]
- [26].Reddy P, Ferrara JLM. Mouse models of graft-versus-host disease StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute, 2008; PMID: 20614594.21558065 [PubMed] [Google Scholar]
- [27].Schroeder MA, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech 2011; 4:318-33; PMID:21558065; http://dx.doi.org/ 10.1242/dmm.006668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Leiva-Juarez MM, Ware HH, Kulkarni VV, Zweidler-McKay PA, Tuvim MJ, Evans SE. Inducible epithelial resistance protects mice against leukemia-associated pneumonia. Blood 2016; 128(7):982-92; PMID:27317793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Waring P, Eichner RD, Mullbacher A. The chemistry and biology of the immunomodulating agent gliotoxin and related epipolythiodioxopiperazines. Med Res Rev 1988; 8:499-524; PMID:2461498; http://dx.doi.org/ 10.1002/med.2610080404 [DOI] [PubMed] [Google Scholar]
- [30].Lewis RE, Wiederhold NP, Chi J, Han XY, Komanduri KV, Kontoyiannis DP, Prince RA. Detection of gliotoxin in experimental and human aspergillosis. Infect Immun 2005; 73:635-7; PMID:15618207; http://dx.doi.org/ 10.1128/IAI.73.1.635-637.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pahl HL, Krauss B, Schulze-Osthoff K, Decker T, Traenckner EB, Vogt M, Myers C, Parks T, Warring P, Muhlbacher A, et al.. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappaB. J Exp Med 1996; 183:1829-40; PMID:8666939; http://dx.doi.org/ 10.1084/jem.183.4.1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tsunawaki S, Yoshida LS, Nishida S, Kobayashi T, Shimoyama T. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect Immun 2004; 72:3373-82; PMID:15155643; http://dx.doi.org/ 10.1128/IAI.72.6.3373-3382.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Comera C, Andre K, Laffitte J, Collet X, Galtier P, Maridonneau-Parini I. Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signalling pathways in human neutrophils. Microbes Infect 2007; 9:47-54; PMID:17196420; http://dx.doi.org/ 10.1016/j.micinf.2006.10.009 [DOI] [PubMed] [Google Scholar]
- [34].Schlam D, Canton J, Carreno M, Kopinski H, Freeman SA, Grinstein S, Fairn GD. Gliotoxin suppresses macrophage immune function by subverting phosphatidylinositol 3,4,5-Trisphosphate Homeostasis. MBio 2016; 7:e02242; PMID:27048806; http://dx.doi.org/ 10.1128/mBio.02242-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Orciuolo E, Stanzani M, Canestraro M, Galimberti S, Carulli G, Lewis R, Petrini M, Komanduri KV. Effects of Aspergillus fumigatus gliotoxin and methylprednisolone on human neutrophils: implications for the pathogenesis of invasive aspergillosis. J Leukoc Biol 2007; 82:839-48; PMID:17626149; http://dx.doi.org/ 10.1189/jlb.0207090 [DOI] [PubMed] [Google Scholar]
- [36].Stanzani M, Orciuolo E, Lewis R, Kontoyiannis DP, Martins SL, St John LS, Komanduri KV. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 2005; 105:2258-65; PMID:15546954; http://dx.doi.org/ 10.1182/blood-2004-09-3421 [DOI] [PubMed] [Google Scholar]
- [37].Bok JW, Chung D, Balajee SA, Marr KA, Andes D, Nielsen KF, Frisvad JC, Kirby KA, Keller NP. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect Immun 2006; 74:6761-8; PMID:17030582; http://dx.doi.org/ 10.1128/IAI.00780-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cramer RA Jr, Gamcsik MP, Brooking RM, Najvar LK, Kirkpatrick WR, Patterson TF, Balibar CJ, Graybill JR, Perfect JR, Abraham SN, et al.. Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot Cell 2006; 5:972-80; PMID:16757745; http://dx.doi.org/ 10.1128/EC.00049-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kupfahl C, Heinekamp T, Geginat G, Ruppert T, Hartl A, Hof H, Brakhage AA. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol Microbiol 2006; 62:292-302; PMID:16956378; http://dx.doi.org/ 10.1111/j.1365-2958.2006.05373.x [DOI] [PubMed] [Google Scholar]
- [40].Spikes S, Xu R, Nguyen CK, Chamilos G, Kontoyiannis DP, Jacobson RH, Ejzykowicz DE, Chiang LY, Filler SG, May GS. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis 2008; 197:479-86; PMID:18199036; http://dx.doi.org/ 10.1086/525044 [DOI] [PubMed] [Google Scholar]
- [41].Sugui JA, Pardo J, Chang YC, Zarember KA, Nardone G, Galvez EM, Mullbacher A, Gallin JI, Simon MM, Kwon-Chung KJ. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell 2007; 6:1562-9; PMID:17601876; http://dx.doi.org/ 10.1128/EC.00141-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bok JW, Balajee SA, Marr KA, Andes D, Nielsen KF, Frisvad JC, Keller NP. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot Cell 2005; 4:1574-82; PMID:16151250; http://dx.doi.org/ 10.1128/EC.4.9.1574-1582.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ben-Ami R, Lewis RE, Leventakos K, Kontoyiannis DP. Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 2009; 114:5393-9; PMID:19843884; http://dx.doi.org/ 10.1182/blood-2009-07-231209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sugui JA, Pardo J, Chang YC, Mullbacher A, Zarember KA, Galvez EM, Brinster L, Zerfas P, Gallin JI, Simon MM, et al.. Role of laeA in the Regulation of alb1, gliP, Conidial Morphology, and Virulence in Aspergillus fumigatus. Eukaryot Cell 2007; 6:1552-61; PMID:17630330; http://dx.doi.org/ 10.1128/EC.00140-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, et al.. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009; 460:1117-21; PMID:19713928; http://dx.doi.org/ 10.1038/nature08264 [DOI] [PubMed] [Google Scholar]
- [46].Carrion Sde J, Leal SM Jr, Ghannoum MA, Aimanianda V, Latge JP, Pearlman E. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J Immunol 2013; 191:2581-8; PMID:23926321; http://dx.doi.org/ 10.4049/jimmunol.1300748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Thau N, Monod M, Crestani B, Rolland C, Tronchin G, Latge JP, Paris S. rodletless mutants of Aspergillus fumigatus. Infect Immun 1994; 62:4380-8; PMID:7927699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chai LY, Netea MG, Sugui J, Vonk AG, van de Sande WW, Warris A, Kwon-Chung KJ, Kullberg BJ. Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 2010; 215:915-20; PMID:19939494; http://dx.doi.org/ 10.1016/j.imbio.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chai LY, Vonk AG, Kullberg BJ, Verweij PE, Verschueren I, van der Meer JW, Joosten LA, Latge JP, Netea MG. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect 2011; 13:151-9; PMID:20971208; http://dx.doi.org/ 10.1016/j.micinf.2010.10.005 [DOI] [PubMed] [Google Scholar]
- [50].Pihet M, Vandeputte P, Tronchin G, Renier G, Saulnier P, Georgeault S, Mallet R, Chabasse D, Symoens F, Bouchara JP. Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol 2009; 9:177; PMID:19703288; http://dx.doi.org/ 10.1186/1471-2180-9-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Heinekamp T, Thywissen A, Macheleidt J, Keller S, Valiante V, Brakhage AA. Aspergillus fumigatus melanins: interference with the host endocytosis pathway and impact on virulence. Front Microbiol 2012; 3:440; PMID:23346079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Thywissen A, Heinekamp T, Dahse HM, Schmaler-Ripcke J, Nietzsche S, Zipfel PF, Brakhage AA. Conidial Dihydroxynaphthalene Melanin of the Human Pathogenic Fungus Aspergillus fumigatus Interferes with the Host Endocytosis Pathway. Front Microbiol 2011; 2:96; PMID:21747802; http://dx.doi.org/ 10.3389/fmicb.2011.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Akoumianaki T, Kyrmizi I, Valsecchi I, Gresnigt MS, Samonis G, Drakos E, Boumpas D, Muszkieta L, Prevost MC, Kontoyiannis DP, et al.. Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity. Cell Host Microbe 2016; 19:79-90; PMID:26749442; http://dx.doi.org/ 10.1016/j.chom.2015.12.002 [DOI] [PubMed] [Google Scholar]
- [54].Chamilos G, Akoumianaki T, Kyrmizi I, Brakhage A, Beauvais A, Latge JP. Melanin Targets LC3-associated phagocytosis (LAP): a novel pathogenetic mechanism in fungal disease. Autophagy 2016; 12(5):888-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jacobson ES. Pathogenic roles for fungal melanins. Clin Microbiol Rev 2000; 13:708-17; PMID:11023965; http://dx.doi.org/ 10.1128/CMR.13.4.708-717.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jahn B, Koch A, Schmidt A, Wanner G, Gehringer H, Bhakdi S, Brakhage AA. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect Immun 1997; 65:5110-7; PMID:9393803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tsai HF, Chang YC, Washburn RG, Wheeler MH, Kwon-Chung KJ. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J Bacteriol 1998; 180:3031-8; PMID:9620950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Beauvais A, Maubon D, Park S, Morelle W, Tanguy M, Huerre M, Perlin DS, Latge JP. Two α(1-3) glucan synthases with different functions in Aspergillus fumigatus. Appl Environ Microbiol 2005; 71:1531-8; PMID:15746357; http://dx.doi.org/ 10.1128/AEM.71.3.1531-1538.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Maubon D, Park S, Tanguy M, Huerre M, Schmitt C, Prevost MC, Perlin DS, Latge JP, Beauvais A. AGS3, an α(1-3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet Biol 2006; 43:366-75; PMID:16531086; http://dx.doi.org/ 10.1016/j.fgb.2006.01.006 [DOI] [PubMed] [Google Scholar]
- [60].Beauvais A, Bozza S, Kniemeyer O, Formosa C, Balloy V, Henry C, Roberson RW, Dague E, Chignard M, Brakhage AA, et al.. Deletion of the α-(1,3)-glucan synthase genes induces a restructuring of the conidial cell wall responsible for the avirulence of Aspergillus fumigatus. PLoS Pathog 2013; 9:e1003716; PMID:24244155; http://dx.doi.org/ 10.1371/journal.ppat.1003716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Beauvais A, Fontaine T, Aimanianda V, Latge JP. Aspergillus cell wall and biofilm. Mycopathologia 2014; 178:371-7; PMID:24947169; http://dx.doi.org/ 10.1007/s11046-014-9766-0 [DOI] [PubMed] [Google Scholar]
- [62].Lee MJ, Geller AM, Bamford NC, Liu H, Gravelat FN, Snarr BD, Le Mauff F, Chabot J, Ralph B, Ostapska H, et al.. Deacetylation of fungal exopolysaccharide mediates adhesion and biofilm formation. MBio 2016; 7:e00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lee MJ, Gravelat FN, Cerone RP, Baptista SD, Campoli PV, Choe SI, Kravtsov I, Vinogradov E, Creuzenet C, Liu H, et al.. Overlapping and distinct roles of Aspergillus fumigatus UDP-glucose 4-epimerases in galactose metabolism and the synthesis of galactose-containing cell wall polysaccharides. J Biol Chem 2014; 289:1243-56; PMID:24257745; http://dx.doi.org/ 10.1074/jbc.M113.522516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, Xu W, Kravtsov I, Hoareau CM, Vanier G, et al.. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog 2013; 9:e1003575; PMID:23990787; http://dx.doi.org/ 10.1371/journal.ppat.1003575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lee MJ, Liu H, Barker BM, Snarr BD, Gravelat FN, Al Abdallah Q, Gavino C, Baistrocchi SR, Ostapska H, Xiao T, et al.. The fungal exopolysaccharide galactosaminogalactan mediates virulence by enhancing resistance to neutrophil extracellular traps. PLoS Pathog 2015; 11:e1005187; PMID:26492565; http://dx.doi.org/ 10.1371/journal.ppat.1005187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Robinet P, Baychelier F, Fontaine T, Picard C, Debre P, Vieillard V, Latge JP, Elbim C. A polysaccharide virulence factor of a human fungal pathogen induces neutrophil apoptosis via NK cells. J Immunol 2014; 192:5332-42; PMID:24790151; http://dx.doi.org/ 10.4049/jimmunol.1303180 [DOI] [PubMed] [Google Scholar]
- [67].Gresnigt MS, Bozza S, Becker KL, Joosten LA, Abdollahi-Roodsaz S, van der Berg WB, Dinarello CA, Netea MG, Fontaine T, De Luca A, et al.. A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist. PLoS Pathog 2014; 10:e1003936; PMID:24603878; http://dx.doi.org/ 10.1371/journal.ppat.1003936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Krappmann S, Braus GH. Nitrogen metabolism of Aspergillus and its role in pathogenicity. Med Mycol 2005; 43 Suppl 1:S31-40; PMID:16110790; http://dx.doi.org/ 10.1080/13693780400024271 [DOI] [PubMed] [Google Scholar]
- [69].Panepinto JC, Oliver BG, Fortwendel JR, Smith DL, Askew DS, Rhodes JC. Deletion of the Aspergillus fumigatus gene encoding the Ras-related protein RhbA reduces virulence in a model of Invasive pulmonary aspergillosis. Infect Immun 2003; 71:2819-26; PMID:12704156; http://dx.doi.org/ 10.1128/IAI.71.5.2819-2826.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hissen AH, Wan AN, Warwas ML, Pinto LJ, Moore MM. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect Immun 2005; 73:5493-503; PMID:16113265; http://dx.doi.org/ 10.1128/IAI.73.9.5493-5503.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, Arst HN Jr, Haynes K, Haas H. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med 2004; 200:1213-9; PMID:15504822; http://dx.doi.org/ 10.1084/jem.20041242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Schrettl M, Ibrahim-Granet O, Droin S, Huerre M, Latge JP, Haas H. The crucial role of the Aspergillus fumigatus siderophore system in interaction with alveolar macrophages. Microbes Infect 2010; 12:1035-41; PMID:20659583; http://dx.doi.org/ 10.1016/j.micinf.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, Wallner A, Arst HN Jr, Haynes K, Haas H. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog 2007; 3:1195-207; PMID:17845073; http://dx.doi.org/ 10.1371/journal.ppat.0030128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gsaller F, Hortschansky P, Beattie SR, Klammer V, Tuppatsch K, Lechner BE, Rietzschel N, Werner ER, Vogan AA, Chung D, et al.. The Janus transcription factor HapX controls fungal adaptation to both iron starvation and iron excess. EMBO J 2014; 33:2261-76; PMID:25092765; http://dx.doi.org/ 10.15252/embj.201489468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Liu H, Gravelat FN, Chiang LY, Chen D, Vanier G, Ejzykowicz DE, Ibrahim AS, Nierman WC, Sheppard DC, Filler SG. Aspergillus fumigatus AcuM regulates both iron acquisition and gluconeogenesis. Mol Microbiol 2010; 78:1038-54; PMID:21062375; http://dx.doi.org/ 10.1111/j.1365-2958.2010.07389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pongpom M, Liu H, Xu W, Snarr BD, Sheppard DC, Mitchell AP, Filler SG. Divergent targets of Aspergillus fumigatus AcuK and AcuM transcription factors during growth in vitro versus invasive disease. Infect Immun 2015; 83:923-33; PMID:25534941; http://dx.doi.org/ 10.1128/IAI.02685-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Altes A, Remacha AF, Sarda P, Sancho FJ, Sureda A, Martino R, Briones J, Brunet S, Canals C, Sierra J. Frequent severe liver iron overload after stem cell transplantation and its possible association with invasive aspergillosis. Bone Marrow Transplant 2004; 34:505-9; PMID:15286693; http://dx.doi.org/ 10.1038/sj.bmt.1704628 [DOI] [PubMed] [Google Scholar]
- [78].Kontoyiannis DP, Chamilos G, Lewis RE, Giralt S, Cortes J, Raad II, Manning JT, Han X. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer 2007; 110:1303-6; PMID:17614303; http://dx.doi.org/ 10.1002/cncr.22909 [DOI] [PubMed] [Google Scholar]
- [79].Hohl TM, Feldmesser M. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot Cell 2007; 6:1953-63; PMID:17890370; http://dx.doi.org/ 10.1128/EC.00274-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Brown NA, Goldman GH. The contribution of Aspergillus fumigatus stress responses to virulence and antifungal resistance. J Microbiol 2016; 54:243-53; PMID:26920884; http://dx.doi.org/ 10.1007/s12275-016-5510-4 [DOI] [PubMed] [Google Scholar]
- [81].Willger SD, Puttikamonkul S, Kim KH, Burritt JB, Grahl N, Metzler LJ, Barbuch R, Bard M, Lawrence CB, Cramer RA Jr. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog 2008; 4:e1000200; PMID:18989462; http://dx.doi.org/ 10.1371/journal.ppat.1000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chung D, Barker BM, Carey CC, Merriman B, Werner ER, Lechner BE, Dhingra S, Cheng C, Xu W, Blosser SJ, et al.. ChIP-seq and in vivo transcriptome analyses of the Aspergillus fumigatus SREBP SrbA reveals a new regulator of the fungal hypoxia response and virulence. PLoS Pathog 2014; 10:e1004487; PMID:25375670; http://dx.doi.org/ 10.1371/journal.ppat.1004487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Blatzer M, Barker BM, Willger SD, Beckmann N, Blosser SJ, Cornish EJ, Mazurie A, Grahl N, Haas H, Cramer RA. SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus. PLoS Genet 2011; 7:e1002374; PMID:22144905; http://dx.doi.org/ 10.1371/journal.pgen.1002374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Schrettl M, Beckmann N, Varga J, Heinekamp T, Jacobsen ID, Jochl C, Moussa TA, Wang S, Gsaller F, Blatzer M, et al.. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog 2010; 6:e1001124; PMID:20941352; http://dx.doi.org/ 10.1371/journal.ppat.1001124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kroll K, Shekhova E, Mattern DJ, Thywissen A, Jacobsen ID, Strassburger M, Heinekamp T, Shelest E, Brakhage AA, Kniemeyer O. The hypoxia-induced dehydrogenase HorA is required for coenzyme Q10 biosynthesis, azole sensitivity and virulence of Aspergillus fumigatus. Mol Microbiol 2016; 101(1):92-108; PMID:26991818 [DOI] [PubMed] [Google Scholar]
- [86].Vaknin Y, Hillmann F, Iannitti R, Ben Baruch N, Sandovsky-Losica H, Shadkchan Y, Romani L, Brakhage A, Kniemeyer O, Osherov N. Identification and characterization of a novel Aspergillus fumigatus rhomboid family putative protease RbdA involved in hypoxia sensing and virulence. Infect Immun 2016; 84(6):1866-78; PMID:27068092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Barkal LJ, Theberge AB, Guo CJ, Spraker J, Rappert L, Berthier J, Brakke KA, Wang CC, Beebe DJ, Keller NP, et al.. Microbial metabolomics in open microscale platforms. Nat Commun 2016; 7:10610; PMID:26842393; http://dx.doi.org/ 10.1038/ncomms10610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Barkal LJ, Walsh NM, Botts MR, Beebe DJ, Hull CM. Leveraging a high resolution microfluidic assay reveals insights into pathogenic fungal spore germination. Integr Biol (Camb) 2016; 8:603-15; PMID:27026574; http://dx.doi.org/ 10.1039/C6IB00012F [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ford S, Friedman L. Experimental study of the pathogenicity of aspergilli for mice. J Bacteriol 1967; 94:928-33; PMID:6051365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Slesiona S, Ibrahim-Granet O, Olias P, Brock M, Jacobsen ID. Murine infection models for Aspergillus terreus pulmonary aspergillosis reveal long-term persistence of conidia and liver degeneration. J Infect Dis 2012; 205:1268-77; PMID:22438397; http://dx.doi.org/ 10.1093/infdis/jis193 [DOI] [PubMed] [Google Scholar]
- [91].Person AK, Kontoyiannis DP, Alexander BD. Fungal infections in transplant and oncology patients. Infect Dis Clin North Am 2010; 24:439-59; PMID:20466278; http://dx.doi.org/ 10.1016/j.idc.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].de Boer MG, Jolink H, Halkes CJ, van der Heiden PL, Kremer D, Falkenburg JH, van de Vosse E, van Dissel JT. Influence of polymorphisms in innate immunity genes on susceptibility to invasive aspergillosis after stem cell transplantation. PLoS One 2011; 6:e18403; PMID:21483748; http://dx.doi.org/ 10.1371/journal.pone.0018403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite role for the dectin-1 β-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 2009; 182:4938-46; PMID:19342673; http://dx.doi.org/ 10.4049/jimmunol.0804250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol 2011; 14:392-9; PMID:21803640; http://dx.doi.org/ 10.1016/j.mib.2011.07.001 [DOI] [PubMed] [Google Scholar]
- [95].Kyrmizi I, Gresnigt MS, Akoumianaki T, Samonis G, Sidiropoulos P, Boumpas D, Netea MG, van de Veerdonk FL, Kontoyiannis DP, Chamilos G. Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting dectin-1/Syk kinase signaling. J Immunol 2013; 191:1287-99; PMID:23817424; http://dx.doi.org/ 10.4049/jimmunol.1300132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tam JM, Mansour MK, Khan NS, Seward M, Puranam S, Tanne A, Sokolovska A, Becker CE, Acharya M, Baird MA, et al.. Dectin-1-dependent LC3 recruitment to phagosomes enhances fungicidal activity in macrophages. J Infect Dis 2014; 210:1844-54; PMID:24842831; http://dx.doi.org/ 10.1093/infdis/jiu290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cramer RA, Rivera A, Hohl TM. Immune responses against Aspergillus fumigatus: what have we learned? Curr Opin Infect Dis 2011; 24:315-22; PMID:21666456; http://dx.doi.org/ 10.1097/QCO.0b013e328348b159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D'Angelo C, Pierini A, Pitzurra L, Falzetti F, et al.. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 2010; 116:5394-402; PMID:20807886; http://dx.doi.org/ 10.1182/blood-2010-04-279307 [DOI] [PubMed] [Google Scholar]
- [99].Gersuk GM, Underhill DM, Zhu L, Marr KA. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J Immunol 2006; 176:3717-24; PMID:16517740; http://dx.doi.org/ 10.4049/jimmunol.176.6.3717 [DOI] [PubMed] [Google Scholar]
- [100].Liu ZC, Wang M, Sun WK, Xia D, Tan MM, Ding Y, Qian Q, Su X, Shi Y. Up-regulation of Dectin-1 in airway epithelial cells promotes mice defense against invasive pulmonary aspergillosis. Int J Clin Exp Med 2015; 8:17489-97; PMID:26770339 [PMC free article] [PubMed] [Google Scholar]
- [101].Mattila PE, Metz AE, Rapaka RR, Bauer LD, Steele C. Dectin-1 Fc targeting of aspergillus fumigatus β-glucans augments innate defense against invasive pulmonary aspergillosis. Antimicrob Agents Chemother 2008; 52:1171-2; PMID:18086835; http://dx.doi.org/ 10.1128/AAC.01274-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Rubino I, Coste A, Le Roy D, Roger T, Jaton K, Boeckh M, Monod M, Latge JP, Calandra T, Bochud PY. Species-specific recognition of Aspergillus fumigatus by Toll-like receptor 1 and Toll-like receptor 6. J Infect Dis 2012; 205:944-54; PMID:22315281; http://dx.doi.org/ 10.1093/infdis/jir882 [DOI] [PubMed] [Google Scholar]
- [103].Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The β-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 2005; 1:e42; PMID:16344862; http://dx.doi.org/ 10.1371/journal.ppat.0010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Balloy V, Si-Tahar M, Takeuchi O, Philippe B, Nahori MA, Tanguy M, Huerre M, Akira S, Latge JP, Chignard M. Involvement of toll-like receptor 2 in experimental invasive pulmonary aspergillosis. Infect Immun 2005; 73:5420-5; PMID:16113258; http://dx.doi.org/ 10.1128/IAI.73.9.5420-5425.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol 2004; 172:3059-69; PMID:14978111; http://dx.doi.org/ 10.4049/jimmunol.172.5.3059 [DOI] [PubMed] [Google Scholar]
- [106].Kasperkovitz PV, Cardenas ML, Vyas JM. TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J Immunol 2010; 185:7614-22; PMID:21059889; http://dx.doi.org/ 10.4049/jimmunol.1002760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Khan NS, Kasperkovitz PV, Timmons AK, Mansour MK, Tam JM, Seward MW, Reedy JL, Puranam S, Feliu M, Vyas JM. Dectin-1 Controls TLR9 Trafficking to Phagosomes Containing β-1,3 Glucan. J Immunol 2016; 196:2249-61; PMID:26829985; http://dx.doi.org/ 10.4049/jimmunol.1401545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, Birrell MA, Saijo S, Mostowy S, Shaunak S, et al.. Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol Med 2015; 7:240-58; PMID:25637383; http://dx.doi.org/ 10.15252/emmm.201404556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bochud PY, Chien JW, Marr KA, Leisenring WM, Upton A, Janer M, Rodrigues SD, Li S, Hansen JA, Zhao LP, et al.. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med 2008; 359:1766-77; PMID:18946062; http://dx.doi.org/ 10.1056/NEJMoa0802629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Carvalho A, Cunha C, Carotti A, Aloisi T, Guarrera O, Di Ianni M, Falzetti F, Bistoni F, Aversa F, Pitzurra L, et al.. Polymorphisms in Toll-like receptor genes and susceptibility to infections in allogeneic stem cell transplantation. Exp Hematol 2009; 37:1022-9; PMID:19539691; http://dx.doi.org/ 10.1016/j.exphem.2009.06.004 [DOI] [PubMed] [Google Scholar]
- [111].Kesh S, Mensah NY, Peterlongo P, Jaffe D, Hsu K, VAN DEN Brink M, O'Reilly R, Pamer E, Satagopan J, Papanicolaou GA. TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive aspergillosis after allogeneic stem cell transplantation. Ann N Y Acad Sci 2005; 1062:95-103; PMID:16461792; http://dx.doi.org/ 10.1196/annals.1358.012 [DOI] [PubMed] [Google Scholar]
- [112].Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B, Peri G, et al.. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 2002; 420:182-6; PMID:12432394; http://dx.doi.org/ 10.1038/nature01195 [DOI] [PubMed] [Google Scholar]
- [113].Bottazzi B, Garlanda C, Cotena A, Moalli F, Jaillon S, Deban L, Mantovani A. The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: interplay with cellular innate immunity. Immunol Rev 2009; 227:9-18; PMID:19120471; http://dx.doi.org/ 10.1111/j.1600-065X.2008.00719.x [DOI] [PubMed] [Google Scholar]
- [114].Gaziano R, Bozza S, Bellocchio S, Perruccio K, Montagnoli C, Pitzurra L, Salvatori G, De Santis R, Carminati P, Mantovani A, et al.. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob Agents Chemother 2004; 48:4414-21; PMID:15504871; http://dx.doi.org/ 10.1128/AAC.48.11.4414-4421.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kaur S, Gupta VK, Thiel S, Sarma PU, Madan T. Protective role of mannan-binding lectin in a murine model of invasive pulmonary aspergillosis. Clin Exp Immunol 2007; 148:382-9; PMID:17335555; http://dx.doi.org/ 10.1111/j.1365-2249.2007.03351.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Madan T, Reid KB, Singh M, Sarma PU, Kishore U. Susceptibility of mice genetically deficient in the surfactant protein (SP)-A or SP-D gene to pulmonary hypersensitivity induced by antigens and allergens of Aspergillus fumigatus. J Immunol 2005; 174:6943-54; PMID:15905537; http://dx.doi.org/ 10.4049/jimmunol.174.11.6943 [DOI] [PubMed] [Google Scholar]
- [117].Cunha C, Aversa F, Lacerda JF, Busca A, Kurzai O, Grube M, Loffler J, Maertens JA, Bell AS, Inforzato A, et al.. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N Engl J Med 2014; 370:421-32; PMID:24476432; http://dx.doi.org/ 10.1056/NEJMoa1211161 [DOI] [PubMed] [Google Scholar]
- [118].Oliveira-Coelho A, Rodrigues F, Campos A Jr, Lacerda JF, Carvalho A, Cunha C. Paving the way for predictive diagnostics and personalized treatment of invasive aspergillosis. Front Microbiol 2015; 6:411; PMID:25999936; http://dx.doi.org/ 10.3389/fmicb.2015.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Karki R, Man SM, Malireddi RK, Gurung P, Vogel P, Lamkanfi M, Kanneganti TD. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 2015; 17:357-68; PMID:25704009; http://dx.doi.org/ 10.1016/j.chom.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Said-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One 2010; 5:e10008; PMID:20368800; http://dx.doi.org/ 10.1371/journal.pone.0010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, Hilmer KM, Thammahong A, Barker BM, Rivera A, et al.. IL-1alpha signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog 2015; 11:e1004625; PMID:25629406; http://dx.doi.org/ 10.1371/journal.ppat.1004625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Cunha C, Galosi C, Puccetti M, Aversa F, Romani L, Carvalho A. The Q705K genetic variant in NLRP3 leads to inflammasome hyperactivation and contributes to invasive aspergillosis after stem cell transplantation. Front. Immunol. Conference Abstract: 15th International Congress of Immunology (ICI); http://dx.doi.org/ 10.3389/conf.fimmu.2013.02.01022 [DOI]
- [123].Croft CA, Culibrk L, Moore MM, Tebbutt SJ. Interactions of Aspergillus fumigatus conidia with airway epithelial cells: A critical review. Front Microbiol 2016; 7:472; PMID:27092126; http://dx.doi.org/ 10.3389/fmicb.2016.00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Sheppard DC, Filler SG. Host cell invasion by medically important fungi. Cold Spring Harb Perspect Med 2015; 5:a019687; http://dx.doi.org/ 10.1101/cshperspect.a019687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog 2006; 2:e129; PMID:17196036; http://dx.doi.org/ 10.1371/journal.ppat.0020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sheppard DC. Molecular mechanism of Aspergillus fumigatus adherence to host constituents. Curr Opin Microbiol 2011; 14:375-9; PMID:21784698; http://dx.doi.org/ 10.1016/j.mib.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Hebart H, Bollinger C, Fisch P, Sarfati J, Meisner C, Baur M, Loeffler J, Monod M, Latge JP, Einsele H. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood 2002; 100:4521-8; PMID:12393638; http://dx.doi.org/ 10.1182/blood-2002-01-0265 [DOI] [PubMed] [Google Scholar]
- [128].Cenci E, Mencacci A, Del Sero G, Bacci A, Montagnoli C, d'Ostiani CF, Mosci P, Bachmann M, Bistoni F, Kopf M, et al.. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J Infect Dis 1999; 180:1957-68; PMID:10558953; http://dx.doi.org/ 10.1086/315142 [DOI] [PubMed] [Google Scholar]
- [129].Bozza S, Gaziano R, Lipford GB, Montagnoli C, Bacci A, Di Francesco P, Kurup VP, Wagner H, Romani L. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect 2002; 4:1281-90; PMID:12443892; http://dx.doi.org/ 10.1016/S1286-4579(02)00007-2 [DOI] [PubMed] [Google Scholar]
- [130].Cenci E, Mencacci A, Bacci A, Bistoni F, Kurup VP, Romani L. T cell vaccination in mice with invasive pulmonary aspergillosis. J Immunol 2000; 165:381-8; PMID:10861075; http://dx.doi.org/ 10.4049/jimmunol.165.1.381 [DOI] [PubMed] [Google Scholar]
- [131].Gafa V, Lande R, Gagliardi MC, Severa M, Giacomini E, Remoli ME, Nisini R, Ramoni C, Di Francesco P, Aldebert D, et al.. Human dendritic cells following Aspergillus fumigatus infection express the CCR7 receptor and a differential pattern of interleukin-12 (IL-12), IL-23, and IL-27 cytokines, which lead to a Th1 response. Infect Immun 2006; 74:1480-9; PMID:16495518; http://dx.doi.org/ 10.1128/IAI.74.3.1480-1489.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Gafa V, Remoli ME, Giacomini E, Gagliardi MC, Lande R, Severa M, Grillot R, Coccia EM. In vitro infection of human dendritic cells by Aspergillus fumigatus conidia triggers the secretion of chemokines for neutrophil and Th1 lymphocyte recruitment. Microbes Infect 2007; 9:971-80; PMID:17556001; http://dx.doi.org/ 10.1016/j.micinf.2007.03.015 [DOI] [PubMed] [Google Scholar]
- [133].Mezger M, Steffens M, Beyer M, Manger C, Eberle J, Toliat MR, Wienker TF, Ljungman P, Hebart H, Dornbusch HJ, et al.. Polymorphisms in the chemokine (C-X-C motif) ligand 10 are associated with invasive aspergillosis after allogeneic stem-cell transplantation and influence CXCL10 expression in monocyte-derived dendritic cells. Blood 2008; 111:534-6; PMID:17957030; http://dx.doi.org/ 10.1182/blood-2007-05-090928 [DOI] [PubMed] [Google Scholar]
- [134].Bozza S, Perruccio K, Montagnoli C, Gaziano R, Bellocchio S, Burchielli E, Nkwanyuo G, Pitzurra L, Velardi A, Romani L. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 2003; 102:3807-14; PMID:12791648; http://dx.doi.org/ 10.1182/blood-2003-03-0748 [DOI] [PubMed] [Google Scholar]
- [135].Montagnoli C, Perruccio K, Bozza S, Bonifazi P, Zelante T, De Luca A, Moretti S, D'Angelo C, Bistoni F, Martelli M, et al.. Provision of antifungal immunity and concomitant alloantigen tolerization by conditioned dendritic cells in experimental hematopoietic transplantation. Blood Cells Mol Dis 2008; 40:55-62; PMID:17827038; http://dx.doi.org/ 10.1016/j.bcmd.2007.06.016 [DOI] [PubMed] [Google Scholar]
- [136].Beck O, Topp MS, Koehl U, Roilides E, Simitsopoulou M, Hanisch M, Sarfati J, Latge JP, Klingebiel T, Einsele H, et al.. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood 2006; 107:2562-9; PMID:16322466; http://dx.doi.org/ 10.1182/blood-2005-04-1660 [DOI] [PubMed] [Google Scholar]
- [137].Kumaresan PR, Manuri PR, Albert ND, Maiti S, Singh H, Mi T, Roszik J, Rabinovich B, Olivares S, Krishnamurthy J, et al.. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc Natl Acad Sci U S A 2014; 111:10660-5; PMID:25002471; http://dx.doi.org/ 10.1073/pnas.1312789111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Clemons KV, Grunig G, Sobel RA, Mirels LF, Rennick DM, Stevens DA. Role of IL-10 in invasive aspergillosis: increased resistance of IL-10 gene knockout mice to lethal systemic aspergillosis. Clin Exp Immunol 2000; 122:186-91; PMID:11091273; http://dx.doi.org/ 10.1046/j.1365-2249.2000.01382.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Del Sero G, Mencacci A, Cenci E, d'Ostiani CF, Montagnoli C, Bacci A, Mosci P, Kopf M, Romani L. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect 1999; 1:1169-80; PMID:10580272; http://dx.doi.org/ 10.1016/S1286-4579(99)00245-2 [DOI] [PubMed] [Google Scholar]
- [140].Keen LJ, DeFor TE, Bidwell JL, Davies SM, Bradley BA, Hows JM. Interleukin-10 and tumor necrosis factor α region haplotypes predict transplant-related mortality after unrelated donor stem cell transplantation. Blood 2004; 103:3599-602; PMID:14701704; http://dx.doi.org/ 10.1182/blood-2002-11-3568 [DOI] [PubMed] [Google Scholar]
- [141].Sainz J, Hassan L, Perez E, Romero A, Moratalla A, Lopez-Fernandez E, Oyonarte S, Jurado M. Interleukin-10 promoter polymorphism as risk factor to develop invasive pulmonary aspergillosis. Immunol Lett 2007; 109:76-82; PMID:17321603; http://dx.doi.org/ 10.1016/j.imlet.2007.01.005 [DOI] [PubMed] [Google Scholar]
- [142].Seo KW, Kim DH, Sohn SK, Lee NY, Chang HH, Kim SW, Jeon SB, Baek JH, Kim JG, Suh JS, et al.. Protective role of interleukin-10 promoter gene polymorphism in the pathogenesis of invasive pulmonary aspergillosis after allogeneic stem cell transplantation. Bone Marrow Transplant 2005; 36:1089-95; PMID:16247433; http://dx.doi.org/ 10.1038/sj.bmt.1705181 [DOI] [PubMed] [Google Scholar]
- [143].Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, et al.. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol 2007; 37:2695-706; PMID:17899546; http://dx.doi.org/ 10.1002/eji.200737409 [DOI] [PubMed] [Google Scholar]
- [144].Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, et al.. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun 2011; 79:3966-77; PMID:21807912; http://dx.doi.org/ 10.1128/IAI.05493-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Urb M, Snarr BD, Wojewodka G, Lehoux M, Lee MJ, Ralph B, Divangahi M, King IL, McGovern TK, Martin JG, et al.. Evolution of the Immune Response to Chronic Airway Colonization with Aspergillus fumigatus Hyphae. Infect Immun 2015; 83:3590-600; PMID:26123803; http://dx.doi.org/ 10.1128/IAI.00359-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Chai LY, van de Veerdonk F, Marijnissen RJ, Cheng SC, Khoo AL, Hectors M, Lagrou K, Vonk AG, Maertens J, Joosten LA, et al.. Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology 2010; 130:46-54; PMID:20002791; http://dx.doi.org/ 10.1111/j.1365-2567.2009.03211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].El-Muzghi AA, Mirkov I, Djokic J, Popov Aleksandrov A, Miljkovic D, Glamoclija J, Kataranovski D, Kataranovski M. Regional cytokine responses to pulmonary aspergillosis in immunocompetent rats. Immunobiology 2013; 218:1514-23; PMID:23810257; http://dx.doi.org/ 10.1016/j.imbio.2013.05.007 [DOI] [PubMed] [Google Scholar]
- [148].Carvalho A, Cunha C, Di Ianni M, Pitzurra L, Aloisi T, Falzetti F, Carotti A, Bistoni F, Aversa F, Romani L. Prognostic significance of genetic variants in the IL-23/Th17 pathway for the outcome of T cell-depleted allogeneic stem cell transplantation. Bone Marrow Transplant 2010; 45:1645-52; PMID:20173782; http://dx.doi.org/ 10.1038/bmt.2010.28 [DOI] [PubMed] [Google Scholar]
- [149].Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, et al.. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 2005; 202:597-606; PMID:16147975; http://dx.doi.org/ 10.1084/jem.20050749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Wharton RE, Stefanov EK, King RG, Kearney JF. Antibodies generated against Streptococci protect in a mouse model of disseminated aspergillosis. J Immunol 2015; 194:4387-96; PMID:25821219; http://dx.doi.org/ 10.4049/jimmunol.1401940 [DOI] [PMC free article] [PubMed] [Google Scholar]