ABSTRACT
In a recent report by Zhang et al., pleiotrophin (PTN) was demonstrated to enhance glioma growth by promoting vascular abnormalization. PTN stimulates glioma vessels through anaplastic lymphoma kinase (Alk)-mediated perivascular deposition of vascular endothelial growth factor (VEGF). Targeting of Alk or VEGF signaling normalizes tumor vessels in PTN-expressing tumors.
KEYWORDS: ALK, GL261, glioblastoma, pleiotrophin, vascular abnormalization, VEGF
Glioma is a group of malignant brain tumors that includes various grades and histopathological and molecular subclasses. Because of their highly invasive growth pattern complete neurosurgical resection is not possible, leading to tumor relapse and progression to more malignant disease. Glioblastoma (WHO grade IV glioma) is the most aggressive type of glioma, with a median survival of only 12–15 months.1 A markedly abnormal tumor vasculature is a hallmark of glioblastoma. Multiple layers of endothelial cells, pericytes, and smooth muscle cells and a thick basement membrane characterize these vessels, which are termed glomeruloid bodies. Vessels in glioblastoma show a distinct gene expression signature compared with normal brain, at least in part due to increased transforming growth factor (TGF)-β and vascular endothelial growth factor (VEGF) signaling in the tumor microenvironment.2 The abnormal glioblastoma vessels are poorly perfused and highly permeable, aggravating the disease by inducing hypoxia and brain edema. In addition, glioblastoma vessels provide a perivascular niche for glioblastoma stem-like cells, which are believed to be resistant to current therapies and important for tumor regrowth after treatment.3
Pleiotrophin (PTN) is a small angiogenic heparin-binding cytokine that is expressed in the brain during embryonic development and is often upregulated in tumors, including glioma.4 PTN binds to and inactivates protein tyrosine phosphatase, receptor-type, Z polypeptide 1 (PTPRZ1), increasing the phosphorylation level of its substrates. In addition, PTN activates the anaplastic lymphoma receptor tyrosine kinase (ALK).4 By ribozyme-targeting of PTN, Grzelinski and colleagues were the first to show that PTN is a rate-limiting growth factor in experimental glioma that intrinsically expresses the PTN receptors PTPRZ1 and ALK.5 In a recent study by Zhang et al., we demonstrated that vascular abnormalization is an additional mechanism through which tumor cell expression of PTN promotes glioma growth.6
We found that increased PTN abundance correlated with malignancy grade in glioma and that a high tumoral expression of PTN was associated with poor survival in patients with grade II–III astrocytoma or glioblastoma. High PTN expression was found in low-grade gliomas harboring wild-type IDH1 (isocitrate dehydrogenase 1) and wild-type ATRX (α-thalassemia/mental retardation syndrome X-linked). This subgroup of gliomas has recently been shown to be molecularly and clinically similar to glioblastoma and has a poor clinical outcome.7 In glioblastoma, PTN was highly expressed in G-CIMP (glioma-CpG island methylator phenotype)-negative tumors, whereas lower levels were found in G-CIMP-positive tumors, which is a feature of secondary glioblastoma.6, 8
Using GL261 and CT2A glioma cell lines, which express negligible levels of PTN receptors Ptprz1 and Alk and do not respond to PTN stimulation in vitro, we showed that PTN can promote tumor growth indirectly by stimulating the tumor microenvironment.6 Tumor growth was accelerated in mice bearing PTN-producing GL261 tumors compared to control GL261 tumors, leading to poor survival. Similarly, knockdown of endogenous Ptn in CT2A cells by shRNA led to significantly decreased tumor growth. PTN-producing GL261 gliomas displayed higher proliferation of tumor endothelial cells and strikingly abnormal tumor vessels, characterized by increased vascular area and vascular diameter. These abnormal vessels showed poor vessel functionality as indicated by a reduced proportion of lectin-perfused vessels.6 The role of PTN in promoting vascular abnormalization was further supported by analysis of human glioma tissue microarrays showing that the presence of PTN in human glioblastoma was significantly correlated to increased vascular area and larger vessel diameter.
We found that the PTN receptor ALK was expressed in mural cells in the glioma vasculature in human tumors and GL261 experimental glioma, but not in control brain vessels. Interestingly, when tumor-bearing mice were treated with ALK inhibitors, tumor growth was reduced and tumor vessels were normalized in PTN-producing GL261 tumors but not in control GL261 tumors, demonstrating that PTN promotes tumor growth and vascular abnormalization through Alk signaling. Notably, an increased deposition of VEGF proximal to the tumor vasculature was detected in PTN-producing GL261 tumors, which was reduced after ALK inhibition. Furthermore, inhibition of VEGF receptor signaling using the small tyrosine kinase inhibitor cediranib reduced tumor growth and inhibited vessel abnormalization in PTN-producing GL261 tumors, but not in control GL261 tumors. Taken together, our results indicate that PTN stimulates glioma growth and vascular abnormalization through an Alk-mediated increase in VEGF in direct proximity to the vasculature (Fig. 1).6
Figure 1.
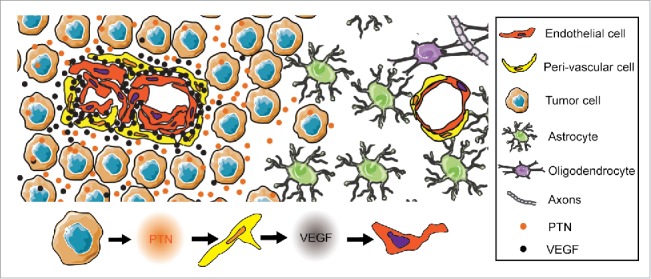
Pleiotrophin (PTN) promotes vascular abnormalization in glioblastoma. PTN stimulates anaplastic lymphoma receptor tyrosine kinase (ALK)-expressing perivascular cells in the glioblastoma vasculature, resulting in increased vascular endothelial growth factor (VEGF) deposition in direct proximity to the vasculature.
Glioblastoma is the most deadly and aggressive type of brain tumors, and novel treatments are urgently needed. Since the glioblastoma vasculature is molecularly and morphologically distinct from normal vessels and contributes to patient morbidity, antiangiogenic treatment has emerged as a promising treatment strategy. However, randomized phase III trials in newly diagnosed glioblastoma have failed to show an overall survival benefit for VEGF targeting alone or in combination with chemotherapy.9 Batchelor and colleagues reported that glioblastoma patients that showed increased tumor perfusion after treatment with the VEGF-receptor tyrosine kinase inhibitor cediranib had improved survival, recognizing the need for predictive molecular biomarkers to identify patients that will respond to antiangiogenic therapy by vascular normalization.10 In our study, PTN-producing GL261 tumors, but not control tumors, responded to cediranib by vascular normalization and decreased tumor growth. This indicates that PTN may serve as a biomarker to identify patients that might benefit from VEGF receptor tyrosine kinase inhibition or anti-VEGF antibody treatment, warranting further studies in clinical cohorts of glioma patients to confirm or discard this hypothesis.
The role of PTN in driving vascular abnormalization in glioma, together with its previously established role in stimulating glioma cells, suggests that PTN and/or its downstream signaling molecules may serve as a potential therapeutic targets for glioblastoma treatment by neutralizing antibodies or small-molecule inhibitors. The ALK inhibitor crizotinib has already been approved for the treatment of non-small cell lung carcinoma and is undergoing clinical trials for treatment of other tumor types. We believe that targeting PTN signaling, in combination with current state-of-the-art treatments, would benefit patients with glioblastoma through vascular normalization, thus increasing drug delivery to the tumor and reducing brain edema.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the Swedish Cancer Society (CAN 2011/862), the Swedish Childhood Cancer Society (PR2013-0107, PROJ11/083) and the Swedish Research Council (2013-3797, 2008-2853).
References
- 1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathologica 2007; 114:97–109; PMID:17618441; http://dx.doi.org/ 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dieterich LC, Mellberg S, Langenkamp E, Zhang L, Zieba A, Salomaki H, Teichert M, Huang H, Edqvist PH, Kraus T, et al. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFbeta2 in vascular abnormalization. J Pathol 2012; 228:378–90; PMID:22786655; http://dx.doi.org/ 10.1002/path.4072 [DOI] [PubMed] [Google Scholar]
- 3. Dimberg A. The glioblastoma vasculature as a target for cancer therapy. Biochem Soc Trans 2014; 42:1647–52; PMID:25399584; http://dx.doi.org/ 10.1042/BST20140278 [DOI] [PubMed] [Google Scholar]
- 4. Perez-Pinera P, Berenson JR, Deuel TF. Pleiotrophin, a multifunctional angiogenic factor: mechanisms and pathways in normal and pathological angiogenesis. Curr Opin Hematol 2008; 15:210–4; PMID:18391787; http://dx.doi.org/ 10.1097/MOH.0b013e3282fdc69e [DOI] [PubMed] [Google Scholar]
- 5. Grzelinski M, Bader N, Czubayko F, Aigner A. Ribozyme-targeting reveals the rate-limiting role of pleiotrophin in glioblastoma. Int J Cancer J Int du Cancer 2005; 117:942–51; PMID:15986444; http://dx.doi.org/ 10.1002/ijc.21276 [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Kundu S, Feenstra T, Li X, Jin C, Laaniste L, El Hassan TE, Ohlin KE, Yu D, Olofsson T, et al. Pleiotrophin promotes vascular abnormalization in gliomas and correlates with poor survival in patients with astrocytomas. Sci Signaling 2015; 8:ra125; PMID:26645582; http://dx.doi.org/ 10.1126/scisignal.aaa1690 [DOI] [PubMed] [Google Scholar]
- 7. Cancer Genome Atlas Research N, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. New Engl J Med 2015; 372:2481–98; PMID:26061751; http://dx.doi.org/ 10.1056/NEJMoa1402121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, et al. The somatic genomic landscape of glioblastoma. Cell 2013; 155:462–77; PMID:24120142; http://dx.doi.org/ 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu-Emerson C, Duda DG, Emblem KE, Taylor JW, Gerstner ER, Loeffler JS, Batchelor TT, Jain RK. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J Clin Oncol: Off J Am Soc Clin Oncol 2015; 33:1197–213; PMID:25713439; http://dx.doi.org/ 10.1200/JCO.2014.55.9575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, Ancukiewicz M, Polaskova P, Pinho MC, Jennings D, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A 2013; 110:19059–64; PMID:24190997; http://dx.doi.org/ 10.1073/pnas.1318022110 [DOI] [PMC free article] [PubMed] [Google Scholar]


